Abstract
Biodiversity assessment demands objective measures, because ultimately conservation decisions must prioritize the use of limited resources for preserving taxa. The most general framework for the objective assessment of conservation worth are those that assess evolutionary distinctiveness, e.g. Genetic (Crozier 1992) and Phylogenetic Diversity (Faith 1992), and Evolutionary History (Nee & May 1997). These measures all attempt to assess the conservation worth of any scheme based on how much of the encompassing phylogeny of organisms is preserved. However, their general applicability is limited by the small proportion of taxa that have been reliably placed in a phylogeny. Given that phylogenizaton of many interesting taxa or important is unlikely to occur soon, we present a framework for using taxonomy as a reasonable surrogate for phylogeny. Combining this framework with exhaustive searches for combinations of sites containing maximal diversity, we provide a proof-of-concept for assessing conservation schemes for systematized but un-phylogenised taxa spread over a series of sites. This is illustrated with data from four studies, on North Queensland flightless insects (Yeates et al. 2002), ants from a Florida Transect (Lubertazzi & Tschinkel 2003), New England bog ants (Gotelli & Ellison 2002) and a simulated distribution of the known New Zealand Lepidosauria (Daugherty et al. 1994). The results support this approach, indicating that species, genus and site numbers predict evolutionary history, to a degree depending on the size of the data set.
Keywords: Evolutionary history, phylogenetic diversity, genetic diversity, biodiversity, phylogeny, systematic nomenclature
Introduction
There is an instinctive and natural desire to preserve all species across the world, but in reality this “Noah’s Ark” approach (Mann & Plummer 1995) is impractical. Resources - financial and otherwise - are limited, the scale of the problem too vast (Agapow et al. 2004), and blanket protection policies are unlikely to be politically successful. Conservation is necessarily a question of economics and prioritization. How can time and money be spent most efficiently? Which species and populations should be targeted for preservation? What metrics can be used for measuring a species importance?
Given the variety of organisms, sites and environments under consideration, it is initially unclear what quality should be measured by any metric of “conservation worth”. Many taxa have qualities that demand their preservation (e.g., being sources of valuable products or other economic benefits, scientific importance, or cultural value), but for the great majority their values are not so clear and are difficult to compare. Even when taxa are clearly “valuable”, questions of priority will arise, because the preservation of one taxon may conflict with that of another. Political success for any conservation scheme is more likely if the proposal is backed by objective measurable data.
Objective criteria for the selection of sites and populations necessary to preserve single species chosen for conservation are relatively well developed (Frankham et al. 2002). But authoritative estimates of the number of species in the world are around 10 million (May 1992, Magurran 2005), so that it is clear that broad-scale solutions are needed rather than dealing with one species at a time.
Ecologists typically regard species richness, the number of species in sites being considered for preservation, as the currency of conservation (Justus & Sarkar 2002, Gaston & Spicer 2004). The consideration of species numbers alone may, however, be insufficient, because of such factors as general imperfect taxonomic knowledge and variation in the level of this knowledge from one group to another. For some time therefore it has been suggested that the phylogenetic distinctiveness of species be taken into account (reviewed by Crozier (1997) and by Mace et al. (2003)), a point of view elegantly encapsulated by Wilson (1992) when he defined biodiversity as the information content in the world’s genomes. The sense of this view is illustrated by the east African great lakes. Some of these are home to more than 1,000 species of cichlid fishes which appear to have evolved over a very short evolutionary time (Meyer 1993). Naively relying on species number alone would value this group more than the ungulates, primates and carnivores combined. Using an approach that weights species by their evolutionary distinctiveness returns the cichlids to a value that intuitively seems more correct and undistorted by “cheap” species.
Early applications of phylogeny to conservation relied purely on topology (reviewed by Crozier (1997)), but the much greater information content in branch-length metrics (recall the cichlid example above) led to their more widespread use and development (Crozier 1992, Faith 1992). Two dimensions can be discerned. One distinction is between measures that consider only the tree connecting the species of interest, as against measures that include the root of the tree connecting the species studied to the rest of life. The other considers the lengths of evolutionary branches (e.g., number of substitutions), as against taking account of saturation of differences (e.g., number of positions with different nucleotides). For example, as two DNA sequences diverge following speciation or gene duplication, differences will accumulate as substitutions occur. With time, substitutions will tend to occur at the same positions as earlier ones, so that the rate of divergence slows even though the rate of evolution does not, a distinction well brought out by the phrase of DeSalle et al. (1987) that eventually Hawaiian Drosophila cease to diverge even while continuing to evolve rapidly. Naturally, saturation occurs for more than DNA sequences–birds and fruit flies continue to evolve, but few would think that they are still becoming more different from each other. “Phylogenetic diversity” (PD) measures retained diversity as the length of tree retained between the group of interest without taking saturation into account (Faith 1992):
| (1) |
where n is the number of species and dk, is the length of branch k in the tree.
“Genetic diversity” (GD) resembles PD but takes saturation into account (Crozier 1992). Specifically, GD estimates the probability that the set of taxa preserves more than one allele per site:
| (2) |
where pk is the proportion of sites different in state at the two ends of branch, hence 0 ≤ pk ≤ 1. For molecular data, dk is derived from pk according to one or other of the models of sequence evolution.
“Evolutionary history” (EH) is similar to PD but includes the connection of the subtree to the rest of life (Nee & May 1997), by always including the node at the root. For symmetry we define a measure “Genetic history” (GH) which uses (2) above but always includes the root node in calculations, thus resembling EH. PD and GD thus deal with unrooted trees whereas EH and GD require rooted ones. Evolutionary history is attractive compared to PD because the analysis then preserves the context within the rest of life, and is appropriate for this study because of the non-molecular nature of the data.
It has been a truism that conservation of habitats, with thousands or more species each, is preferable to concentrating on conserving particular species, necessarily small in number. The phylogenetic approach goes further, and asks about the evolutionary distinctiveness of species to be conserved. Phylogenetic methods involving whole communities have been applied to aquatic eukaryotic microbes using denaturing gradient gel electrophoresis of total extracted environmental DNA (van Hannen et al. 1998) and to subterranean bacteria via 16S rDNA sequences (Crozier et al. 1999).
There is, however, a major impediment to a more general application of phylogenetic methods to conservation, and that is that the vast majority of groups lack complete phylogenies and this situation is unlikely to be corrected in the near future. A workaround for this problem already exists but has yet to be applied to conservation biology problems.
Systematists generally try to make the arrangement of species into taxa mirror the topology of an inferred evolutionary tree, and the various classificatory levels similarly reflect the systematist’s judgement as to the degree of difference. Thus, surrogate phylogenies can be inferred from systematic nomenclature, and these phylogenies applied in biodiversity assessment. We here illustrate this method and, using species by site (location) data from four other studies, demonstrate its application using multi-platform computer programs.
Estimates of confidence in biodiversity estimates are desirable when they can be made (Crozier 1997). Where surveys are not claimed to yield complete data, the survey data could be used to estimate statistical sufficiency, such as by using bootstrap or jackknife methods to derive confidence limits for EH, PD, GD or GH, and sample coverage methods (Chao & Lee 1992, Chao 2004) to obtain confidence limits for species richness. The entities used for such estimates will differ between groups. For example for social insects the correct unit is closer to the number of colonies (Wilson 1963, Pamilo & Crozier 1997, Chapman & Bourke 2001) because these better approximate the number of reproductives than does the number of sterile or infertile workers. In turn, the number of colonies of a species is approximated by the number of pitfall traps with its workers, rather than the absolute number of workers. Such measures are available in one of the programs discussed here, MeSA, and we discuss their use below.
Methods
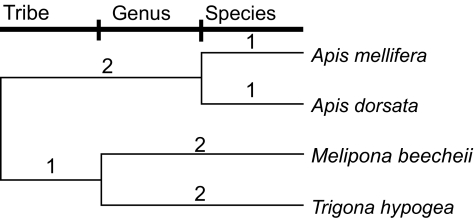
Systematic nomenclature is used to infer a phylogeny of the species under consideration. A branch of equal length is allowed for each level in the hierarchy. An example is shown for a selection of social bees with the systematic nomenclature shown in Table 1, yielding the phylogeny of Figure 1.
Table 1.
Systematic nomenclature for some social bees (Michener 2000).
| Subfamily | Tribe | Genus | Species |
|---|---|---|---|
| Apinae | Apini | Apis | mellifera |
| dorsata | |||
| Meliponini | Melipona | beecheii | |
| Trigona | hypogea |
Figure 1.
Phylogeny of some social bees inferred from the systematic nomenclature shown in Table 1.
The program TreeMaker allows the conversion of systematic nomenclature into an inferred phylogeny (or the importing of an actual phylogeny, if known) and the recording of the presence of the various species across collection sites, either as presence or absence or as abundance data. Branch lengths can either be one for each change of systematic level, or the distance from the root of the tree to the tips can be divided equally. Biodiversity for different combinations of sites is then determined by the species and resultant phylogeny that would be preserved if the sites are retained, according to whichever metric (e.g. PD, GD, GH or EH) is used. The absolute value of the preserved biodiversity varies with the metric used, but the ranks of combinations of sites are the same (Krajewski 1994) and there is for any particular data set (e.g., that of Crozier et al. (1999)) a simple interconversion between PD and GD unique to that data set. The absolute values can be important in intuitive evaluations - for example EH will tend to indicate that more biodiversity is preserved than does PD for the same data.
We have used EH in our calculations here. For the set of bees, a set with Apis mellifera and A. dorsata preserved will have an EH of 4 and one which also preserves Melipona beechei one of 7 (the PD values of these sets are 2 and 7).
The biodiversity preserved by conserving a set of sites is the EH of the species preserved. The program MeSA allows an exhaustive search of combinations of sites, calculating the species richness and EH (and other measures if desired, such as various estimates of species diversity and complementarity) of each combination. Confidence limits for species richness are asymmetric ones obtained via sample coverage methods. For example the estimator Chao84 (Chao 1984) uses information on the abundance of species which are rare but present to estimate the number of species which are rare but absent. Confidence limits for the diversity measure used in an analysis (e.g., EH) are obtained by standard jackknife and bootstrap methods, namely by subsampling from the observations seen in a combination and determining EH for each subsample (see Sokal and Rohlf (1995) for a review). Our implementation of jack-knifing followed standard practice, with each observation being omitted in turn to create a sub-sample.
The algorithm for converting systematic nomenclature into an inferred phylogeny is implemented in two freely available programs, both called TreeMaker. The first is a Java program storing its data in an SQL database, and is available from http://homes.jcu.edu.au/~jc125033/Treemaker.htm. The second, available in Windows and Macintosh versions, stores its data in a structured format in files and is available from http://www.agapow.net/software/treemaker. MeSA is available from http://www.agapow.net/software/mesa.
We used four data sets to explore the properties of our approach. The first of these example data sets contains information on the presence or absence of 273 species of flightless insects in 86 genera from 14 North Queensland localities resulting from a long-running Queensland Museum study directed by G. E. Monteith (Yeates et al. 2002). The tree inferred from systematics is given in the Appendix as a NEXUS file readable by TREEVIEW X. The second data set comes from a transect surveying the occurrences of northern Florida ants in a longleaf pine habitat, involving 72 species in 24 genera from 12 sites (Lubertazzi & Tschinkel 2003). The third data set stems from a study of New England bog ants (Gotelli & Ellison 2002) using an updated data set recording abundances of 34 species at 22 localities. The fourth data set was inspired by the discovery of a second species of the genus Sphenodon, which as the sister group to all other lepidosaurs is highly isolated phylogenetically (Daugherty et al. 1990, May 1990). Sphenodon is now largely limited to sites lacking introduced rats, with the rate of loss dependent on the particular invasive rat species (C. E. Daugherty, pers. comm.), rendering problematic any examination of the impact of Sphenodon on the conservation worth of sites. We therefore used the list of New Zealand lepidosaurs (Sphenodon and lizards) given by Daugherty et al. (1994), comprising 62 species placed in five genera, and simulated a set of 15 sites. Each species occurs three times and these occurrences were distributed at random to the 15 sites. The phylogenetic trees and occurrences at sites for the four data sets are given in NEXUS files in the Appendix.
For each dataset, all possible combinations of included sites were generated. From the resultant ensemble of sites the genera, species and EH preserved were calculated. These analyses were performed by MeSA. Including the set of all sites, there are 16,383 combinations for the North Queensland Flightless Insects (NQFI) data, 4,095 for the Florida ants (FLA) data, 4,194,303 for the New England bog ants (NEBA) data and 32,767 for the New Zealand Lepidosauria (NZL) data. All the NQFI and FLA data can be meaningfully graphed, but it was necessary to sample from the NEBA and NZL results to yield a more tractable number of points, chosen to be 20,000.
In order to investigate the effects on EH of phylogenetically divergent species, for each data set we distinguished between site combination-shaving remarkably divergent taxa and those without. The impact of a species on EH is expected to reflect the length of the branch connecting it to the rest of the tree (Crozier 1992, Faith 1992). For the NQFI data we selected Austrovelia queenslandica (abbreviated Austrovelia AV01 in the NEXUS file), the sole member of the Mesoveliidae in this data set, for FLA we selected Myrmecina americana, sole representative of its tribe, for the NEBA data we selected Amblyopone pallipes, sole member of its subfamily in this ant data set, and for NZL we selected the genus Sphenodon.
To illustrate the use of confidence limit calculations, we used the Chao84 estimator for the number of species and its confidence limit, and for estimating the confidence limits for EH we estimated its standard error (SE) using the jackknife and derived confidence limits as 1.96 × SE. We used the NEBA data set for this demonstration; but we caution that that although the data are of the right form for the calculation they represent capture records of individual ants, not colonies as we have argued above would be more appropriate. Regression analyses were made using Statview 4.5 (Abacus Concepts).
Results
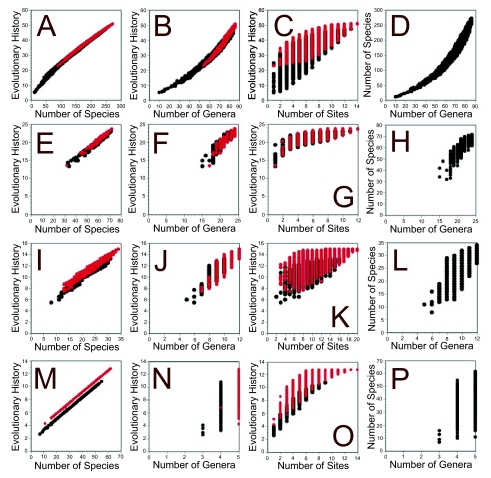
Graphs of species number and preserved evolutionary history (figure 2) show a strong relationship between these quantities. In every case there is a strong tendency for site combinations with the divergent taxa selected to preserve more evolutionary history than combinations with the same number of species but lacking these divergent taxa.
Figure 2.
Relationships between species richness, generic richness, number of sites and evolutionary history preserved, and between the number of genera and number of species preserved, for the data sets of the flightless insects of North Queensland (A–D), Florida ants (E–H), New England bog ants (I–L) and New Zealand lepidosaurs (M–P). Where applicable, combinations of sites preserving a selected phylogenetically divergent taxon are given in red and others in black; the rare taxa are Austrovelia queenslandica (A–C), Myrmecina americana (E–G), Amblyopone pallipes (I–K) and the genus Sphenodon (M–O).
The number of genera is predictive of evolutionary history preserved (figure 2) but with the effect most marked when the number of genera is large (as in the NQFI data set). The relationship between evolutionary history preserved and the number of sites is often not a close one, but there is an evident significant payoff to selecting sites with the selected divergent taxa (figure 2). The advantage to selecting sites with these divergent taxa is marked for all data sets except FLA.
The relationship between number of species and number of genera varies between data sets, apparently in proportion to the range of numbers of genera preserved by different site combinations (figure 2). There is a very strong relationship for NQFI (a range of 10 to 86 genera preserved) and the weakest relationship is seen for the NZL data (three to five genera preserved).
Statistical analyses are problematic because each site enters into many site combinations, but regression analyses can be at least indicative. For each data set all three independent variables (number of sites, number of genera, number of species) were highly significant under multiple regression (Table 2) and all were retained in the model under stepwise regression (Table 3). For the stepwise regression, the order of entry of terms into the model was number of species > number of genera > number of sites for all data sets except NZL (with a very small number of genera), in which the order was number of species > number of sites > number of genera.
Table 2.
ANOVA table for the four data sets, for the independent variables shown and the dependent variable Evolutionary History. The data sets are North Queensland Flightless Insects (NQFI), Florida Ants (FLA), New England Bog Ants (NEBA), and New Zealand Lepidosaurs (NZL). In each case the regression was significant with P < 0.0001. The regression in each case had 3 degrees of freedom and the total number of degrees of freedom is given after each dataset abbreviation.
| Data/parameter | Coefficient | Standard Error | Standard Coefficient | t-value | P |
|---|---|---|---|---|---|
| NQFI (16382) | |||||
| Intercept | 3.097 | 0.032 | 3.097 | 95.657 | <0.0001 |
| Number of Sites | −0.026 | 0.002 | −0.008 | −16.876 | <0.0001 |
| Number of Genera | 0.163 | 0.001 | 0.209 | 197.165 | <0.0001 |
| Number of Species | 0.125 | <0.001 | 0.802 | 689.038 | <0.0001 |
| FLA (4094) | |||||
| Intercept | 2.600 | 0.029 | 2.600 | 90.273 | <0.0001 |
| Number of Sites | 0.011 | 0.001 | 0.014 | 8.241 | <0.0001 |
| Number of Genera | 0.310 | 0.002 | 0.319 | 135.947 | <0.0001 |
| Number of Species | 0.190 | 0.001 | 0.698 | 251.929 | <0.0001 |
| NEBA (17464) | |||||
| Intercept | 2.417 | 0.021 | 2.427 | 115.492 | <0.0001 |
| Number of Sites | 0.005 | 0.001 | 0.011 | 5.806 | <0.0001 |
| Number of Genera | 0.333 | 0.003 | 0.272 | 119.329 | <0.0001 |
| Number of Species | 0.252 | 0.001 | 0.758 | 296.172 | <0.0001 |
| NZL (19729) | |||||
| Intercept | −7.551 | 1.779 | −7.551 | −4.243 | <0.0001 |
| Number of Sites | 0.115 | 0.007 | 0.185 | 16.224 | <0.0001 |
| Number of Genera | 2.931 | 0.357 | 0.049 | 8.216 | <0.0001 |
| Number of Species | 0.066 | 0.002 | 0.366 | 32.096 | <0.0001 |
Table 3.
Final ANOVA tables after all three independent variables (Number of Sites, Number of Genera and Number of Species) were entered into the stepwise regression analysis. In all cases the regressions were significant with P < 0.0001. The degrees of freedom were as given in Table 2. The adjusted R2 value for each regression is given in parentheses after each dataset abbreviation.
| Data/parameter | Coefficient | Standard Error | Std. Coefficient | F-to-Remove | |
|---|---|---|---|---|---|
| NQFI (0.999) | |||||
| Intercept | 3.097 | 0.032 | 3.097 | 9150.323 | |
| Number of Sites | −0.026 | 0.002 | −0.008 | 284.800 | |
| Number of Genera | 0.163 | 0.001 | 0.209 | 38874.218 | |
| Number of Species | 0.125 | <0.001 | 0.802 | 474773.447 | |
| FLA (0.994) | |||||
| Intercept | 2.600 | 0.029 | 2.600 | 8149.174 | |
| Number of Sites | 0.011 | 0.001 | 0.014 | 67.909 | |
| Number of Genera | 0.310 | 0.002 | 0.319 | 18481.488 | |
| Number of Species | 0.190 | 0.001 | 0.698 | 63468.304 | |
| NEBA (0.956) | |||||
| Intercept | 2.427 | 0.021 | 2.427 | 13338.354 | |
| Number of Sites | 0.005 | 0.001 | 0.011 | 33.710 | |
| Number of Genera | 0.333 | 0.003 | 0.272 | 14239.333 | |
| Number of Species | 0.252 | 0.001 | 0.758 | 87717.638 | |
| NZL (0.288) | |||||
| Intercept | −7.551 | 1.779 | −7.551 | 18.007 | |
| Number of Sites | 0.115 | 0.007 | 0.185 | 263.220 | |
| Number of Genera | 2.931 | 0.357 | 0.049 | 67.494 | |
| Number of Species | 0.006 | 0.002 | 0.366 | 1030.168 | |
Because giving all results for the confidence limits for EH and species richness for all sites of an would make for a voluminous table, we present the results of all combinations of dropping one site at a time for the NEBA data, in Table 4.
Table 4.
Confidence intervals for species richness calculated using the Chao84 estimator and for EH using the jackknife. The 22 combinations obtained by dropping each site in turn from Goltelli’s NEBA data are shown. For the data, see the Appendix.
| Site Omitted | n | S | EH |
|---|---|---|---|
| ARC | 34 | 37.961<46.000<70.349 | 13.419<15.000<16.581 |
| BH | 34 | 41.538<54.000<87.063 | 12.865<15.000<17.135 |
| CB | 33 | 34.597<39.000<55.548 | 13.250<14.750<16.250 |
| CKB | 33 | 46.566<57.000<75.458 | 13.177<14.500<15.823 |
| HAW | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| HBC | 33 | 36.962<45.000<69.349 | 13.169<14.750<16.331 |
| OB | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| PK | 34 | 37.961<46.000<70.349 | 13.419<15.000<16.581 |
| QP | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| RP | 33 | 40.538<53.000<86.063 | 13.092<14.750<16.408 |
| SKP | 33 | 34.597<39.000<55.548 | 13.276<14.500<15.724 |
| SW | 34 | 35.991<44.000<84.238 | 13.342<15.000<16.658 |
| TPB | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| WIN | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| SPR | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| SNA | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| PEA | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| CHI | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| MOL | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
| COL | 33 | 36.962<45.000<69.349 | 13.169<14.750<16.331 |
| CAR | 34 | 37.962<46.000<70.349 | 13.419<15.000<16.581 |
Terms: n is the observed species richness, S the estimated value and its 95% confidence limits and EH is Evolutionary History and its 95% confidence limits.
Discussion
We have demonstrated a method of using phylogenetic information implicit in systematic nomenclature to assess the conservation worth of sets of reserves using large proportions of their species, in fact potentially all of them. The method is not divorced from direct phylogenetic knowledge because systematists generally seek to make systematic nomenclature reflect this knowledge, and as it advances will modify the nomenclature. The information already being collected from surveys can be readily entered into the programs TREEMAKER and MeSA, and the results for moderate numbers of reserved (as in the NQFI case) readily sorted using popular spreadsheet programs such as EXCEL, enabling the most bio-diverse sets to be easily identified. The number of possible combinations does rise steeply with increasing number of locations, so that obtaining and listing all of these becomes prohibitive in computer time and effort, whether for identifying just species richness or EH. Simulated annealing has been proposed for identifying sets of locations maximising species richness (McDonnell et al. 2002) and this approach can also be used for maximising EH (Agapow & Crozier 2005).
The estimates of statistical sufficiency in Table 4 are not strictly correct for these data, as discussed above, but the results bring out an important point. For some sites 34 species were recorded and others 33, but the 22 combinations formed by dropping one site each time yielded results which did not differ significantly: all the various combinations are not significantly different with respect either to the number of species preserved or the EH. The management implication is that the criteria for choosing between those combinations which do not differ significantly can rest on other grounds than species richness or EH.
The identification of species is commonly a laborious and difficult process, so that it is natural that short cuts have been sought that avoid this task. One such short cut is “higher taxon richness”, in which higher taxa (such as genera or even families) are counted rather than species. Because higher taxa are more easily identified than species, this method is naturally attractive (reviewed by Crozier (1997)). In a study of subterranean bacterial communities related through an rRNA phylogeny, Crozier et al. (1999) found that higher taxon richness correlated well with GD. The present results indicate that the number of genera is highly predictive of EH (as gauged using systematic nomenclature) for large data sets. For small to medium sized data sets the predictiveness of EH drops off markedly as the range of number of genera preserved by site combinations decreases. For large data sets, such as NQFI, genus number is highly predictive of species number, a result suggesting that for such studies there could be a saving of effort through identifying specimens to genus only.
Phylogenies or surrogates based on systematic nomenclature have been used in or recommended for ecological studies on community structure (Warwick & Clarke 1994, 1998, Clarke & Warwick 2001, Webb et al. 2002, Cattin et al. 2004, Gotelli 2004), and estimated functional divergence has been used instead of phylogeny in examining community structure (Petchey & Gaston 2002, Petchey et al. 2004). There seems therefore to be a widespread move towards going beyond species richness in biodiversity assessment and similar endeavors, as also shown by the use of unit-length morphological phylogenies (Faith et al. 2004).
The methods suggested here have limitations. Groups in which there is minimal systematic structure, perhaps because they have radiated recently and not yet evolved high degrees of divergence, will have a poor reflection of phylogeny in their nomenclature. There are grounds for optimism, in that a study of the effects of phylogenetic inaccuracy on comparative analysis (Symonds 2002) found that the process is fairly robust against such errors. More serious, given the ambition to cover a significant proportion of the species in habitats (Humphries et al. 1995), is the lack of consistency across broad taxonomic groups, such as insects and mammals. If a consistent standard could be applied for systematics across at least the metazoa, such as a correspondence between systematic rank and time since origin (Avise & Johns 1999), then a broad array of animal groups could be included in such analyses. However, as it is, use of the NQFI data set shows that most terrestrial species could be included in analyses.
The argument in favor of a phylogenetic basis for setting conservation priorities was put persuasively by Wilson (1992) and implemented in various metrics by others (reviewed by Crozier (1997)). However the idea that the object of conservation is to preserve the widest diversity of features in the biota shows that a phylogenetic rationale has long been implicit. But even if the underlying rationale for biodiversity preservation is phylogenetic, need the methods for achieving it be? If large numbers of species are involved, does a phylogenetic approach to assessment still matter (Humphries et al. 1995, Crozier 1997)? Our results indicate that phylogeny (gauged through its surrogate of systematic nomenclature) will make the most difference when the number of species is small. However, given that it is much more difficult and labor-intensive to collect the data than to analyse them, it would seem negligent not to investigate the effects of phylogeny now that there are adequate tools for doing so.
Acknowledgements
We thank Alan N. Andersen, Jon D. Majer, David Lubertazzi, Charles Daugherty, Raghavendra Gadagkar, Nick J. Gotelli and Phil S. Ward for discussions and data, and Nick J. Gotelli, Brian E. Heterick, David Lubertazzi, Geoff E. Monteith, Helge Schlüns, Matt R. E. Symonds, Phil S Ward, Brian Heterick, Alan N. Andersen, David K. Yeates and two anonymous reviewers for comments on drafts of the manuscript. Part of this work was included in a James Cook University honours thesis by LJD. RHC’s work on social insect evolution is supported by grants from the Australian Research Council.
Appendix
NEXUS files for the analyses used in this paper. The trees involved can be readily viewed using TREE-VIEW X if the DATA block is removed or commented out.
#NEXUS
[! Created by TreeMakerB v1.0.8d 9/7/04]
[Data from Yeates, Bouchard & Monteith, 2002, who also give]
[the complete names for each species used]
[The systematic levels used were superorder, order, suborder, infraorder, superfamily, family, genus, and species]
BEGIN DATA;
DIMENSIONS NTAX=273 NCHAR=14;
FORMAT DATATYPE=CONTINUOUS;
CHARSTATELABELS
| 1 | site_FU, |
| 2 | site_TU, |
| 3 | site_WU, |
| 4 | site_CU, |
| 5 | site_BM, |
| 6 | site_LU, |
| 7 | site_AU, |
| 8 | site_BK, |
| 9 | site_MT, |
| 10 | site_KU, |
| 11 | site_LE, |
| 12 | site_SU, |
| 13 | site_HU, |
| 14 | site_EU |
| ; |
MATRIX
| TargaremineA_LY08 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D083 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tryonicus_BL02 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Notuchus_DE02 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| PeloridiidA_PE02 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hackeriella_PE01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Schizopteromiris_MI01 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Grosshygia_GR03 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mesophloeobia_A078 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Austrovelia_AV01 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kumaressa_A001 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A026 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Euricoris_A027 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Glyptoaptera_A030 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Drakiessa_A066 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| GenusE_A041 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GenusH_A043 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chelonoderus_A070 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Aegisocoris_A072 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drakiessa_A088 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grosshygioides_GR04 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tomocoris_LY01 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Australotarma_LY03 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Targarops_LY06 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Darodilia_C069 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TargaremineC_LY10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mystropomus_C001 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Pamborus_C005 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Migadopine_C006 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Laccopterum_C007 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Mecyclothorax_C010 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raphetis_C011 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sitaphe_C012 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Coptocarpus_C016 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Illaphanus_C017 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Castelnaudia_C021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Feronista_C022 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C031 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Loxogenius_C032 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C043 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nurus_C044 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Setalis_C046 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lecanomerus_C057 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Harpaline_C060 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anomotarus_C063 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Loxandrus_C065 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Lacordairia_C067 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chariotheca_CM53 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Terradessus_DY02 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Athemistus_AT03 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Blepegenes_AD01 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cardiothorax_AD02 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bluops_AD03 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Seirotrana_AD15 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Adelium_AD18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Adelodemus_AD19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bellendenum_AD22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Monteithium_AD24 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nolicima_AD25 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Licinoma_AD26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Dicyrtodes_AD29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Epomidus_AD33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diaspirus_AD34 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coripera_AD42 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dicyrtodes_AD49 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM08 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM11 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM16 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydissus_CM17 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM51 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Mychestes_MY05 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Lissapterus_LU02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Amphistomus_D005 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacopris_D007 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aptenocanthon_D012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Temnoplectron_D065 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Tryonicus_BL01 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML01 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML02 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML03 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML04 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myerslopella_ML05 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notuchus_DE01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A019 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A020 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A021 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| Aellocoris_A022 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A023 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A024 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aellocoris_A025 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glyptoaptera_A028 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glyptoaptera_A029 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Spinandra_A031 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Spinandra_A032 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spinandra_A033 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Spinandra_A034 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| GenusA_A037 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| GenusB_A038 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| GenusA_A035 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GenusA_A036 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| GenusE_A039 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| GenusE_A040 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drakiessa_A064 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drakiessa_A065 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chelonoderus_A067 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chelonoderus_A068 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chelonoderus_A069 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aegisocoris_A071 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Neophloeobia_A073 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Neophloeobia_A074 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neophloeobia_A075 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mesophloeobia_A077 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Granulaptera_A079 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Granulaptera_A080 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Granulaptera_A081 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Granulaptera_A082 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Granulaptera_A083 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Granulaptera_A084 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grosshygia_GR01 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grosshygia_GR02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| TargaremineA_LY07 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pamborus_C003 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Pamborus_C004 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mecyclothorax_C008 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mecyclothorax_C009 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coptocarpus_C015 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Castelnaudia_C018 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Castelnaudia_C019 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Castelnaudia_C020 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Leiradira_C023 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C024 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C025 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C026 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C027 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C028 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C029 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiradira_C030 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C033 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C034 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| Notonomus_C035 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Notonomus_C036 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C037 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C038 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C039 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C040 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C041 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Notonomus_C042 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C047 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C048 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C049 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Trichosternus_C050 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C051 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C052 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Trichosternus_C053 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trichosternus_C055 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Trichosternus_C056 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Harpaline_C058 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Harpaline_C059 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anomotarus_C061 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Carenum_C068 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP15 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP16 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP19 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP20 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Philipis_CP27 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP32 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP33 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP34 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Philipis_CP36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Terradessus_DY01 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Athemistus_AT01 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Athemistus_AT02 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD04 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Adelium_AD06 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD07 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD08 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Adelium_AD09 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Adelium_AD11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Adelium_AD12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Adelium_AD13 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coripera_AD16 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD17 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bellendenum_AD20 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bellendenum_AD21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Monteithium_AD23 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dicyrtodes_AD28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epomidus_AD30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diaspirus_AD31 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptogastrus_AD35 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptogastrus_AD36 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coripera_AD40 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coripera_AD41 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apocryphodes_AD43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Leptogastrus_AD44 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adelium_AD45 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bellendenum_AD47 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM01 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM02 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM03 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM06 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM07 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM09 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM10 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cuemus_CM12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cuemus_CM13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM14 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM15 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Omolipus_CM18 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Apterotheca_CM23 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM24 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM25 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Apterotheca_CM26 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM27 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Apterotheca_CM29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Apterotheca_CM30 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM32 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM33 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM35a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM35b | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM35c | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM37 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM38 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Apterotheca_CM39 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM40 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caxtonana_CM41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM42 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Omolipus_CM43 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Apterotheca_CM50 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mychestes_MY01 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mychestes_MY03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lissapterus_LU01 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amphistomus_D004 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aptenocanthon_D008 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aptenocanthon_D009 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Aptenocanthon_D010 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aptenocanthon_D011 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Temnoplectron_D019 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lepanus_D088 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Temnoplectron_D097 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Temnoplectron_D138 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D139 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D140 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D141 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D142 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D143 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D144 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D146 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pseudignambia_D147 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D148 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D149 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D151 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D152 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D153 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D154 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D155 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pseudignambia_D156 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ; |
END;
BEGIN TREES;
TRANSLATE
| 203 | TargaremineA_LY08, |
| 388 | Pseudignambia_D083, |
| 138 | Myerslopella_ML06, |
| 132 | Tryonicus_BL02, |
| 140 | Notuchus_DE02, |
| 143 | PeloridiidA_PE02, |
| 142 | Hackeriella_PE01, |
| 141 | Schizopteromiris_MI01, |
| 197 | Grosshygia_GR03, |
| 187 | Mesophloeobia_A078, |
| 144 | Austrovelia_AV01, |
| 145 | Kumaressa_A001, |
| 153 | Aellocoris_A026, |
| 154 | Euricoris_A027, |
| 157 | Glyptoaptera_A030, |
| 174 | Drakiessa_A066, |
| 171 | GenusE_A041, |
| 175 | GenusH_A043, |
| 179 | Chelonoderus_A070, |
| 181 | Aegisocoris_A072, |
| 194 | Drakiessa_A088, |
| 198 | Grosshygioides_GR04, |
| 199 | Tomocoris_LY01, |
| 200 | Australotarma_LY03, |
| 201 | Targarops_LY06, |
| 284 | Philipis_CP37, |
| 267 | Darodilia_C069, |
| 204 | TargaremineC_LY10, |
| 205 | Mystropomus_C001, |
| 208 | Pamborus_C005, |
| 209 | Migadopine_C006, |
| 210 | Laccopterum_C007, |
| 213 | Mecyclothorax_C010, |
| 214 | Raphetis_C011, |
| 215 | Sitaphe_C012, |
| 217 | Coptocarpus_C016, |
| 218 | Illaphanus_C017, |
| 222 | Castelnaudia_C021, |
| 223 | Feronista_C022, |
| 232 | Leiradira_C031, |
| 233 | Loxogenius_C032, |
| 244 | Notonomus_C043, |
| 245 | Nurus_C044, |
| 246 | Setalis_C046, |
| 257 | Lecanomerus_C057, |
| 260 | Harpaline_C060, |
| 262 | Anomotarus_C063, |
| 263 | Loxandrus_C065, |
| 264 | Lacordairia_C067, |
| 372 | Chariotheca_CM53, |
| 286 | Terradessus_DY02, |
| 289 | Athemistus_AT03, |
| 290 | Blepegenes_AD01, |
| 291 | Cardiothorax_AD02, |
| 292 | Bluops_AD03, |
| 301 | Adelium_AD14, |
| 302 | Seirotrana_AD15, |
| 305 | Adelium_AD18, |
| 306 | Adelodemus_AD19, |
| 309 | Bellendenum_AD22, |
| 311 | Monteithium_AD24, |
| 312 | Nolicima_AD25, |
| 313 | Licinoma_AD26, |
| 315 | Dicyrtodes_AD29, |
| 317 | Epomidus_AD33, |
| 319 | Diaspirus_AD34, |
| 324 | Coripera_AD42, |
| 329 | Dicyrtodes_AD49, |
| 337 | Caxtonana_CM08, |
| 340 | Apterotheca_CM11, |
| 345 | Apterotheca_CM16, |
| 346 | Hydissus_CM17, |
| 371 | Apterotheca_CM51, |
| 375 | Mychestes_MY05, |
| 377 | Lissapterus_LU02, |
| 379 | Amphistomus_D005, |
| 380 | Aulacopris_D007, |
| 385 | Aptenocanthon_D012, |
| 387 | Temnoplectron_D065, |
| 131 | Tryonicus_BL01, |
| 133 | Myerslopella_ML01, |
| 134 | Myerslopella_ML02, |
| 135 | Myerslopella_ML03, |
| 136 | Myerslopella_ML04, |
| 137 | Myerslopella_ML05, |
| 139 | Notuchus_DE01, |
| 146 | Aellocoris_A019, |
| 147 | Aellocoris_A020, |
| 148 | Aellocoris_A021, |
| 149 | Aellocoris_A022, |
| 150 | Aellocoris_A023, |
| 151 | Aellocoris_A024, |
| 152 | Aellocoris_A025, |
| 155 | Glyptoaptera_A028, |
| 156 | Glyptoaptera_A029, |
| 158 | Spinandra_A031, |
| 159 | Spinandra_A032, |
| 160 | Spinandra_A033, |
| 161 | Spinandra_A034, |
| 167 | GenusA_A037, |
| 168 | GenusB_A038, |
| 165 | GenusA_A035, |
| 166 | GenusA_A036, |
| 169 | GenusE_A039, |
| 170 | GenusE_A040, |
| 172 | Drakiessa_A064, |
| 173 | Drakiessa_A065, |
| 176 | Chelonoderus_A067, |
| 177 | Chelonoderus_A068, |
| 178 | Chelonoderus_A069, |
| 180 | Aegisocoris_A071, |
| 182 | Neophloeobia_A073, |
| 183 | Neophloeobia_A074, |
| 184 | Neophloeobia_A075, |
| 186 | Mesophloeobia_A077, |
| 188 | Granulaptera_A079, |
| 189 | Granulaptera_A080, |
| 190 | Granulaptera_A081, |
| 191 | Granulaptera_A082, |
| 192 | Granulaptera_A083, |
| 193 | Granulaptera_A084, |
| 195 | Grosshygia_GR01, |
| 196 | Grosshygia_GR02, |
| 202 | TargaremineA_LY07, |
| 206 | Pamborus_C003, |
| 207 | Pamborus_C004, |
| 211 | Mecyclothorax_C008, |
| 212 | Mecyclothorax_C009, |
| 216 | Coptocarpus_C015, |
| 219 | Castelnaudia_C018, |
| 220 | Castelnaudia_C019, |
| 221 | Castelnaudia_C020, |
| 224 | Leiradira_C023, |
| 225 | Leiradira_C024, |
| 226 | Leiradira_C025, |
| 227 | Leiradira_C026, |
| 228 | Leiradira_C027, |
| 229 | Leiradira_C028, |
| 230 | Leiradira_C029, |
| 231 | Leiradira_C030, |
| 234 | Notonomus_C033, |
| 235 | Notonomus_C034, |
| 236 | Notonomus_C035, |
| 237 | Notonomus_C036, |
| 238 | Notonomus_C037, |
| 239 | Notonomus_C038, |
| 240 | Notonomus_C039, |
| 241 | Notonomus_C040, |
| 242 | Notonomus_C041, |
| 243 | Notonomus_C042, |
| 247 | Trichosternus_C047, |
| 248 | Trichosternus_C048, |
| 249 | Trichosternus_C049, |
| 250 | Trichosternus_C050, |
| 251 | Trichosternus_C051, |
| 252 | Trichosternus_C052, |
| 253 | Trichosternus_C053, |
| 254 | Trichosternus_C055, |
| 255 | Trichosternus_C056, |
| 258 | Harpaline_C058, |
| 259 | Harpaline_C059, |
| 261 | Anomotarus_C061, |
| 265 | Carenum_C068, |
| 268 | Philipis_CP07, |
| 269 | Philipis_CP10, |
| 270 | Philipis_CP15, |
| 271 | Philipis_CP16, |
| 272 | Philipis_CP19, |
| 273 | Philipis_CP20, |
| 274 | Philipis_CP21, |
| 275 | Philipis_CP23, |
| 276 | Philipis_CP24, |
| 277 | Philipis_CP25, |
| 278 | Philipis_CP26, |
| 279 | Philipis_CP27, |
| 280 | Philipis_CP32, |
| 281 | Philipis_CP33, |
| 282 | Philipis_CP34, |
| 283 | Philipis_CP36, |
| 285 | Terradessus_DY01, |
| 287 | Athemistus_AT01, |
| 288 | Athemistus_AT02, |
| 293 | Adelium_AD04, |
| 294 | Adelium_AD06, |
| 295 | Adelium_AD07, |
| 296 | Adelium_AD08, |
| 297 | Adelium_AD09, |
| 298 | Adelium_AD11, |
| 299 | Adelium_AD12, |
| 300 | Adelium_AD13, |
| 303 | Coripera_AD16, |
| 304 | Adelium_AD17, |
| 307 | Bellendenum_AD20, |
| 308 | Bellendenum_AD21, |
| 310 | Monteithium_AD23, |
| 314 | Dicyrtodes_AD28, |
| 316 | Epomidus_AD30, |
| 318 | Diaspirus_AD31, |
| 320 | Leptogastrus_AD35, |
| 321 | Leptogastrus_AD36, |
| 322 | Coripera_AD40, |
| 323 | Coripera_AD41, |
| 325 | Apocryphodes_AD43, |
| 326 | Leptogastrus_AD44, |
| 327 | Adelium_AD45, |
| 328 | Bellendenum_AD47, |
| 330 | Caxtonana_CM01, |
| 331 | Caxtonana_CM02, |
| 332 | Caxtonana_CM03, |
| 333 | Caxtonana_CM04, |
| 334 | Caxtonana_CM05, |
| 335 | Caxtonana_CM06, |
| 336 | Caxtonana_CM07, |
| 338 | Apterotheca_CM09, |
| 339 | Apterotheca_CM10, |
| 341 | Cuemus_CM12, |
| 342 | Cuemus_CM13, |
| 343 | Caxtonana_CM14, |
| 344 | Caxtonana_CM15, |
| 347 | Omolipus_CM18, |
| 348 | Caxtonana_CM21, |
| 349 | Apterotheca_CM23, |
| 350 | Apterotheca_CM24, |
| 351 | Apterotheca_CM25, |
| 352 | Apterotheca_CM26, |
| 353 | Apterotheca_CM27, |
| 354 | Apterotheca_CM28, |
| 355 | Apterotheca_CM29, |
| 356 | Apterotheca_CM30, |
| 357 | Apterotheca_CM32, |
| 358 | Apterotheca_CM33, |
| 359 | Apterotheca_CM35a, |
| 360 | Apterotheca_CM35b, |
| 361 | Apterotheca_CM35c, |
| 362 | Apterotheca_CM36, |
| 363 | Apterotheca_CM37, |
| 364 | Apterotheca_CM38, |
| 365 | Apterotheca_CM39, |
| 366 | Apterotheca_CM40, |
| 367 | Caxtonana_CM41, |
| 368 | Apterotheca_CM42, |
| 369 | Omolipus_CM43, |
| 370 | Apterotheca_CM50, |
| 373 | Mychestes_MY01, |
| 374 | Mychestes_MY03, |
| 376 | Lissapterus_LU01, |
| 378 | Amphistomus_D004, |
| 381 | Aptenocanthon_D008, |
| 382 | Aptenocanthon_D009, |
| 383 | Aptenocanthon_D010, |
| 384 | Aptenocanthon_D011, |
| 386 | Temnoplectron_D019, |
| 389 | Lepanus_D088, |
| 390 | Temnoplectron_D097, |
| 391 | Temnoplectron_D138, |
| 392 | Pseudignambia_D139, |
| 393 | Pseudignambia_D140, |
| 394 | Pseudignambia_D141, |
| 395 | Pseudignambia_D142, |
| 396 | Pseudignambia_D143, |
| 397 | Pseudignambia_D144, |
| 398 | Pseudignambia_D145, |
| 399 | Pseudignambia_D146, |
| 400 | Pseudignambia_D147, |
| 401 | Pseudignambia_D148, |
| 402 | Pseudignambia_D149, |
| 403 | Pseudignambia_D150, |
| 404 | Pseudignambia_D151, |
| 405 | Pseudignambia_D152, |
| 406 | Pseudignambia_D153, |
| 407 | Pseudignambia_D154, |
| 408 | Pseudignambia_D155, |
| 409 | Pseudignambia_D156 |
| ; |
TREE tree_1 = ((((((((131:0.125,132:0.125):0.125):0.125):0.125):0.125):0.125):0.125):0.125, (((((((133:0.125,134:0.125,135:0.125,136:0.125,137:0.125,138:0.125):0.125):0.125):0.125): 0.125,((((139:0.125,140:0.125):0.125):0.125):0.125):0.125):0.125, (((((141:0.125):0.125):0.125):0.125):0.125,((((142:0.125):0.125, (143:0.125):0.125):0.125):0.125):0.125,((((144:0.125):0.125):0.125):0.125):0.125, ((((145:0.125):0.125, (146:0.125,147:0.125,148:0.125,149:0.125,150:0.125,151:0.125,152:0.125,153:0.125):0.125, (154:0.125):0.125,(155:0.125,156:0.125,157:0.125):0.125, (158:0.125,159:0.125,160:0.125,161:0.125):0.125,(165:0.125,166:0.125,167:0.125):0.125, (172:0.125,173:0.125,174:0.125,194:0.125):0.125, (176:0.125,177:0.125,178:0.125,179:0.125):0.125,(180:0.125,181:0.125):0.125, (182:0.125,183:0.125,184:0.125):0.125, (188:0.125,189:0.125,190:0.125,191:0.125,192:0.125,193:0.125):0.125,(168:0.125):0.125, (169:0.125,170:0.125,171:0.125):0.125,(175:0.125):0.125, (186:0.125,187:0.125):0.125):0.125):0.125,(((195:0.125,196:0.125,197:0.125):0.125, (198:0.125):0.125):0.125):0.125,(((199:0.125):0.125,(200:0.125):0.125,(201:0.125):0.125, (202:0.125,203:0.125):0.125,(204:0.125):0.125):0.125):0.125):0.125):0.125):0.125):0.125, (((((((205:0.125):0.125,(206:0.125,207:0.125,208:0.125):0.125,(209:0.125):0.125, (210:0.125):0.125,(211:0.125,212:0.125,213:0.125):0.125,(214:0.125):0.125, (215:0.125):0.125,(216:0.125,217:0.125):0.125,(218:0.125):0.125, (219:0.125,220:0.125,221:0.125,222:0.125):0.125,(223:0.125):0.125, (224:0.125,225:0.125,226:0.125,227:0.125,228:0.125,229:0.125,230:0.125,231:0.125,232:0.125):0.125,(233:0.125):0.125, (234:0.125,235:0.125,236:0.125,237:0.125,238:0.125,239:0.125,240:0.125,241:0.125,242:0.125,243:0.125,244:0.125):0.125,(245:0.125):0.125,(246:0.125):0.125, (247:0.125,248:0.125,249:0.125,250:0.125,251:0.125,252:0.125,253:0.125,254:0.125,255:0.125):0.125,(258:0.125,259:0.125,260:0.125):0.125,(261:0.125,262:0.125):0.125, (263:0.125):0.125,(264:0.125):0.125,(265:0.125):0.125, (268:0.125,269:0.125,270:0.125,271:0.125,272:0.125,273:0.125,274:0.125,275:0.125,276:0.125,277:0.125,278:0.125,279:0.125,280:0.125,281:0.125,282:0.125,283:0.125,284:0.125):0.125, (257:0.125):0.125,(267:0.125):0.125):0.125, ((285:0.125,286:0.125):0.125):0.125):0.125):0.125):0.125, (((((287:0.125,288:0.125,289:0.125):0.125):0.125):0.125,(((290:0.125):0.125, (291:0.125):0.125,(292:0.125):0.125, (293:0.125,294:0.125,295:0.125,296:0.125,297:0.125,298:0.125,299:0.125,300:0.125,301:0.125,304:0.125,305:0.125,327:0.125):0.125,(302:0.125):0.125, (303:0.125,322:0.125,323:0.125,324:0.125):0.125,(306:0.125):0.125, (307:0.125,308:0.125,309:0.125,328:0.125):0.125,(310:0.125,311:0.125):0.125, (312:0.125):0.125,(313:0.125):0.125,(314:0.125,315:0.125,329:0.125):0.125, (316:0.125,317:0.125):0.125,(318:0.125,319:0.125):0.125, (320:0.125,321:0.125,326:0.125):0.125,(325:0.125):0.125, (330:0.125,331:0.125,332:0.125,333:0.125,334:0.125,335:0.125,336:0.125,337:0.125,343:0.125,344:0.125,348:0.125,367:0.125):0.125, (338:0.125,339:0.125,340:0.125,345:0.125,349:0.125,350:0.125,351:0.125,352:0.125,353:0.125,354:0.125,355:0.125,356:0.125,357:0.125,358:0.125,359:0.125,360:0.125,361:0.125,362:0.125,363:0.125,364:0.125,365:0.125,366:0.125,368:0.125,370:0.125,371:0.125):0.125, (341:0.125,342:0.125):0.125,(346:0.125):0.125,(347:0.125,369:0.125):0.125, (372:0.125):0.125,(373:0.125,374:0.125,375:0.125):0.125):0.125):0.125):0.125, ((((376:0.125,377:0.125):0.125):0.125,((378:0.125,379:0.125):0.125,(380:0.125):0.125, (381:0.125,382:0.125,383:0.125,384:0.125,385:0.125):0.125, (386:0.125,387:0.125,390:0.125,391:0.125):0.125, (388:0.125,392:0.125,393:0.125,394:0.125,395:0.125,396:0.125,397:0.125,398:0.125,399:0.125,400:0.125,401:0.125,402:0.125,403:0.125,404:0.125,405:0.125,406:0.125,407:0.125,408:0.125,409:0.125):0.125,(389:0.125):0.125):0.125):0.125):0.125):0.125):0.125):0.125); END;
#NEXUS
[! Created by TreeMakerB v1.0.7d 12/4/04]
[Data courtesy of NJ Gottelli from the study by Gottelli & Ellison 2002]
[Data are from ‘Most Recent Bog Ant Matrices.xls’]
[panel ‘Forests. Abundance data from pitfall traps only]
[The systematic divisions used were subfamily, tribe, genus and species]
BEGIN DATA;
DIMENSIONS NTAX=34 NCHAR=22;
FORMAT DATATYPE=CONTINUOUS;
CHARSTATELABELS
| 1 | site_ARC, |
| 2 | site_BH, |
| 3 | site_CB, |
| 4 | site_CKB, |
| 5 | site_HAW, |
| 6 | site_HBC, |
| 7 | site_OB, |
| 8 | site_PK, |
| 9 | site_QP, |
| 10 | site_RP, |
| 11 | site_SKP, |
| 12 | site_SW, |
| 13 | site_TPB, |
| 14 | site_WIN, |
| 15 | site_SPR, |
| 16 | site_SNA, |
| 17 | site_PEA, |
| 18 | site_CHI, |
| 19 | site_MOL, |
| 20 | site_COL, |
| 21 | site_MOO, |
| 22 | site_CAR |
| ; |
MATRIX
| Amblypone_pallipes | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Aphaenogaster_rudis | 48 | 1 | 2 | 63 | 15 | 98 | 41 | 5 | 5 | 22 | 35 | 16 | 2 | 0 | 11 | 0 | 0 | 7 | 0 | 23 | 0 | 0 |
| Leptothorax_curvispinosus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptothorax_ambiguus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Leptothorax_longispinosus | 0 | 18 | 2 | 3 | 0 | 0 | 16 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 16 | 0 | 0 |
| Myrmecina_americana | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myrmica_cfbrevispinosus | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myrmica_detrinodis | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 59 | 12 |
| Myrmica_punctiventris | 2 | 0 | 115 | 44 | 0 | 50 | 1 | 17 | 1 | 202 | 8 | 16 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | 139 | 0 | 0 |
| Myrmica_sculptilis | 0 | 2 | 1 | 24 | 0 | 9 | 0 | 1 | 23 | 60 | 7 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myrmica_smithana | 0 | 0 | 0 | 5 | 0 | 3 | 0 | 0 | 1 | 56 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stenamma_diecki | 0 | 3 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Stenamma_impar | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Stenamma_schmitti | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Camponotus_herculeanus | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 5 | 39 |
| Camponotus_noveborencensis | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Camponotus_nearcticus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Camponotus_pennsylvanicus | 3 | 5 | 0 | 1 | 2 | 16 | 17 | 15 | 0 | 122 | 1 | 7 | 56 | 0 | 250 | 0 | 0 | 0 | 8 | 0 | 0 | |
| Formica_argentea | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Formica_fusca | 0 | 0 | 0 | 20 | 0 | 1 | 5 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Formica_glacialis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Formica_neogagates | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Formica_obscuriventris | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Formica_subintegra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 209 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Formica_subsericea | 0 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 | 18 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lasius_alienus | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 9 | 1 | 5 | 0 | 0 | 0 | 0 | 3 | 3 | 5 | 0 | 4 | 4 | 4 |
| Lasius_flavus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lasius_neoniger | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Lasius_speculiventris | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lasius_umbratus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 0 |
| Prenolepis_imparis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dolichoderus_plagiatus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tapinoma_sessile | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stenamma_brevicorne | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ; |
END;
BEGIN TREES;
TRANSLATE
| 6 | Amblypone_pallipes, |
| 12 | Aphaenogaster_rudis, |
| 15 | Leptothorax_curvispinosus, |
| 16 | Leptothorax_ambiguus, |
| 17 | Leptothorax_longispinosus, |
| 22 | Myrmecina_americana, |
| 25 | Myrmica_cfbrevispinosus, |
| 26 | Myrmica_detrinodis, |
| 27 | Myrmica_punctiventris, |
| 28 | Myrmica_sculptilis, |
| 29 | Myrmica_smithana, |
| 32 | Stenamma_diecki, |
| 33 | Stenamma_impar, |
| 34 | Stenamma_schmitti, |
| 41 | Camponotus_herculeanus, |
| 42 | Camponotus_noveborencensis, |
| 43 | Camponotus_nearcticus, |
| 44 | Camponotus_pennsylvanicus, |
| 48 | Formica_argentea, |
| 49 | Formica_fusca, |
| 50 | Formica_glacialis, |
| 51 | Formica_neogagates, |
| 53 | Formica_obscuriventris, |
| 54 | Formica_subintegra, |
| 55 | Formica_subsericea, |
| 58 | Lasius_alienus, |
| 59 | Lasius_flavus, |
| 60 | Lasius_neoniger, |
| 61 | Lasius_speculiventris, |
| 62 | Lasius_umbratus, |
| 64 | Prenolepis_imparis, |
| 69 | Dolichoderus_plagiatus, |
| 71 | Tapinoma_sessile, |
| 72 | Stenamma_brevicorne |
| ; |
TREE tree_1 = ((((6:0.25):0.25):0.25):0.25,(((12:0.25):0.25):0.25, ((15:0.25,16:0.25,17:0.25):0.25):0.25,((22:0.25):0.25):0.25, ((25:0.25,26:0.25,27:0.25,28:0.25,29:0.25):0.25):0.25, ((32:0.25,33:0.25,34:0.25,72:0.25):0.25):0.25):0.25, (((41:0.25,42:0.25,43:0.25,44:0.25):0.25):0.25, ((48:0.25,49:0.25,50:0.25,51:0.25,53:0.25,54:0.25,55:0.25):0.25):0.25, ((58:0.25,59:0.25,60:0.25,61:0.25,62:0.25):0.25,(64:0.25):0.25):0.25):0.25, (((69:0.25):0.25,(71:0.25):0.25):0.25):0.25); END;
#NEXUS
[! Created by TreeMakerB v1.1.1 31/5/05]
[Species list taken from Daugherty et al 1994, distributions were simulated]
[The systematic levels used were order, suborder, infraorder, family, genus and species].
BEGIN DATA;
DIMENSIONS NTAX=62 NCHAR=15;
FORMAT DATATYPE=CONTINUOUS;
CHARSTATELABELS
| 1 | site_1, |
| 2 | site_2, |
| 3 | site_3, |
| 4 | site_4, |
| 5 | site_5, |
| 6 | site_6, |
| 7 | site_7, |
| 8 | site_8, |
| 9 | site_9, |
| 10 | site_10, |
| 11 | site_11, |
| 12 | site_12, |
| 13 | site_13, |
| 14 | site_14, |
| 15 | site_15 |
| ; |
MATRIX
| Sphenodon_guntheri | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Sphenodon_punctulatus | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spPKI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Cyclodina_spTwo | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Naultinus_tuberculatus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Hoplodactylus_chrysosireticus | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_duvaucelii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hoplodactylus_granulatus | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_kahutarae | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hoplodactylus_maculatus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hoplodactylus_nebulosus | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_pacificus | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hoplodactylus_stephensi | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Hoplodactylus_spNK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spMa | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spMtA | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spCan | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Hoplodactylus_spSoA | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Hoplodactylus_spDap | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spEaO | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hoplodactylus_spWeO | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hoplodactylus_spSoM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Hoplodactylus_spWes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Hoplodactylus_sp3KI | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hoplodactylus_spMaI | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hoplodactylus_rakiurae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Naultinus_elegans | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Naultinus_gemmeus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Naultinus_grayii | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Naultinus_manukanus | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Naultinus_rudis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Naultinus_stellatus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cyclodina_aenea | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cyclodina_alani | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Cyclodina_macgregori | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclodina_oliveri | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Cyclodina_ornata | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Cyclodina_whitakeri | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Cyclodina_spOne | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Leiolopisma_acrinasum | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Leiolopisma_chloronoton | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Leiolopisma_fallai | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Leiolopisma_grande | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_homalanotum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Leiolopisma_inconspicuum | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Leiolopisma_infrapunctatum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Leiolopisma_lineoocellatum | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_maccanni | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_microlepis | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_moco | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Leiolopisma_nigriplantare | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Leiolopisma_polychroma | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Leiolopisma_notosaurus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Leiolopisma_otagense | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Leiolopisma_waimatense | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Leiolopisma_smithi | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_stenotis | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_striatum | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_suteri | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Leiolopisma_zelandicum | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiolopisma_spOne | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Leiolopisma_spTwo | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| ; |
END;
BEGIN TREES;
TRANSLATE
| 9 | Sphenodon_guntheri, |
| 8 | Sphenodon_punctulatus, |
| 40 | Hoplodactylus_spPKI, |
| 55 | Cyclodina_spTwo, |
| 47 | Naultinus_tuberculatus, |
| 19 | Hoplodactylus_chrysosireticus, |
| 20 | Hoplodactylus_duvaucelii, |
| 21 | Hoplodactylus_granulatus, |
| 22 | Hoplodactylus_kahutarae, |
| 23 | Hoplodactylus_maculatus, |
| 24 | Hoplodactylus_nebulosus, |
| 25 | Hoplodactylus_pacificus, |
| 26 | Hoplodactylus_stephensi, |
| 27 | Hoplodactylus_spNK, |
| 28 | Hoplodactylus_spMa, |
| 29 | Hoplodactylus_spMtA, |
| 30 | Hoplodactylus_spCan, |
| 31 | Hoplodactylus_spSoA, |
| 32 | Hoplodactylus_spDap, |
| 33 | Hoplodactylus_spEaO, |
| 34 | Hoplodactylus_spWeO, |
| 35 | Hoplodactylus_spSoM, |
| 36 | Hoplodactylus_spWes, |
| 37 | Hoplodactylus_sp3KI, |
| 38 | Hoplodactylus_spMaI, |
| 39 | Hoplodactylus_rakiurae, |
| 41 | Naultinus_elegans, |
| 42 | Naultinus_gemmeus, |
| 43 | Naultinus_grayii, |
| 44 | Naultinus_manukanus, |
| 45 | Naultinus_rudis, |
| 46 | Naultinus_stellatus, |
| 48 | Cyclodina_aenea, |
| 49 | Cyclodina_alani, |
| 50 | Cyclodina_macgregori, |
| 51 | Cyclodina_oliveri, |
| 52 | Cyclodina_ornata, |
| 53 | Cyclodina_whitakeri, |
| 54 | Cyclodina_spOne, |
| 56 | Leiolopisma_acrinasum, |
| 57 | Leiolopisma_chloronoton, |
| 58 | Leiolopisma_fallai, |
| 59 | Leiolopisma_grande, |
| 60 | Leiolopisma_homalanotum, |
| 61 | Leiolopisma_inconspicuum, |
| 62 | Leiolopisma_infrapunctatum, |
| 63 | Leiolopisma_lineoocellatum, |
| 64 | Leiolopisma_maccanni, |
| 65 | Leiolopisma_microlepis, |
| 66 | Leiolopisma_moco, |
| 67 | Leiolopisma_nigriplantare, |
| 68 | Leiolopisma_polychroma, |
| 69 | Leiolopisma_notosaurus, |
| 70 | Leiolopisma_otagense, |
| 71 | Leiolopisma_waimatense, |
| 72 | Leiolopisma_smithi, |
| 73 | Leiolopisma_stenotis, |
| 74 | Leiolopisma_striatum, |
| 75 | Leiolopisma_suteri, |
| 76 | Leiolopisma_zelandicum, |
| 77 | Leiolopisma_spOne, |
| 78 | Leiolopisma_spTwo |
| ; |
TREE tree_1 = ((((((8:0.1666667,9:0.1666667):0.1666667):0.1666667):0.1666667):0.1666667):0.1666667, (((((19:0.1666667,20:0.1666667,21:0.1666667,22:0.1666667,23:0.1666667,24:0.1666667,25:0.1 666667,26:0.1666667,27:0.1666667,28:0.1666667,29:0.1666667,30:0.1666667,31:0.1666667,32:0.1666667,33:0.1666667,34:0.1666667,35:0.1666667,36:0.1666667,37:0.1666667,38:0.1666667,39:0.1666667,40:0.1666667):0.1666667, (41:0.1666667,42:0.1666667,43:0.1666667,44:0.1666667,45:0.1666667,46:0.1666667,47:0.1666667):0.1666667):0.1666667):0.1666667, (((48:0.1666667,49:0.1666667,50:0.1666667,51:0.1666667,52:0.1666667,53:0.1666667,54:0.1666667,55:0.1666667):0.1666667, (56:0.1666667,57:0.1666667,58:0.1666667,59:0.1666667,60:0.1666667,61:0.1666667,62:0.1666667,63:0.1666667,64:0.1666667,65:0.1666667,66:0.1666667,67:0.1666667,68:0.1666667,69:0.1666667,70:0.1666667,71:0.1666667,72:0.1666667,73:0.1666667,74:0.1666667,75:0.1666667,76:0.1666667,77:0.1666667,78:0.1666667):0.1666667):0.1666667):0.1666667):0.1666667):0.1666667);
END;
References
- Agapow P-M, Crozier RH. Efficient optimization of complex conservation priorities. in prep 2005 [Google Scholar]
- Agapow PM, Bininda-Emonds ORP, Crandall KA, et al. The impact of species concept on biodiversity studies. Q Rev Biol. 2004;79:161–179. doi: 10.1086/383542. [DOI] [PubMed] [Google Scholar]
- Avise JC, Johns GC. Proposal for a standardized temporal scheme of biological classification for extant species. Proc. Nat. Acad. Sci. USA. 1999;96:7358–7363. doi: 10.1073/pnas.96.13.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin MF, Bersier LF, Banasek-Richter C, et al. Phylogenetic constraints and adaptation explain food-web structure. Nature. 2004;427:835–839. doi: 10.1038/nature02327. [DOI] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand. J. Stats. Theor. Appl. 1984;11:265–270. [Google Scholar]
- Chao A. Species richness estimation. In: Balakrishnan N, Read CB, Vidakovic B, editors. Encyclopedia of Statistical Sciences. Wiley; New York, N Y: 2004. in press. [Google Scholar]
- Chao A, Lee SM. Estimating the number of classes via sample coverage. J Amer Stat Assoc. 1992;87:210–217. [Google Scholar]
- Chapman RE, Bourke AFG. The influence of sociality on the conservation biology of social insects. Ecol Letts. 2001;4:650–662. [Google Scholar]
- Clarke KR, Warwick RM. A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Progr Ser. 2001;216:265–278. [Google Scholar]
- Crozier RH. Genetic diversity and the agony of choice. Biol Conserv. 1992;61:11–15. [Google Scholar]
- Crozier RH. Preserving the information content of species: genetic diversity, phylogeny and conservation worth. Annu Rev Ecol Syst. 1997;24:243–268. [Google Scholar]
- Crozier RH, Agapow PM, Pedersen K. Towards complete biodiversity assessment: an evaluation of the subterranean bacterial communities in the Oklo region of the sole surviving natural nuclear reactor. FEMS Microbial Ecol. 1999;28:325–334. [Google Scholar]
- Daugherty CH, Cree A, Hay JM, et al. Neglected taxonomy and continuing extinctions of tuatara (Sphenodon). . Nature. 1990;347:177–179. [Google Scholar]
- Daugherty CH, Patterson GB, Hitchmough RA. Taxonomic and conservation review of the New Zealand herpetofauna. N Z J Zool. 1994;21:317–323. [Google Scholar]
- DeSalle R, Freedman T, Prager EM, et al. Tempo and mode of sequence evolution in mitochondrial DNA of Hawaiian. Drosophila J Mol Evol. 1987;26:157–164. doi: 10.1007/BF02111289. [DOI] [PubMed] [Google Scholar]
- Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1–10. [Google Scholar]
- Faith DP, Reid CAM, Hunter J. Integrating phylogenetic diversity, complementarity, and endemism for conservation assessment. Conserv Biol. 2004;18:255–261. [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to conservation genetics. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Gaston KJ, Spicer JI. Biodiversity: An introduction. Oxford, UK: Blackwell Publishing; 2004. [Google Scholar]
- Gotelli NJ. A taxonomic wish-list for community ecology. Philos Trans R Soc Lond Ser B-Biol Sci. 2004;359:585–597. doi: 10.1098/rstb.2003.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli NJ, Ellison AM. Biogeography at a regional scale: determinants of ant species density in New England bogs and forests. Ecology. 2002;83:1604–1609. [Google Scholar]
- Humphries CJ, Williams PH, Vane-Wright RI. Measuring biodiversity value for conservation. Annu Rev Ecol Syst. 1995;26:93–111. [Google Scholar]
- Justus J, Sarkar S. The principle of complementarity in the design of reserve networks to conserve biodiversity: a preliminary history. J Biosci. 2002;27:421–435. doi: 10.1007/BF02704970. [DOI] [PubMed] [Google Scholar]
- Krajewski C. Phylogenetic measures of biodiversity: a comparison and critique. Biol Cons. 1994;69:33–39. [Google Scholar]
- Lubertazzi D, Tschinkel WR. Ant community change across a ground vegetation gradient in north Florida’s longleaf pine flatwoods. J Insect Sci. 2003;3 doi: 10.1093/jis/3.1.21. Available online: insectscience.org/3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace GM, Gittleman JL, Purvis A. Preserving the Tree of Life. Science. 2003;300:1707–1709. doi: 10.1126/science.1085510. [DOI] [PubMed] [Google Scholar]
- Magurran AE. Biological diversity. Curr Biol. 2005;15:R116–118. doi: 10.1016/j.cub.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Mann CC, Plummer ML. Noah’s Choice. New York: Knopf; 1995. [Google Scholar]
- May RM. Taxonomy as destiny. Nature. 1990;347:129–130. [Google Scholar]
- May RM. How many species inhabit the earth. Sci Amer. 1992;267:42–48. [Google Scholar]
- McDonnell MD, Possingham HP, Ball IR, et al. Mathematical methods for spatially cohesive reserve design. Environ Model Assess. 2002;7:107–114. [Google Scholar]
- Meyer A. Phylogenetic relationships and evolutionary processes In East African cichlid fishes. Trends Ecol Evol. 1993;8:279–284. doi: 10.1016/0169-5347(93)90255-N. [DOI] [PubMed] [Google Scholar]
- Michener CD. The bees of the world. Baltimore: Johns Hopkins University Press; 2000. [Google Scholar]
- Nee S, May RM. Extinction and the loss of evolutionary history. Science. 1997;278:692–694. doi: 10.1126/science.278.5338.692. [DOI] [PubMed] [Google Scholar]
- Pamilo P, Crozier RH. Population biology of social insect conservation. Mem Mus Vict. 1997;56:411–419. [Google Scholar]
- Petchey OL, Gaston KJ. Extinction and the loss of functional diversity. Proc. R. Soc. Lond. B. 2002;269:1721–1727. doi: 10.1098/rspb.2002.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchey OL, Hector A, Gaston KJ. How do different measures of functional diversity perform? Ecology. 2004;85:847–857. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry The principles and practice of statistics in biological research. New York, NY: W H Freeman; 1995. [Google Scholar]
- Symonds MRE. The effects of topological inaccuracy in evolutionary trees on the phylogenetic comparative method of independent contrasts. Syst Biol. 2002;51:541–553. doi: 10.1080/10635150290069977. [DOI] [PubMed] [Google Scholar]
- van Hannen EJ, van Agterveld MP, Gons HJ, et al. Revealing genetic diversity of eukaryotic microorganisms in aquatic environments by denaturing gradient gel electrophoresis. J Phycol. 1998;34:206–213. [Google Scholar]
- Warwick RM, Clarke KR. Relearning the ABC: taxonomic changes and abundance biomass relationships in disturbed benthic communities. Mar Biol. 1994;118:739–744. [Google Scholar]
- Warwick RM, Clarke KR. Taxonomic distinctness and environmental assessment. J Appl Ecol. 1998;35:532–543. [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, et al. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- Wilson EO. Social modifications related to rareness in ant species. Evolution. 1963;17:249–253. [Google Scholar]
- Wilson EO. The Diversity of Life. Cambridge, MA: Harvard University Press; 1992. [Google Scholar]
- Yeates DK, Bouchard P, Monteith GB. Patterns and levels of endemism in the Australian Wet Tropics rainforest: evidence from flightless insects. Invertebr Syst. 2002;16:605–619. [Google Scholar]