Abstract
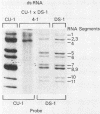
The SB-1A rotavirus recovered from a diarrheic piglet in the United States is a naturally occurring intertypic rotavirus. When studied by reciprocal neutralization tests, the SB-1A virus was similar, if not identical, to the porcine Gottfried virus (serotype 4) and the porcine OSU virus (serotype 5). Analysis of reassortant viruses prepared from the SB-1A virus and the serotype 2 human DS-1 virus revealed that the antigenic specificity of the outer capsid protein VP3 of SB-1A was shared with the OSU virus, while the antigenic specificity of another outer capsid protein, VP7, of SB-1A appeared to be shared with the Gottfried virus. This suggests that SB-1A is a naturally occurring reassortant rotavirus between OSU-like and Gottfried-like porcine rotaviruses. In addition, using a genetic approach, we found evidence that the fourth gene was responsible for the predominantly one-way cross-neutralizing reactivity between canine rotavirus strain CU-1 (serotype 3) and porcine rotavirus strains SB-1A (serotypes 4 and 5) and OSU (serotype 5). Assignment of hemagglutination function to the fourth genome segment of porcine rotaviruses SB-1A and OSU and canine rotavirus CU-1 confirmed a similar previous gene assignment established for certain rotaviruses. Analysis of single gene 4 substitution reassortants confirmed our previous finding that VP3 was as potent in stimulating neutralizing antibodies as VP7. The observations confirm the need for a binary system of rotavirus classification and nomenclature similar to that used for the influenza A viruses; in such a system the neutralization specificity of both VP3 and VP7 would be indicated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridger J. C., Brown J. F. Antigenic and pathogenic relationships of three bovine rotaviruses and a porcine rotavirus. J Gen Virol. 1984 Jul;65(Pt 7):1151–1158. doi: 10.1099/0022-1317-65-7-1151. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986 Jan;57(1):110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Flores J., Midthun K., Kapikian A. Z. Serotypic characterization of rotaviruses derived from asymptomatic human neonatal infections. J Clin Microbiol. 1985 Mar;21(3):425–430. doi: 10.1128/jcm.21.3.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Scott F. W., Appel M. J. Isolation and characterization of a canine rotavirus. Arch Virol. 1982;72(1-2):113–125. doi: 10.1007/BF01314456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Fukuhara N., Tazawa F., Suzuki H., Sato T., Konno T., Ebina T., Ishida N. Characterization of monoclonal antibodies against human rotavirus hemagglutinin. J Med Virol. 1986 Aug;19(4):313–323. doi: 10.1002/jmv.1890190404. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Suzuki H., Numazaki T., Sato T., Konno T., Ebina T., Ishida N., Nakagomi O., Nakagomi T. Hemagglutination by human rotavirus strains. J Med Virol. 1984;13(3):215–222. doi: 10.1002/jmv.1890130303. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A. Z., Chanock R. M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Daoud G., White L., Urbina G., Daoud N., Perez M., Flores J. Rotavirus shedding by newborn children. J Med Virol. 1984;14(2):127–136. doi: 10.1002/jmv.1890140206. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]