Abstract
We assessed effects of alcohol consumption on different types of working memory (WM) tasks in an attempt to characterize the nature of alcohol effects on cognition. The WM tasks varied in two properties of materials to be retained in a two-stimulus comparison procedure. Conditions included (1) spatial arrays of colors, (2) temporal sequences of colors, (3) spatial arrays of spoken digits, and (4) temporal sequences of spoken digits. Alcohol consumption impaired memory for auditory and visual sequences, but not memory for simultaneous arrays of auditory or visual stimuli. These results suggest that processes needed to encode and maintain stimulus sequences, such as rehearsal, are more sensitive to alcohol intoxication than other WM mechanisms needed to maintain multiple concurrent items, such as focusing attention on them. These findings help to resolve disparate findings from prior research into alcohol’s effect on WM and on divided attention. The results suggest that moderate doses of alcohol impair WM by affecting certain mnemonic strategies and executive processes rather than by shrinking the basic holding capacity of WM.
Keywords: Alcohol, Ethanol, Intoxication, Working Memory, Rehearsal, Attention, Scope of Attention
There is widespread agreement that acute alcohol intoxication affects cognitive functioning and that this cognitive impairment could mediate many diverse behavioral and affective consequences of alcohol intoxication. However, there has been considerable disagreement on how to characterize the cognitive effects of alcohol. This study addresses that question by documenting that a key component of cognition, working memory (WM), includes at least one process that is affected by acute alcohol intoxication and at least one process that is relatively spared. This detailed understanding is important, inasmuch as WM may be critically involved in most complex behaviors (e.g., Baddeley, 1986, 2001; Cowan, 1999, 2001).
We define WM as the temporary maintenance of a limited amount of information in a heightened state of availability for use in cognitive tasks (cf. Cowan, 1999). Some consider WM to include the processes used to reactivate information in storage, such as covert rehearsal, or to manipulate the stored information (e.g., Baddeley, 1986, 2001). The exact definition is not critical for our purposes, provided that one keeps in mind that either strategic processing or storage theoretically could be affected by alcohol.
The maintenance of some of the information in WM is thought to require attention (Cowan, 1999, 2001) as well as strategic processing such as rehearsal (Baddeley, 1986, 2001). (The amount of attention needed for rehearsal depends on the type of rehearsal; and later we discuss contributions of automatic storage processes, like sensory memory, that do not require attention.) Attention and rehearsal also play prominent roles in leading theories of alcohol intoxication. Steele and Josephs (1990) proposed an attention-allocation hypothesis in which alcohol impairs cognitive processing in such a way that the drinker can perceive and focus on only the most immediate cues and a situation's most salient features. This may be tantamount to having less information in an attention-dependent portion of WM (Cowan, 2001). In his appraisal-disruption model, Sayette (1993, 1999) argued that alcohol’s most potent effects occur during the encoding of new information. If alcohol is consumed before the onset of anxiety-eliciting cues, it will disrupt the cognitive appraisal of those cues, and we believe that this could emerge from impaired storage and/or processing of those cues in WM.
Curtin, Patrick, Lang, Cacioppo, and Birbaumer (2001) demonstrated that alcohol reduced anticipatory fear and response inhibition in human participants not by directly suppressing emotion centers but instead by impairing cognitive-processing capacity. The hypothesis that the impairment in response inhibition involves components of WM is consistent with the finding of Finn, Justus, Mazas, and Steinmetz (1999) that alcohol increased impulsive responding in subjects with low WM capacity, as well as the finding by Finn and Hall (2004) that WM capacity moderated the association between social deviance and alcohol problems.
Nevertheless, the few studies that have examined alcohol’s acute effects on WM have had mixed results. For example, Finn et al. (1999) found that alcohol (mean BACs of 0.07 and 0.09) reduced WM, measured by a backward digit span, but only in participants with a high baseline WM capacity (based on a median split). Grattan-Miscio and Vogel-Sprott (2005) assessed WM using a memory scanning task and found impaired speed and accuracy during moderate rising BACs (from 0.07 to 0.08). On the other hand, Schweizer et al. (2006) found no significant impairment of an immediate verbal WM task (testing recall of three consonants after 18-s counting backwards) during ascending or descending BACs which ranged from 0.08 to 0.09. Schweizer et al. did find that alcohol impaired performance on a visual-spatial WM task (testing memory of three locations after a 30-s visual-spatial distraction task) during declining BAC (from 0.09 to 0.08), suggesting that alcohol effects on WM may be modality specific. However, Paulus, Tapert, Pulido, and Schuckit (2006) used a different visual-spatial WM task than Schweizer et al. (a visual array comparison task after Luck and Vogel, 1997) and found no significant effect of alcohol (at a mean BAC of 0.06). Similarly, Weissenborn and Duka (2003) failed to find that alcohol impaired spatial working memory (remembering search locations in a self-ordered search task) or pattern recognition (recognizing patterns from a previous sequence of 12 patterns), but did find that it impaired planning (in a Tower of London test) and spatial recognition (recognizing locations from a previous sequence of 5 locations). Weissenborn and Duka reported mean BACs of 0.06 when testing began and 0.05 after it ended 30 min later.
The variability across studies in the effects of acute alcohol intoxication on WM appears to be contingent upon one or more individual difference or task factors. It seems likely that certain kinds or components of WM might be differentially sensitive to alcohol. Alcohol’s effects could depend on WM tasks’ encoding or response demands. Varied results also could be related to experimental procedures that assessed performance at different rising or falling blood alcohol concentrations (BACs). Differences among sample populations might further contribute to inconsistent results across studies. For example, sample groups with different baseline WM capacities might be differentially impaired by alcohol, based on the findings of Finn et al. (1999). Other participant characteristics, like drinking experience, tolerance, age, and health, also might differ across studies and influence their outcomes.
Although it is important to understand the range of individual differences to the effects of alcohol, the focus of this study is on task variables, so we restricted our sample to young, healthy, moderate drinkers. The aim of the present research is to clarify alcohol’s effect on WM by directly comparing its differential effects on several relatively simple tasks chosen to tap particular WM processes. One can distinguish between the transient, strategy-free maintenance of information in storage on one hand, and effects of strategic processing like grouping (Hitch, Burgess, Towse, & Culpin, 1996) and covert verbal rehearsal (Baddeley, 1986, 2001), on the other. In one type of WM task, verbal items are presented in a spoken list to be remembered. In these tasks, rehearsal is critical; preventing rehearsal by articulatory suppression during reception of the list compromises performance in several ways (e.g., Baddeley, Lewis, & Vallar, 1984) with both printed and spoken lists (e.g., Cowan, Cartwright, Winterowd, & Sherk, 1987). Moreover, lists of nonverbal items that can be rehearsed using verbal labels also benefit from rehearsal (e.g., Hitch, Halliday, Dodd, & Littler, 1989).
In another type of WM task (Luck & Vogel, 1997) nonverbal items to be remembered are presented in a simultaneous visual array. On each trial, a randomly arranged array of different colored squares is briefly presented, followed shortly by a second array, identical to the first or differing only in the color of one square. The memory test is to detect whether a color change between the two arrays has occurred. There is clear evidence that this type of memory does not require rehearsal (Morey & Cowan, 2004), but there does seem to be a heavy demand on general encoding and storage processes in order to encode and retain multiple items concurrently. Reflecting this general demand, Morey and Cowan found a strong effect from imposing a random 7-digit memory load, which not only should have blocked rehearsal but also should have engaged the storage mechanisms of working memory. The effect of a load on array comparison performance was strongest when an error was made on the 7-digit load, which would elicit error-monitoring processes. This general pattern of results suggests that array memory depends on a general attention mechanism that is used to encode and retain multiple items in arrays concurrently (e.g., Cowan et al., 2005).
Few studies of short-term memory have utilized spatial arrays of three or more sounds. Darwin, Turvey, & Crowder (1972) and Moray, Bates, and Barnett (1964) simulated three and four locations, respectively, over headphones, and examined the recall of concurrent lists from the different locations. Moray et al. found that several list items from the same location could be recalled in order, but the order of items from different locations was poorly recalled. This suggest that it may have been impossible for participants to rehearse items across spatial locations within a concurrent array, Darwin et al. found rapid forgetting over several seconds indicating that concurrent auditory stimuli were not rehearsed or rapid forgetting would not occur. More recently, Saults and Cowan (in press) used the same auditory array and visual array tasks as the current study and found that both tasks share a general storage capacity limit that is supplemented, in the absence of sensory masks, by automatic, modality-specific, sensory storage.
In the present study, we varied two properties of materials to be retained in a common, two-stimulus comparison procedure modeled after Luck and Vogel (1997). Specifically, we manipulated the sequential versus simultaneous presentation and the sensory modality of items, keeping other task parameters constant. We thus included (1) spatial arrays of colors, (2) a temporal sequence of colors, (3) a spatial array of spoken digits, and (4) a temporal sequence of spoken digits. In this way, we could distinguish between effects of alcohol intoxication on WM in each stimulus modality, and effects of WM on arrays versus sequences. In most studies of working memory, the simultaneous versus sequential nature of item presentation is confounded with the visual versus auditory modality of the stimuli. Our design compared presentation methods using similar tasks without this confound and allowed us to independently estimate effects attributable to modality and the timing of presentation.
In selecting tasks and optimizing them for the study, we chose relatively simple tasks that should not place much demand on executive processes required to allocate, switch, share, or maintain attention. This contrasts with more complex WM tasks that have become popular in studies of individual differences in cognition (for a review see Conway et al., 2005). Based on the assumption that a common set of resources is used within working memory to store and process information, many tests have included separate components to engage both storage and processing. For example, in the reading span task (Daneman & Carpenter, 1980), participants must comprehend several sentences while remembering the last word of each sentence for later recall. Recently, some have questioned the value of such complex tasks because: (1) individuals differ in the ability to engage in covert verbal rehearsal, especially across age groups, and (2) when the possibility of rehearsing is removed, individuals differ in basic capacity in a way that is rather strongly related to attention and cognitive aptitude differences (Cowan et al., 2005, 2006; Lépine, Barrouillet, & Camos, 2005). The latter tasks can be simple ones in which so much information is presented at once that rehearsal is impractical, as in the present array tasks.
Theory and prior research leads to several alternative predictions. If alcohol intoxication affects the basic holding mechanisms of WM, it should affect performance on all four types of WM task. However, if alcohol intoxication affects the use of attention to encode and retain multiple items concurrently, it should have a much stronger effect on arrays than on sequences. Alternatively, if alcohol intoxication affects the ability to rehearse or in some other way to overcome the disadvantages of extending information over time in stimulus sequences, then it should affect performance only on sequences. If either effect is mostly central, then modality should make little difference. However, modality effects would suggest that modality-specific storage or peripheral sensory/perceptual processes are important. For example, alcohol might selectively affect visual-spatial storage because its control might take more attention than in audition or because alcohol disrupts eye movements (Nawrot, Nordenstrom, & Olson, 2004), which could impede visual WM tasks. Conversely, though, alcohol can impair processing of tones and frequency change (Kähkönen, Marttinen, & Yamashita, 2005), which could impede auditory WM tasks.
Method
Participants
Participants were 72 moderate social drinkers (36 female), 21–30 years old, who qualified according to a telephone screening interview which excluded anyone who reported that they ever had been in treatment for substance abuse problems, had been arrested for any alcohol-related offense or any violence-related offense, had any medical conditions resulting from alcohol or drug abuse, or deliberately attempted to abstain from alcohol due to a fear of having or developing alcoholism or other alcohol-related health problems. Also excluded was anyone who reported a history of chronic psychological problems, diabetes, hyperglycemia, hypoglycemia, hemophilia, hepatitis, jaundice, gastritis, seizure disorder, neurological disorder, heart trouble, high blood pressure, or fainting. To ensure that the alcohol dose received in the study would be within participants’ normal range of experience, light drinkers (i.e. individuals reporting an average of less than 2 drinks/week) and very heavy drinkers (individuals reporting an average of 25 or more drinks/week) were excluded from the study sample. Equal numbers of men and women were assigned randomly to alcohol, placebo, and no-alcohol conditions.
Participants were to follow a pre-experimental protocol that included refraining from any alcohol or drug use for 24 hours prior to their appointment, eating a light meal 4–6 hours prior to their appointment, and refraining from strenuous physical exercise within 3 hours of their appointment. Upon arrival at the lab, participants signed affidavits that assured their compliance to the pre-experimental protocol and their reported general health, drinking habits, and absence of major medical conditions. One participant was disqualified for failure to comply with pre-experimental protocol because she had taken a prescription medication that morning before her session. Female participants took a hormonal pregnancy test prior to their experimental session; no positive results were obtained. All participants were administered one BAC measurement via breath analysis (see below) before testing to exclude individuals who evidenced recent drinking in contradiction to their self-report.
The quantity and frequency of alcohol use by the 72 participants were assessed by a questionnaire assessing typical frequency and typical quantity over the past year. Our participants reported consuming alcohol an average of about 2.1 (SD=1.9) occasions per week and 4.8 (SD=2.0) drinks per occasion. Also, a composite alcohol quantity/frequency estimate was created by summing per week alcohol quantity estimates for beer, wine, liquor, and wine coolers during the past 30 days. Based on this composite, our participants consumed an average of about 12 (SD=10.3) drinks per week during the past 30 days.
Apparatus, Stimuli, and Procedure
Beverage administration
Testing included a phase to establish baseline, a pre-intoxication phase, and two successive post-intoxication phases (or tests at equivalent times in control groups). The beverage was administered immediately after the second of four tests. In the alcohol condition, the experimenter mixed a beverage of 1 part 100 proof Smirnoff™’s vodka to 4 parts tonic, measured to yield a dose of 0.72 g ethanol per kg of weight for men, and 0.65 g/kg for women. The placebo dose was 5% of that, achieved by administered vodka–diluted, flattened tonic water poured from a 100 proof Smirnoff™ vodka bottle in the same proportions as that used in the moderate dose condition. In the no-alcohol condition, the experimenter poured the beverage directly from a newly opened bottle of tonic. The volume of beverage by body weight was the same in all three conditions (except as moderated by sex as described). The mixed beverage was divided into three drinks and topped with lime juice for flavor. Participants finished each drink in consecutive 5-minute intervals and then sat idle for a 15-minute absorption period before beginning the first post-treatment memory test.
Measurement of blood alcohol concentration (BAC) levels
BAC levels were measured using an Alco-Sensor IV Intoximeter (Intoximeters, Inc., St. Louis, MO), calibrated using a dry gas standard mix containing .08% ethanol. All participants were administered one BAC measurement before testing, to ensure they were free of alcohol. Participants in the alcohol and placebo conditions were administered additional BAC measurements just before and just after the third and fourth tests. Thereafter, participants in the alcohol condition were administered BAC measurements until it was below the release threshold of 0.02%.
Subjective intoxication measures
All participants completed two questionnaires intended to measure their subjective experience of intoxication. The first questionnaire, the Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993), was administered 5 minutes before the BAC measurements preceding the first, third, and fourth test batteries (described below). The BAES is a self-report measure in which participants use a 10-point scale to rate the extent to which they are experiencing seven states associated with stimulation (e.g., elated, excited) and seven states associated with sedation (e.g., down, sluggish). We revised the instructions of Martin et al. by omitting any reference to alcohol and simply asking participants to “Please use the following 1–10 scale to indicate how you feel RIGHT NOW.” This change allows the scale to be used during the pre-treatment phase and by the no-alcohol group. A post-experimental questionnaire, designed to assess participants’ subjective intoxication and motivation during the study, was administered 5 minutes after the end of the last test battery. Five items asked participants to rate how intoxicated they felt during different phases of the experiment. Five more items asked participants to rate how they felt about various aspects of the procedures. Participants also used a 0 to 20 scale to estimate the number of standard alcohol drinks they believed they consumed.
Memory testing
Memory tests were conducted using a Pentium 4 computer with a 17 inch color monitor. Auditory stimuli were reproduced by four small speakers arranged in a semicircle. When participants faced the screen at a viewing distance of 50 cm, the speakers were 30 and 90 degrees to the left and to the right of their line-of-sight, at a distance of 1 meter.
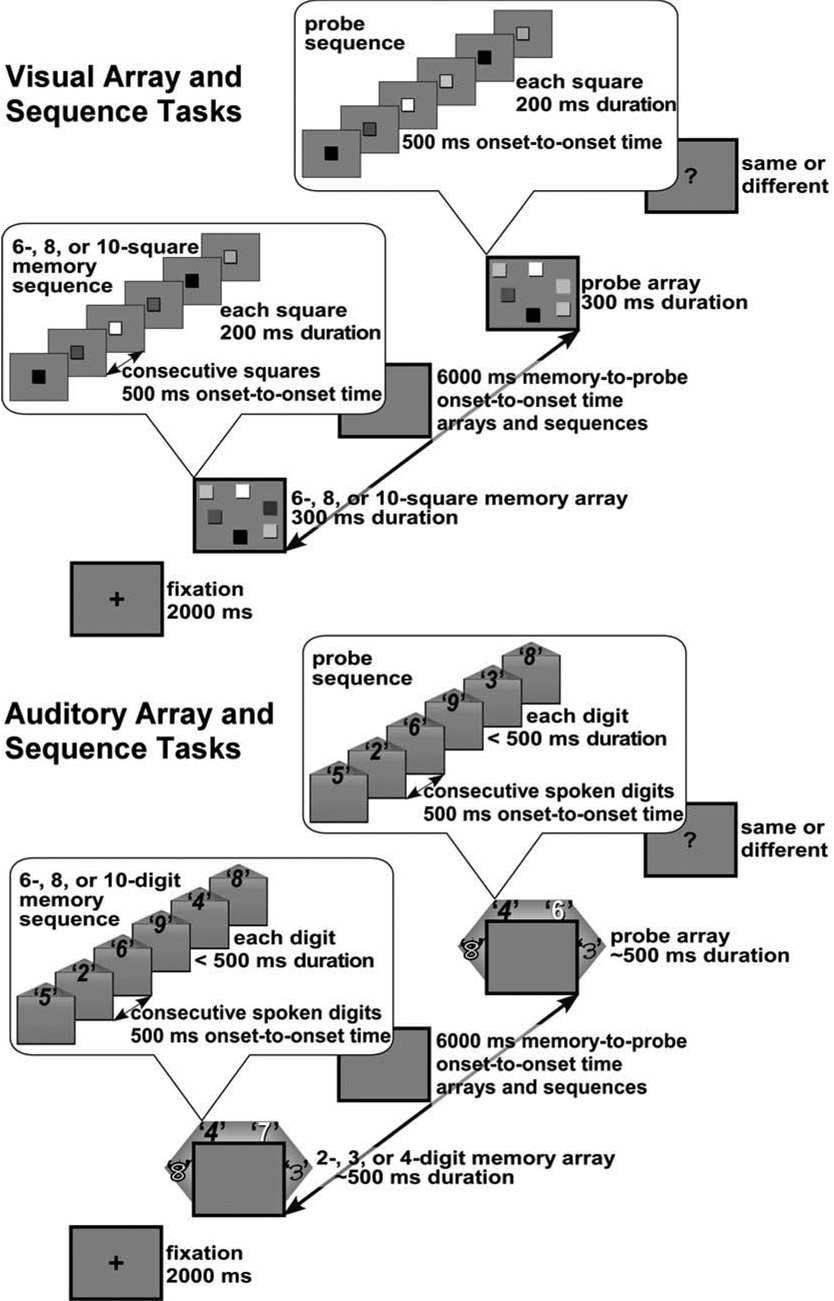
Each of the four memory tasks, illustrated in Figure 1, involved the presentation of two sets of stimuli, either both as spatial arrays or both as temporal sequences, with 6s between the onset times of the two sets. The tasks were designed to be as similar as possible except for the contrasting modalities, visual or auditory, and presentation styles, simultaneous or sequential. The Visual Array (VA) task and Auditory Array (AA) task used simultaneous arrays of colored squares or spoken digits, respectively, and the Visual Sequence (VS) task and Auditory Sequence (AS) task used lists of these same stimuli. In each task, an initial set of memory stimuli was to be compared to a subsequent set of probe stimuli to determine whether one item had changed or whether the two arrays were identical. The subject initiated each trial and later responded with a key press. Feedback was displayed after every trial.
Figure 1.
Schematic illustration of the visual (top panel) and auditory (bottom panel) memory tasks. In each panel, the enclosed inserts show how the sequential task corresponds to the array task, except that memory and probe sequential stimuli replace memory and probe array stimuli, with the same onset times. For visual stimuli, shading of squares represents different colors. For auditory stimuli, text styles represent different voices. In an auditory array, each voice came from a different loudspeaker. In an auditory sequence, only one voice was used and it came from the two front speakers and thus seemed to come from the center.
The number of stimuli, or memory set size, varied from trial to trial, randomly intermixed within each block of trials for each task. Half of the trials at each set size within a block were no-change trials, on which the memory and probe stimuli were identical. The other half were change trials, in which only one stimulus differed between the memory and probe stimuli. New trials were generated for each block of trials for each subject.
The VA task was adapted from Luck and Vogel (1997) and used similar stimuli: arrays of 6, 8, or 10 colored squares (6.0 mm × 6.0 mm), on a gray background, arranged in random locations within a rectangular display area (74 mm wide by 56 mm high) centered on the screen. Both memory and probe arrays were displayed for 300 ms.
The VS task used the same colored squares as the visual array task but presented them one at a time in the center of the screen, with a 200-ms duration and 500-ms onset-to-onset time.
The AA task was based on the 4-item auditory array task used by Saults and Cowan (in press). Stimuli in the AA task consisted of 2, 3, or 4 digitally-recorded spoken digits from the set 1 – 9, presented simultaneously, each from a different loudspeaker. With participants 50 cm from the viewing screen, the speakers were arranged within a circle, −90, −30, +30 and +90 degrees from the line of sight, about 1 meter from the participant's head, and 121 cm off the floor. To help distinguish the spatial channels and reduce mutual interference from simultaneous masking, each speaker location was consistently associated with stimuli from a particular human voice. The words were spoken by female child, a male child, a female adult, and a male adult. The combined intensity of the four voices was about 70–75 dB(A) at each of the subjects' ears. The digits in each array were randomly selected, with replacement. On change trials, the loudspeaker with the stimulus change was balanced across trials so that the new digit occurred equally often in each voice and location within a block. For array sizes of 2 and 3 words, the loudspeakers used in each trial were randomly selected but balanced across trials.
The AS task used the same stimuli as the AA task, but the spoken digits were presented one at a time. The two front speakers played each digit in a sequence simultaneously and with equal intensity so that it seemed to come from the center. The auditory sequences consisted of 6, 8, or 10 digits, randomly selected with replacement. All digits in each trial were spoken in the same voice and presented with a 500-ms onset-to-onset time.
The experiment included four test blocks, each containing all four tasks (VA, VS, AA, and AS). The order of the four tasks within each test block for each participant was determined by a Latin square, replicated three times for each gender and treatment group. The tasks then occurred in the same order in all four test blocks within a participant.
At the start of each task in test block 1, the experimenter read the task instructions for the first task while they were displayed on the computer screen. After completing four practice trials under the experimenter’s supervision, the task continued with 36 experimental trials, including 6 change and 6 no-change trials at each of three set sizes in a random order. This procedure was then repeated for all four tasks in the first test block. Sets sizes were 6, 8, and 10 for the VA, VS, and AS tasks, and 2, 3, and 4 for the AA task because of the greater difficulty of perceiving acoustic arrays. Test block 1 lasted about 30 to 45 minutes.
Test block 2 began 60 minutes after the start of the first test. Test block 2 and the two subsequent test blocks were like the first, but shorter. Each of the four tasks within a test block had one practice trial and 24 experimental trials, 12 at each of two set sizes, determined by the participant’s performance in the first test. For each task, if a participant was correct on at least 75% of the trials at the highest set size in test block 1, then the two higher set sizes were used in subsequent test blocks. Otherwise, the two lower set sizes were used. Test blocks 2, 3, and 4 each took about 20 to 25 minutes.
Test block 2 began 60 minutes after the start of test block 1. As soon as it was completed, the beverage was administered. Test block 3 began after the BAC measurement following the absorption period. The fourth and final test block began 120 minutes after the start of test block 3. Except for the item composition of individual trials, the third and fourth test blocks were identical to the second.
Results
Manipulation Checks
Alcohol dose
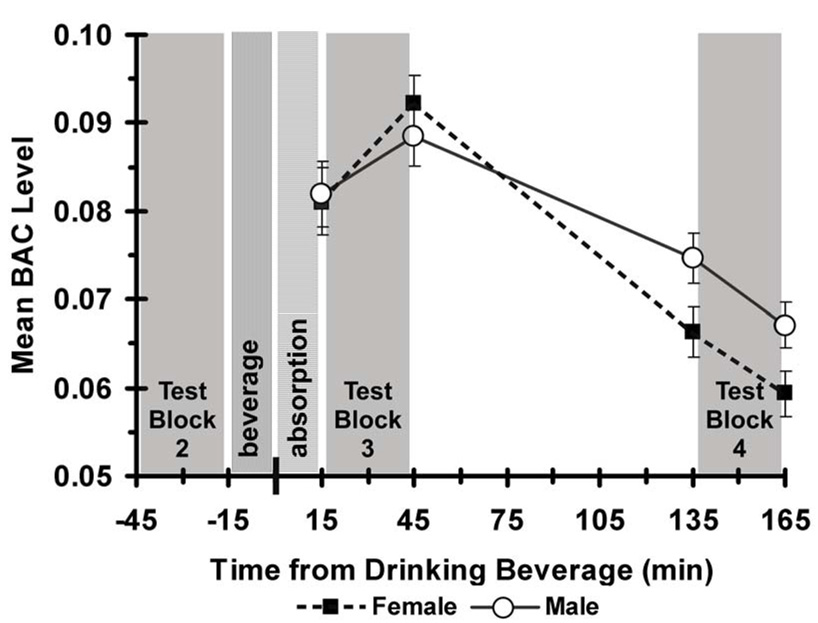
The pre-intoxication BAC measurements in all groups, and all measurements in the placebo and the no-alcohol groups, were 0.00%. The administration of alcohol produced the expected BAC levels. As intended, the mean BAC after the absorption period and just before test block 3 was 0.082 (SD=0.013), just over the legal driving limit of intoxication in Missouri. It increased to 0.090 (SD=0.011) just after test block 3, and fell to 0.070% (SD=0.010) just before test block 4, and to 0.063 (SD=0.009) just after test block 4.
BAC levels attained by the alcohol group after drinking were analyzed using a 2 (Gender) × 4 (Time) analysis of variance (ANOVA). Assessment time had a statistically significant effect, F(3,66)=55.3, p<0.01. Tukey HSD tests showed all pairwise comparisons of BAC levels to be significantly different, p<0.05. Also, the Gender × Time interaction was statistically significant, F(3,66)=3.22, p<0.05. Compared to the average male BAC level, the average female BAC level increased faster from the first to the second post-drinking assessment, just after test block 3, when it was 0.004% more than the average male BAC, and then decreased faster to the third post-drinking assessment, just before test block 4, when it was 0.009% less than the average male BAC (See Figure 2). The main effect of Gender was not significant.
Figure 2.
Mean blood alcohol concentrations (BAC) for males (solid line) and females (dashed line) in the alcohol treatment group, measured just before and after each post-treatment test block. Error bars show the standard error of the means. The x-axis shows the time from when participants finished drinking.
Subjective Intoxication
BAES Stimulation and Sedation subscale scores were examined using a 3 (Treatment Group: No-alcohol, Placebo, Alcohol) × 2 (Subscale: Stimulation, Sedation) × 3 (Time: prior to 1st, 3rd, and 4th test battery) mixed ANOVA. There were no significant interactions with Subscale. The Time × Treatment Group interaction was significant, F(4,132)=9.92, p<0.01. Tukey HSD tests showed that only the three assessments of the alcohol group were significantly different from each other (see Table 1).
Table 1.
Means and Standard Deviations of Subjective Intoxication by Treatment Group, from BAES Subscales
| Test Block1 | Test Block 3 | Test Block 4 | ||||
|---|---|---|---|---|---|---|
| Treatment Group | Mean | SD | Mean | SD | Mean | SD |
| BAES Stimulation Subscale | ||||||
| Alcohol | 31.0 | 12.61 | 37.0 | 11.18 | 33.5 | 11.53 |
| Placebo | 34.3 | 13.45 | 34.6 | 13.32 | 28.3 | 12.86 |
| No-Alcohol | 31.5 | 14.49 | 30.5 | 12.03 | 31.0 | 13.38 |
| BAES Sedation Subscale | ||||||
| Alcohol | 15.7 | 8.63 | 21.8 | 8.80 | 22.1 | 10.67 |
| Placebo | 17.0 | 11.40 | 19.7 | 9.71 | 21.5 | 14.16 |
| No-Alcohol | 19.5 | 9.94 | 18.2 | 8.51 | 17.7 | 11.02 |
Note. Subscale scores on the Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993), are averaged sums of ratings, on 10-point scales, of the extent to which a participant was experiencing seven states associated with stimulation (e.g., elated, excited) and seven states associated with sedation (e.g., down, sluggish). Each BAES was administered 5 minutes before BAC measurements preceding the indicated test block.
In the post-experimental questionnaire, on a 0 to 4 rating of how drunk they felt “just after drinking” and “during the first block of computer tasks after drinking,” placebo participants provided ratings significantly above 0 (M=0.6, SD=0.65 and M=0.5, SD=0.66, respectively). Their estimate of the number of standard drinks they drank in the study (M=2.0, SD=1.16) was significantly greater than 0 (p<0.01) though significantly (p< 0.01) less than the alcohol group’s response (M=3.9, SD=1.56).
Memory Tests
As noted above, the set sizes administered to each subject in tests blocks 2 – 4 were determined on the basis of performance in test block 1. There were no significant differences in set sizes selected for the alcohol, no-alcohol, and placebo groups for any of the stimulus types. The mean set size was 7.5 (SD=0.86)) for the VA task, 3.2 (SD=0.48) for the AA task, 7.4 (SD=0.80) for the VS task, and 7.8 (SD=0.99) for the AS task.
Performance on test block 1 was used as a pretest to individualize task difficulty by selecting two set sizes for each task for the remaining three test blocks. Because test block 1 included a different size than the other blocks, the following analyses only include data from test blocks 2, 3, and 4. Proportions correct were analyzed in a 3 (Treatment Group) × 2 (Gender) × 3 (Test Block) × 2 (Modality) × 2 (Presentation Type) mixed ANOVA. The most important finding was a significant three-way interaction of Treatment Group × Test Block × Presentation Type, F(4,132)=3.54, p<0.01. As shown in Table 2, this interaction was due to accuracy for sequences by the alcohol and placebo groups diverging in test block 3 and converging again in the last test block. When proportions correct were analyzed in separate ANOVAs for each of the three test blocks that included individualized set sizes, the ANOVA for test block 3, the block with peak alcohol intoxication, produced the only significant effect, a Treatment Group × Presentation Type interaction, F(2,66)=3.83, p<0.05, due to group differences in performance for the sequence condition. The proportions correct for VS and AS, averaged together, were .75 (SD=0.07), .72 (SD=0.07), and .68 (SD=0.09) for the placebo, the no-alcohol, and the alcohol groups, respectively. Tukey HSD pairwise tests indicated that the difference between the alcohol and placebo groups was significant, p<0.05. In test block 4, this effect was no longer significant.
Table 2.
Means and Standard Deviations of Proportions Correct for Each Memory Task by Treatment Group and Test Block.
| Test Block1 | Test Block 2 | Test Block 3 | Test Block 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Visual Arrays | ||||||||
| Alcohol | 0.69 | 0.13 | 0.74 | 0.10 | 0.73 | 0.11 | 0.72 | 0.11 |
| Placebo | 0.70 | 0.11 | 0.74 | 0.12 | 0.70 | 0.10 | 0.75 | 0.10 |
| No-Alcohol | 0.69 | 0.08 | 0.72 | 0.12 | 0.75 | 0.10 | 0.75 | 0.11 |
| Auditory Arrays | ||||||||
| Alcohol | 0.86 | 0.07 | 0.85 | 0.08 | 0.85 | 0.10 | 0.85 | 0.09 |
| Placebo | 0.86 | 0.06 | 0.83 | 0.12 | 0.85 | 0.10 | 0.87 | 0.09 |
| No-Alcohol | 0.85 | 0.08 | 0.85 | 0.09 | 0.84 | 0.10 | 0.82 | 0.10 |
| Visual Sequences | ||||||||
| Alcohol | 0.66 | 0.11 | 0.66 | 0.11 | 0.63 | 0.09 | 0.66 | 0.11 |
| Placebo | 0.65 | 0.08 | 0.67 | 0.12 | 0.70 | 0.08 | 0.67 | 0.13 |
| No-Alcohol | 0.64 | 0.11 | 0.69 | 0.09 | 0.65 | 0.12 | 0.69 | 0.13 |
| Auditory Sequences | ||||||||
| Alcohol | 0.77 | 0.08 | 0.76 | 0.13 | 0.74 | 0.13 | 0.80 | 0.10 |
| Placebo | 0.79 | 0.08 | 0.78 | 0.10 | 0.80 | 0.13 | 0.78 | 0.12 |
| No-Alcohol | 0.77 | 0.07 | 0.77 | 0.08 | 0.79 | 0.08 | 0.82 | 0.10 |
Note. The total N (72) included 12 males and 12 females in each treatment group.
A theoretically more meaningful measure of performance was the memory capacity estimate (k), calculated as described previously (Cowan, 2001; Cowan et al., 2005): k=N*[p(hits)- p(false alarms)], where N is the set size, p(hits) is the proportion of change trials in which the change was correctly detected, and p(false alarms) is the proportion of no-change trials in which the response was incorrect. This formula is based on the assumption that k items are apprehended and that, if not all items are apprehended, the result is influenced by a rate of guessing that a change had occurred. For set sizes above capacity, it produces a nearly flat function of capacity. Therefore, for the sake of simplicity, Table 3 presents capacity estimates for all conditions of the experiment averaged across set sizes.
Table 3.
Means and Standard Deviations of Capacity Estimates for Each Memory Task by Treatment Group and Test Block.
| Test Block1 | Test Block 2 | Test Block 3 | Test Block 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Visual Arrays | ||||||||
| Alcohol | 2.76 | 2.14 | 3.58 | 1.62 | 3.30 | 1.68 | 3.13 | 1.47 |
| Placebo | 2.97 | 1.85 | 3.36 | 1.67 | 2.84 | 1.48 | 3.57 | 1.48 |
| No-Alcohol | 2.70 | 1.36 | 3.19 | 1.89 | 3.60 | 1.46 | 3.72 | 1.72 |
| Auditory Arrays | ||||||||
| Alcohol | 2.01 | 0.49 | 2.10 | 0.47 | 2.07 | 0.50 | 2.11 | 0.55 |
| Placebo | 2.03 | 0.36 | 2.02 | 0.73 | 2.15 | 0.64 | 2.33 | 0.62 |
| No-Alcohol | 1.97 | 0.55 | 2.10 | 0.52 | 2.03 | 0.56 | 1.91 | 0.56 |
| Visual Sequences | ||||||||
| Alcohol | 2.38 | 1.75 | 2.23 | 1.47 | 1.88 | 1.36 | 2.25 | 1.57 |
| Placebo | 2.14 | 1.27 | 2.43 | 1.73 | 2.83 | 1.25 | 2.42 | 1.88 |
| No-Alcohol | 2.06 | 1.76 | 2.77 | 1.39 | 2.13 | 1.69 | 2.85 | 1.97 |
| Auditory Sequences | ||||||||
| Alcohol | 4.13 | 1.28 | 3.81 | 1.89 | 3.41 | 1.77 | 4.69 | 1.67 |
| Placebo | 4.28 | 1.43 | 4.28 | 1.39 | 4.35 | 1.75 | 4.20 | 1.69 |
| No-Alcohol | 3.97 | 1.13 | 3.90 | 1.10 | 4.31 | 1.15 | 4.64 | 1.30 |
Note. The total N (72) included 12 males and 12 females in each treatment group. Capacity estimates were calculated for each set size and then averaged across set sizes, separately for each subject (see Cowan et al.., 2005).
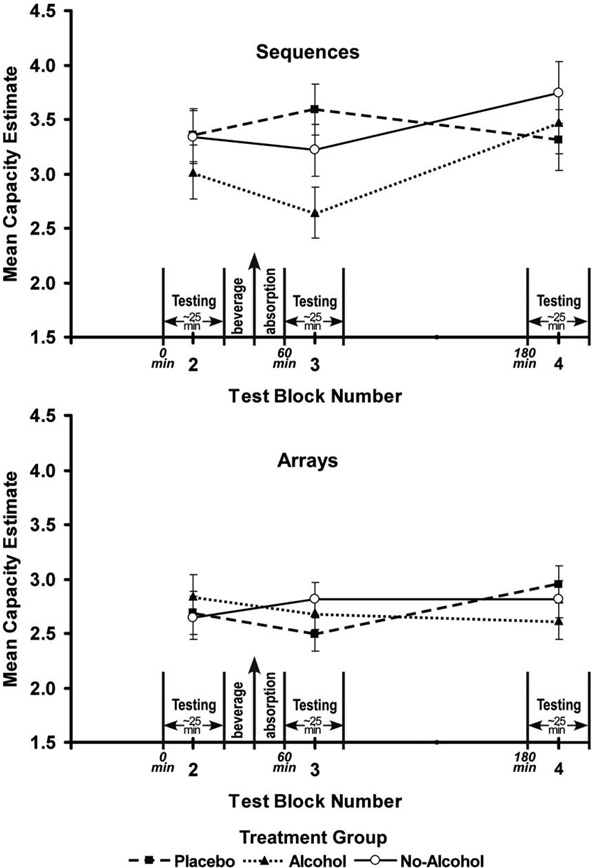
Like proportions correct, capacity values were analyzed in a 3 (Treatment Group) × 2 (Gender) × 3 (Test Block) × 2 (Modality) × 2 (Presentation Type) mixed ANOVA that omitted test block 1. The critical result was a significant three-way interaction of Treatment Group × Test Block × Presentation Type, F(4,132)=3.52, p<0.01. This interaction was the result of memory for sequences by the alcohol and placebo groups diverging in test block 3 and then converging again in test block 4 (as shown in the top panel of Figure 3), with no comparable separation of the groups for arrays (bottom panel). For sequences, a separate ANOVA for each test block produced a significant effect for Treatment Group only in test block 3, the block with peak alcohol intoxication, F(2,66)=4.03, p<0.05. The capacities for VS and AS, averaged together, were 3.6 (SD=1.04), 3.2 (SD=1.12), and 2.6 (SD=1.25) for the placebo, the no-alcohol, and the alcohol groups, respectively. Tukey HSD pairwise tests for test block 3 showed a significant difference between the alcohol and placebo groups, p<0.05 but not between the alcohol and no-alcohol groups. The first comparison is more relevant to the effect of intoxication because the placebo and alcohol groups shared similar expectations (See Testa et al., 2006, for more regarding this rationale for a placebo control.). Although analysis of BACs, reported above, suggested that female BAC fell more slowly than male BAC, we detected no performance differences related to gender for any test blocks.
Figure 3.
Mean memory capacities for sequences (top panel) and arrays (bottom panel), averaged across modalities, in three treatment groups, alcohol (dotted line), placebo (dashed line), and no-alcohol (solid line), as a function of test block. Error bars show the standard error of the means. The test blocks are spaced according to their relative timings. The x-axis shows elapsed time from the start of test block 1. A vertical arrow marks the time when participants finished drinking. Test block 2 occurred before any beverage was administered. Test block 3 began 15 minutes after, and test block 4 began 135 minutes after, participants finished drinking.
There were also main effects of Modality, F(1,66)=4.42, p<0.05 and Presentation Type, F(1,66)=24.19, p<0.01, and a significant interaction of these two factors, F(4,132)=226.19, p<0.01. For auditory stimuli, the capacity for sequences (M=4.2, SD=1.17) was greater than the capacity for arrays (M=2.1, SD=0.41) but for visual stimuli, the capacity for arrays (M=3.4, SD=1.15) was greater than the capacity for sequences (M=2.4, SD=1.27). This finding is consistent with theories that visual and auditory subsystems have different capabilities for processing spatial versus temporal information (e.g., see Penney, 1989). The main effect of Presentation Type was due to capacity for arrays, (M=2.7, SD=0.63), being less than capacity for sequences (M=3.3, SD=1.00). The main effect of Modality was due to auditory capacity (M=3.1, SD=0.64) being greater than visual capacity (M=2.9, SD=1.00). No other effects were significant. Including data from test block 1 did not alter the pattern of effects for any of the above analyses.
Results of the capacity ANOVA, above, were identical to the results of the corresponding ANOVA of proportions correct, with two exceptions caused by how the calculation of capacity takes into account the number of items to be remembered. First, proportions correct did not exhibit a Modality × Presentation Type interaction. Second, the main effect of Presentation Type was reversed. Accuracy for arrays (M=0.79, SD=0.05) was greater than accuracy for sequences (M=0.73, SD=0.07).
Group differences in test block 3 possibly could be due to rapid sequential encoding being especially vulnerable to slower processing caused by alcohol. To test this possibility, reaction times (RTs) were analyzed for the AS and VS tasks in test block 3. Instructions did not stress the need to respond quickly, so RTs were quite variable. Also, pauses were occasionally caused by premature or inadequate key presses requiring a second response. To reduce the effects of such mechanical problems, all RTs greater than 5 s (less than 1% overall) were excluded from the analysis. To compensate for the typically skewed distribution of RTs, medians were calculated for each subject by task and set size. The median RTs for the VS and AS tasks in test block 3 were then analyzed in a 2 × 3 ANOVA, with Treatment Group and Gender as between-subject factors and Modality as the within-subject factor. Treatment Group had no statistically reliable effects. The only significant effect in this ANOVA was for Modality; VS reaction times (M=956 ms, SD=400 were slower than AS reaction times (M=771 ms, SD=259), F(1,66)=30.2, p<0.01. However, the distribution of median RTs failed a Shapiro-Wilk test of normality, W=.956, p<.05 (Shapiro, Wilk, & Chen, 1968). Because the initial analysis removed any concerns that scaling problems might conceal an interaction, a natural logarithmic transformation was performed on the raw RT data. The log-transformed RTs passed the normality test, W=.99, p>.05. Still, an ANOVA of these normalized data produced the same results as the parallel ANOVA of median RTs, reported above. If alcohol did not slow RTs for the sequential tasks, then slower processing cannot explain the sequential task performance deficit of the alcohol group, compared to the placebo group.
Discussion
Alcohol consumption, at the dosage employed, had no apparent effect on memory for visual or auditory arrays. In contrast, alcohol consumption did reduce memory for sequences of visual or auditory stimuli, when compared to the performance of the placebo group. This, along with our experimental rationale explained above, suggests that acute alcohol intoxication had little or no effect on any general WM holding mechanism used to retain multiple concurrent items (e.g., the scope of attention: Cowan et al., 2005), but had a more substantial effect on mnemonic strategies that are needed to retain sequences (e.g., covert verbal rehearsal: Baddeley et al., 1984).
The alcohol effect that we observed might depend critically on BAC level. Treatment groups only differed in performance on test block 3, when the alcohol group had an average BAC of about .08 before and .09 after testing. No differences were detected for test block 4, when the average BAC fell from about .07 to .06. This difference in BAC is confounded with the ascending versus descending limbs of the BAC curve and potential practice and fatigue effects. However, Grattan-Miscio and Vogel-Sprott (2005) found that alcohol impaired accuracy on a memory scanning task during ascending and descending BACs, until BAC had declined to .064, close to the BAC level of our participants during the last test block. Future studies should examine performance on our tasks at multiple BACs on both ascending and descending limbs to distinguish effects of dose from acute tolerance.
Our findings might not generalize to some populations outside the restricted sample that we used. We screened out of our sample anyone who reported health, legal, or psychological problems related to alcohol. Therefore, our participants were not the people clinicians typically see in therapy. It might be that people who have had alcohol problems are more sensitive or react differently to alcohol. They also might have other problems that could interact with alcohol to modify its cognitive effects. Our participants also were accustomed to having several drinks per occasion. Therefore, they probably were relatively tolerant to intoxicating doses, compared to lighter drinkers. Less experienced drinkers could exhibit different patterns of WM deficits than what we found in our sample, especially if different WM processes are differentially responsive to compensatory efforts.
The finding of a difference between an alcohol and placebo treatment, but not between an alcohol and no-alcohol treatment, frequently has been reported, according to Testa et al. (2006). Although the placebo and no-alcohol groups were not significantly different in our experiment, alcohol expectancy has been found to improve performance on cognitive and psychomotor tasks (e.g., George, Raynor, and Nochajski, 1990, 1992; Vogel-Sprott, 1992). Although the large sample size employed should have allowed us to detect large effects of compensation, we were insufficiently powered to detect small magnitude effects of compensation, clearly an area where more investigation is needed.
Although numerous studies have investigated the effects of alcohol on WM and other cognitive processes, to our knowledge this is the first experiment to compare the effects of alcohol on several WM tasks systematically designed to differ only in specific parameters relevant to particular WM processes. This unique design has allowed us to distinguish among WM processes and identify certain alcohol-sensitive processes that have not been sufficiently recognized in previous research. Our findings suggest it is likely that that alcohol caused a differential deficit in memory for sequences compared to arrays by impairing mnemonic processes involving rehearsal. Our findings are also notable because alcohol affected tasks requiring only concentrated attention, rather than divided attention or sustained vigilance. Therefore, unlike the results of most previous alcohol challenge research, the relative deficit that we observed cannot be attributed to alcohol’s effects on complex executive processes needed to allocate and control attention. Rather, this deficit can be explained simply in terms of alcohol’s effect on mnemonic processes of WM.
Although some alternative explanations for our findings cannot be ruled out, these seem unlikely. For example, the sequential stimuli could have been more susceptible to interference and masking, made worse by alcohol consumption. Moskowitz and Murray (1976) reported that alcohol consumption affected visual masking, but their masking intervals of 15 to 75 ms were much less than the 500 ms between visual stimuli in the present experiment. Moskowitz and Murray further argued that alcohol causes a generalized slowing of cognitive processes, but analyses of reaction times in our experiment showed no evidence that the alcohol group was any slower to respond. Another phenomenon associated with sequential presentation is the attentional blink (Raymond, Shapiro, & Arnell, 1992), which occurs when a person fails to detect the second of two successive targets during rapid serial presentations. Even though the attentional blink has a longer time course than masking, it has only been reported for stimuli with onsets separated by less than half a second. This still is too short to account for our results, unless alcohol substantially lengthens this refractory period, a possibility that has not been investigated as far as we know. Perhaps the most important problem with an encoding-speed hypothesis is that it is unclear how it would explain the insignificant alcohol effects for visual arrays. Nevertheless, we do not discount the possibility that alcohol could affect other interference-related processes, like temporal distinctiveness (e.g., Brown, Neath, & Chater, 2007; Crowder, 1993), which operate with sequential stimuli over a longer time frame.
Most critically, the results of this experiment help resolve inconsistencies in previous studies of alcohol’s effect on WM. Based on our findings, alcohol is more likely to impair a WM task that involves sequential presentation of material that can be verbally encoded or recoded and maintained using rehearsal than it is to impair a WM task that requires focused, but not divided, attention and does not rely on rehearsal or related mnemonic strategies. Although we intentionally avoided divided attention in our WM tasks, other researchers incorporate divided attention in WM tasks to measure a combined storage-plus-processing capacity (See Daneman & Carpenter, 1980, and Daneman & Merikle, 1996, for a rationale of the latter dual-task approach and Cowan et al., 2005, for a rationale of the alternative single-task approach and comparisons between the two.). Several previous studies of alcohol and attention have shown the alcohol impairs performance on divided-attention tasks (e.g., Lex et al., 1996; Maylor et al., 1990; Leigh et al., 1977; Fisk & Scerbo, 1987; Moskowitz & DePry, 1968) but has less consistent effects on concentrated attention tasks (Koelega, 1995). Therefore, we expect that most WM measures using a dual-task procedure, like the visual-spatial WM task used by Schweizer et al. (2006), would be more sensitive to the effects of alcohol than a focused-attention task, like the array tasks used in the present experiment and the visual-spatial WM task used by Paulus et al. (2006), that do not benefit from rehearsal, or the immediate verbal WM task that Schweizer et al. (2006) combined with rehearsal-blocking activity. On the other hand, we still expect alcohol to affect certain focused attention tasks that profit from rehearsal and grouping, like the sequential tasks in our experiment and the backward digit span used by Finn et al. (1999).
The results of this experiment help clarify the specific cognitive deficits that could constitute alcohol myopia. Used metaphorically, myopia (short-sightedness) connotes a contraction of attention in which cues of the most interest are apprehended clearly, but without a full consideration of less salient cues and the accompanying context. Steele and Josephs (1990) proposed the attention-allocation hypothesis to explain many important and diverse behavioral effects of alcohol in terms of alcohol myopia. Although this has been a useful and influential theory, its underlying cognitive mechanism is unclear. One possibility is that alcohol simply reduces attentional capacity, so that a person cannot pay attention to as many different stimuli at one time when intoxicated as they can when sober. Cowan et al (2005) referred to this limit to the number of items that can be attended to at one time as the “scope of attention”. Cowan et al found that this capacity, indexed by a visual array task like the one we used in this study, correlated with aptitude as well as other, more traditional measures of WM. Yet, at least at the dosage we studied, alcohol did not reduce the scope of attention. It did, however, impair memory for stimuli presented one after another, which also could be recoded and rehearsed. This finding does not contradict the attention-allocation hypotheses of Steele and Josephs (1990), but does suggest that the relevant deficit caused by alcohol might have more to do with the ability to allocate attention, as discussed above, than with the amount of information that can be simultaneously attended. Furthermore, the sequential memory deficit that we found suggests that a diminished ability to use mnemonic strategies to maintain successive stimuli also could contribute to alcohol myopia by reducing the number of different cues considered over time.
Our findings also are potentially relevant to understanding individual differences in the behavioral consequences of alcohol use. The role of WM capacity was specifically implicated by Finn and Hall (2004), who found that working memory capacity, but not other executive cognitive functions, moderated the association between social deviance and alcohol problems. However, their measure of WM capacity was a forward digit span test, which confounds storage and rehearsal processes, making the precise mediating mechanism(s) uncertain. High-span individuals might be so much better at rehearsal that they still can muster adequate control even when their rehearsal is impaired by intoxication. On the other hand, high-span individual might have more storage capacity, and this resource is relatively less vulnerable to alcohol than rehearsal processes, as our finding suggest. If so, then their superior storage capacity might help mitigate other effects of intoxications. To better understand the behavioral consequence of alcohol, future research on how working memory moderates the relationship between behavior and alcohol can combine decision making tasks, like those reviewed in Finn (2002), with working memory measures that distinguish among specific WM processes. Our experiment shows that this is a feasible and potentially informative undertaking.
The cognitive effects of alcohol are certainly complex, but they might be better understood if alcohol challenge studies were more closely linked to basic cognitive research and WM theory. Although there are surely more pieces to this puzzle, we believe that our research begins the task of distinguishing the alcohol-sensitive processes of WM (including sequential memory) from WM processes that are less sensitive (including array memory). Much more research along these lines is needed to determine the specific mechanisms linking the cognitive impairments and behavioral consequences of alcohol intoxication.
Acknowledgements
This research was supported by a grant to Nelson Cowan by the Alcoholic Beverage Medical Research Foundation (Award # 002965) and a grant to Kenneth J. Sher from the National Institute on Alcohol Abuse and Alcoholism, (RO1 AA7231). The authors wish to thank Bruce Bartholow for his helpful comments and Samuel T. Mattox and Pinky Bomb for their assistance with data collection.
References
- Baddeley AD. Working memory. Oxford: Clarendon Press; 1986. Oxford Psychology Series #11. [Google Scholar]
- Baddeley AD. Is working memory still working? American Psychologist. 2001;56:851–864. doi: 10.1037/0003-066x.56.11.851. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Lewis V, Vallar G. Exploring the articulatory loop. The Quarterly Journal of Experimental Psychology. 1984;36A:233–252. [Google Scholar]
- Brown GDA, Neath I, Chater N. A temporal ratio model of memory. Psychological Review. 2007;114:539–576. doi: 10.1037/0033-295X.114.3.539. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review & user's guide. Psychonomic Bulletin # Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, U.K: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Cartwright C, Winterowd C, Sherk M. An adult model of preschool children's speech memory. Memory # Cognition. 1987;15:511–517. doi: 10.3758/bf03198385. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliot EM, Saults JS, Morey C, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Fristoe NM, Elliott EM, Brunner RP, Saults JS. Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition. 2006;34:1754–1768. doi: 10.3758/bf03195936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder RG. Short-term memory: Where do we stand? Memory # Cognition. 1993;21:142–145. doi: 10.3758/bf03202725. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Patrick CJ, Lang AR, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12:527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning # Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: A Meta-Analysis. Psychonomic Bulletin # Review. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Darwin C, Turvey M, Crowder R. An auditory analogue of the Sperling partial report procedure: Evidence for brief auditory storage. Cognitive Psychology. 1972;3:255–267. [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Reviews. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Hall J. Cognitive ability and risk for alcoholism: Short-term memory capacity and intelligence moderate personality risk for alcohol problems. Journal of Abnormal Psychology. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on go/no-go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Scerbo MW. Automatic and control processing approach to interpreting vigilance performance: A review and reevaluation. Human Factors. 1987;29:653–660. doi: 10.1177/001872088702900605. [DOI] [PubMed] [Google Scholar]
- George W, Raynor J, Nochajski T. Resistance to alcohol imp airment of visual-motor performance II: Effects for attentional set and self-reported concentration (1990) Pharmacology Biochemistry # Behavior. 1990;35:261–266. doi: 10.1016/0091-3057(90)90401-3. [DOI] [PubMed] [Google Scholar]
- George W, Raynor J, Nochajski T. Resistance to alcohol impairment of visual-motor performance: Does it help to pay attention? Journal of Studies on Alcohol. 1992;35:507–513. doi: 10.15288/jsa.1992.53.507. [DOI] [PubMed] [Google Scholar]
- Grattan-Miscio K, Vogel-Sprott M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology. 2005;181:188–196. doi: 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- Hitch GJ, Burgess N, Towse JN, Culpin V. Temporal grouping effects in immediate recall: A working memory analysis. Quarterly Journal of Experimental Psychology. 1996;49A:116–139. [Google Scholar]
- Hitch GJ, Halliday MS, Dodd A, Littler JE. Development of rehearsal in short-term memory: Differences between pictorial and spoken stimuli. British Journal of Developmental Psychology. 1989;7:347–362. [Google Scholar]
- Kähkönen S, Marttinen RE, Yamashita H. Alcohol impairs auditory processing of frequency changes and novel sounds: a combined MEG and EEG study. Psychopharmacology. 2005;117:366–372. doi: 10.1007/s00213-004-1960-1. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Alcohol and vigilance performance: A review. Psychopharmacology. 1995;118:233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- Leigh G, Tong J, Campbell J. Effects of ethanol and tobacco on divided attention. Journal of Studies on Alcohol. 1977;38:1233–1239. doi: 10.15288/jsa.1977.38.1233. [DOI] [PubMed] [Google Scholar]
- Lépine R, Barrouillet P, Camos V. What makes working memory spans so predictive of high level cognition? Psychonomic Bulletin # Review. 2005;12:165–170. doi: 10.3758/bf03196363. [DOI] [PubMed] [Google Scholar]
- Lex BW, Rhoades EM, Teoh SK, Mendelson JH. Divided attention task performance and subjective effects following alcohol and placebo: differences between women with and without a family history of alcoholism. Drug # Alcohol Dependence. 1994;35:95–105. doi: 10.1016/0376-8716(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbit PM, James GH, Kerr SA. Effects of alcohol and extended practice on divided-attention performance. Perception # Psychophysics. 1990;48:445–452. doi: 10.3758/bf03211588. [DOI] [PubMed] [Google Scholar]
- Moray N, Bates A, Barnett T. Experiments on the four-eared man. Journal of the Acoustical Society of America. 1965;38:196–201. doi: 10.1121/1.1909631. [DOI] [PubMed] [Google Scholar]
- Morey CC, Cowan N. When visual and verbal memories compete: Evidence of cross-domain limits in working memory. Psychonomic Bulletin # Review. 2004;11:296–301. doi: 10.3758/bf03196573. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, DePry D. Differential effect of alcohol on auditory vigilance and divided-attention tasks. Quarterly Journal of Studies on Alcohol. 1968;29:54–63. [Google Scholar]
- Moskowitz H, Murray JT. Alcohol and backward masking of visual information. Journal of Studies on Alcohol. 1976;37:41–45. doi: 10.15288/jsa.1976.37.40. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Nordenstrom B, Olson A. Disruption of eye movements by ethanol intoxication affects perception of depth from motion parallax. Psychological Science. 2004;15:858–865. doi: 10.1111/j.0956-7976.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Paulus M, Tapert S, Pulido C, Schuckit M. Alcohol attenuates load-related activation during a working memory task: Relation to level of response to alcohol. Alcoholism: Clinical and Experimental Research. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney C. Modality effects and the structure of short-term verbal memory. Memory # Cognition. 1989;17:398–422. doi: 10.3758/bf03202613. [DOI] [PubMed] [Google Scholar]
- Raymond J, Shapiro K, Arnell K. Temporary suppression of visual processing in an RSVP task: an attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1992;21:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Saults JS, Cowan N. A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General. doi: 10.1037/0096-3445.136.4.663. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effects on stress responses in social drinkers. Psychological Bulletin. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Cognitive theory and research. In: Leonard K, Blane H, editors. Psychological Theories of Drinking and Alcoholism. New York: Guilford Press; 1999. pp. 247–291. [Google Scholar]
- Schweizer T, Vogel-Sprott M, Danckert J, Roy E, Skakum A, Broderick C. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Wilk M, Chen H. A comparative study of various tests of normality. Journal of the American Statistical Association. 1968;63:1343–1372. [Google Scholar]
- Sperling G. The information available in brief visual presentations. Psychological Monographs. 1960;74 (Whole No. 498.) [Google Scholar]
- Steele C, Josephs R. Alcohol myopia: Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Testa M, Fillmore M, Norris J, Abbey A, Curtin J, Leonard K, Mariano K, Thomas M, Nomensen K, George W, VanZile-Tamsen C, Livingston J, Saenz C, Buck P, Zawacki T, Parkhill M, Jacques A, Hayman L. Understanding alcohol expectancy effects: Revisiting the placebo condition. Alcoholism: Clinical and Experimental Research. 2006;30:339–348. doi: 10.1111/j.1530-0277.2006.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Sprott M. Alcohol Tolerance and Social Drinking. New York: Guilford Press; 1992. [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]