Abstract
Objective
To describe how women with fecal incontinence (FI) respond to combined pharmacologic therapy and pelvic floor muscle exercises (PFME).
Study Design
Validated questionnaires (Fecal Incontinence Severity Index and Modified Manchester Health Questionnaire) were mailed to 80 women who received combined therapy for FI and had complete baseline assessments. Regression models were constructed to identify predictors of change in questionnaire scores.
Results
Response rate was 69%. Mean age was 59 ± 12 years. All women were taught PFME with digital palpation and 87% of the women received medications. FI severity scores and quality of life (QOL) improved from baseline to follow-up (p < .001 and p = .02, respectively). A fair/normal external anal sphincter (EAS) contraction resulted in greater improvements in FI severity (13 points, p=0.006) and QOL scores (22 points, p<0.001).
Conclusion
FI severity and QOL improved after combination therapies and a fair/normal EAS contraction predicted greater improvement.
Condensation
Treating fecal incontinence in women with combination therapy including pelvic floor muscle exercises and medications improved fecal incontinence symptom severity and health-related quality of life.
Keywords: Fecal Incontinence, Treatment Outcomes, Quality of Life, Behavioral Medicine
INTRODUCTION
The International Consultation on Incontinence definition of fecal incontinence (FI) is the involuntary loss of feces - solid or liquid that is a social or hygienic problem.1 When analyzing FI prevalence “in the last year” among adults in the community, rates vary from 4–24% and may increase to one-third of women 70 years of age and older.2–6 FI can be a devastating condition that has a negative on impact quality of life (QOL) in a few large, epidemiologic studies.2, 4, 7
While some studies have found FI to negatively impact QOL among women, few studies have taken the next step and examined the impact of treatment on FI symptoms.8, 9 Existing studies show improvement with surgical treatment, behavioral modification, and biofeedback, but further information, regarding combination treatment with medications and pelvic floor muscle exercises, is needed.8, 10, 11
Given the paucity of data on outcomes of combination therapy for FI, this study provides data on treatment outcomes using validated questionnaires for women receiving combination FI treatment at a specialty-based clinic.
MATERIALS AND METHODS
Participants
Participants for this study included 170 women who presented to the Genito-Rectal Disorders Clinic at the University of Alabama at Birmingham’s (UAB) since2003. Data on symptoms and severity of FI, as well as potential risk factors, were collected and entered into an IRB approved database. Ninety-seven women were referred and/or elected to have non-surgical therapy for FI, and 80 had at least one visit by either a nurse practitioner or one of two physicians (authors ADM and PSG) specializing in behavioral and pharmacologic treatments for urinary incontinence and FI. The UAB Institutional Review Board approved all data collection.
Baseline questionnaires
Women were mailed questionnaires on fecal and urinary incontinence to complete prior to presenting the Genito-Rectal Disorders Clinic. Questionnaires to assess FI included the Modified Manchester Health Questionnaire (MMHQ), which is a validated questionnaire that also includes the Fecal Incontinence Severity Index (FISI).12 The MMHQ measures health related quality of life (HR-QOL) for FI and includes 8 subscales on the impact of FI on: overall impact, role, physical, social, relationships, emotion, sleep/energy, and severity/adaptation. The MMHQ is scaled from 0–100, for total and subscale scores, where higher scores represent greater impact on HR-QOL. The FISI measures the severity of liquid, solid, mucous, or gas incontinence that occurs from “two of more times per day,” “once per day,” “two or more times per week,” “once a week,” to “one to three times per month.” Patient-weighted scores were used to determine severity and scores ranged from 0–61, with higher scores indicating worse FI severity. A FISI score of 0 indicated continence. Women who responded to any level of flatus incontinence, without having incontinence to liquid, solid, or mucous stool, were considered incontinent to flatus only and not considered to have fecal incontinence. Women with incontinence to liquid, solid, or mucous stool loss were included in this study. The Medical, Epidemiologic, and Social Aspects of Aging Questionnaire (MESA) was used to evaluate symptoms of urinary incontinence.13 The MESA has two subscales; one for stress urinary incontinence symptoms and one for urge urinary incontinence symptoms. Questions referring to loss of urine at times of exertion such as laughing, sneezing, lifting, or bending over define symptoms of stress urinary incontinence. Questions referring to urine loss preceded by an urge to void, or uncontrollable voiding with little or no warning define symptoms of urge incontinence. The questionnaire has a range of scores of 0–27 for stress UI symptoms and 0–18 for urge UI symptoms. Higher scores represent greater symptom severity.
Clinical Evaluation
At the UAB Genito-rectal Disorders Clinic, women referred for FI treatment provided baseline data on their medical and surgical histories and questionnaires were reviewed Women had evaluation of their FI with anorectal manometry and endoanal ultrasound. Anorectal manometry was completed by physicians (authors ADM and HER) using a water-perfused disposable catheter system (Medtronic, Inc Minneapolis, MN). Pressures were recorded during resting, squeezing, and pushing at 1 cm intervals starting at 5–6 cm from the anal verge. Rectal capacity was measured in milliliters using an air-filled balloon. To evaluate for tears or disruption of the internal anal sphincter and the external anal sphincter, endoanal ultrasound using a 10 MHz, 360° window endoanal probe at 5mm intervals (B & K Medical Systems, Inc. Willmington, MA) was completed by the same physicians (ADM and HER). If women had full-thickness tears in the external anal sphincter with or without tears in the internal anal sphincter, they were given the option to have surgical repair of the sphincter. However, if women had scarring, thinning, or attenuation of the anal sphincter, they were referred to non-surgical treatment for their FI.
At the first treatment visit for combination treatment, physical examinations were performed by the behavioral specialists, including pelvic examinations with prolapse assessment (above or through the hymen), Brink’s assessment (strength and duration of vaginal pelvic floor muscle contraction14), and rectal examinations (resting anal sphincter tone and assessment of voluntary contraction). Prolapse was dichotomized based on the presence or absence on examination. Anal sphincter tone was assessed as “diminished” or “normal.” External anal sphincter contraction was rated as: “none,” “very weak,” “fair,” or “strong.” Responses were dichotomized as “none/very weak” and “fair/strong.”
Combination Treatment
Treatment for FI consisted of medications and multi-component behavioral therapy (Figure1).. The multi-component behavioral therapy approach included dietary recommendations (information on dietary fiber), bowel habit strategies, including barrier cream use, and pelvic floor muscle exercises. Women were taught to contract pelvic floor muscles both vaginally and rectally during the examination. Women were instructed to squeeze and relax equal amounts of time, usually 2–4 seconds with 15 repetitions, 3-times per day, slowly increasing squeeze/relaxation periods weekly, for a goal of 10 seconds. Women also received instruction in behavioral strategies to control urgency sensations.
Figure 1.
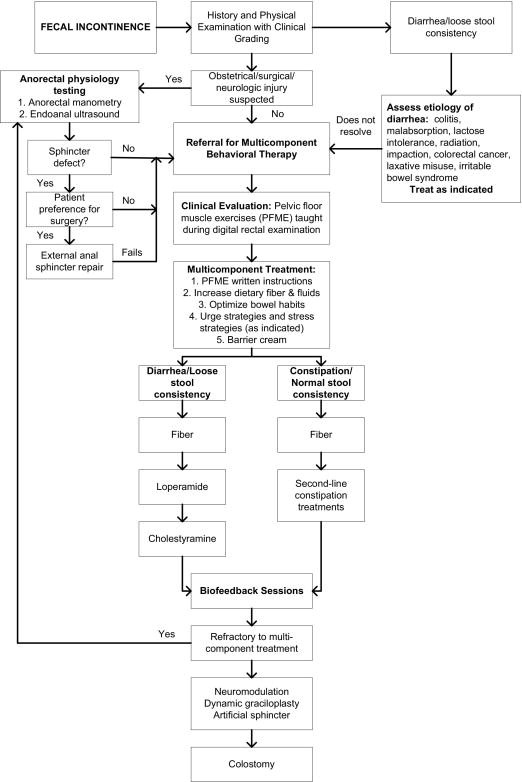
Algorithm for a Combination Treatment of Women with Fecal Incontinence in a Tertiary Referral Center
If indicated, medications were prescribed. As first-line therapy, supplemental fiber (over-the-counter) was initiated to improve stool consistency, if needed. If women had previously failed therapy with fiber and/or could not tolerate the side-effects of abdominal bloating and increased flatus, they were given instructions to take loperamide. If women with chronic loose or mushy stools had previously failed therapy with both fiber and loperamide, they were given cholestyramine, a bile acid-binding resin.15 In some women, a combination of fiber and loperamide was given based on symptoms and severity of FI. Any patient with diarrhea, constipation, or alteration in bowel habits who had not received an adequate medical evaluation, was evaluated or referred. This step-wise approach was done by all behavioral specialists. Women were asked to return to clinic in 4 week intervals for subsequent evaluations.
Treatment outcomes
After receiving combination treatment, women were mailed the following questionnaires in a self-addressed stamped envelope: Patient Satisfaction Question (PSQ),16 Global Perception of Improvement (GPI),16 Estimated Percent Improvement (EPI),16 MMHQ, and FISI. If women did not respond to the initial mailed questionnaires after 3 weeks, we telephoned the women to remind them to send the completed questionnaires. Additional data from a chart review on women who were sent the questionnaires included: information on the number of visits, final types of treatment (behavioral and pharmacological), and types of medications given. Data from the physical examination included presence/absence and type of pelvic organ prolapse, Brink’s assessment of pelvic floor muscle strength, and anal sphincter tone/strength of contraction.
Data Collection and Analysis
Women who responded to the mailed follow-up questionnaire were compared to women who did not respond. Chi-square analyses were conducted for categorical variables and Student’s T-test for continuous variables. The Fisher exact test was used for categorical analysis when chi-square assumptions did not hold. Chi-square analysis was used to compare the results of the external anal sphincter examination by the individual providers. Student’s T-test identified differences in the baseline assessment for the FISI and the MMHQ compared to follow-up scores. Multivariable linear regression models were constructed, controlling for age, to identify factors that were associated with a change in FISI scores and MMHQ scores. Software used for analyses was STATA 8.2 (College Station, TX).
RESULTS
The survey response rate was 69% (55/80) with one woman excluded for having an ileostomy. Age ranged from 31 to 85 years (mean age ± standard deviation was 56 ± 13 years). The sample was predominately Non-Hispanic White participants (89%), with 8% African American, 2% Asian American, and 1% self-reported as “Other”. Table 1 depicts baseline clinical characteristics of the women (n = 54) who responded to the questionnaire compared to women who did not respond (n = 26). Survey responders were more likely (P < .05) to be older, have more stress urinary incontinence symptoms, more pelvic organ prolapse, and a lower body mass index. Survey responders were less likely (P = .002) to have a fair or strong external anal sphincter contraction on physical examination. Baseline FISI scores (28.1 vs 32.6, P = .06) were slightly higher in survey responders compared to non-responders and MMHQ scores (39.4 vs 51.2, P = .02) were significantly higher for survey responders compared to non-responders.
Table 1.
Baseline Characteristics of Women Who Received Combination Treatment for Fecal Incontinence
| Demographics | N | Responders | N | Non-responders | P value |
|---|---|---|---|---|---|
| Age (years) | 54 | 59.2 ± 12.1 | 26 | 52.5 ± 14.9 | .03 |
| White race/ethnicity | 54 | 46 (85) | 25 | 25 (96) | .50 |
| Medical History | |||||
| Diabetes | 41 | 3 (7) | 25 | 4 (16) | .27 |
| Hypertension | 39 | 18 (46) | 25 | 10 (40) | .63 |
| Inflammatory Bowel Disease | 40 | 4 (10) | 24 | 2 (8) | .83 |
| Prior recto-vaginal fistula | 39 | 4 (10) | 24 | 4 (17) | .76 |
| Urinary Incontinence | 54 | 37 (67) | 26 | 12 (52) | .21 |
| MESA urge symptoms | 36 | 9.6 ± 5.2 | 23 | 7.8 ± 5.3 | .10 |
| MESA stress symptoms | 36 | 11.9 ± 8.1 | 23 | 8.3 ± 7.3 | .04 |
| Cholecystectomy | 38 | 11 (29) | 21 | 6 (29) | 1.00 |
| Prior ano-rectal surgery | 39 | 20 (37) | 23 | 4 (17) | .60 |
| Hysterectomy | 41 | 32 (78) | 25 | 16 (64) | .21 |
| Vaginal deliveries (number) | 54 | 2.3 ± 1.3 | 26 | 2.1 ± 1.2 | .74 |
| Physical Examination | |||||
| Brink’s Score | 30 | 7.9 ± 3.2 | 23 | 6.9 ± 3.4 | .14 |
| Prolapse (yes/no) | 54 | 42 (78) | 26 | 26 (38) | .01 |
| Diminished rectal tone at rest | 54 | 30 (57) | 26 | 10 (38) | .19 |
| Fair or strong external anal sphincter contraction on physical exam | 54 | 38 (70) | 26 | 26 (100) | .002 |
| Body Mass Index (kg/m2) | 39 | 29.4 ± 7.7 | 26 | 33.5 ± 8.5 | .005 |
| Anorectal Manometry | |||||
| Resting pressure (mmH2O) | 29 | 35.7 ±19.7 | 23 | 33.5 ± 20.9 | .34 |
| Squeeze pressure (mm H2O) | 29 | 63.3 ± 32.6 | 23 | 70.8 ± 32.1 | .20 |
| Capacity (ml) | 29 | 122.5 ± 67.3 | 23 | 144.1 ± 74.5 | .13 |
| Endoanal Ultrasound | |||||
| External anal sphincter tear/disruption | 36 | 16 (44) | 23 | 8 (35) | .46 |
| Internal anal sphincter tear | 36 | 5 (14) | 23 | 5 (22) | .43 |
| Questionnaires | |||||
| Baseline FISI | 50 | 28.1 ± 11.0 | 19 | 32.6 ± 9.6 | .06 |
| Baseline MMHQ | 46 | 39.4 ± 21.5 | 21 | 51.2 ± 23.0 | .02 |
KEY: FISI = Fecal Incontinence Severity Index; MMHQ = Modified Manchester Health Questionnaire
All data presented as n (%) or mean ± standard deviation
Two-thirds (67%) of the women who responded to the survey had concurrent urinary incontinence, with greater stress than urge symptoms. Women who responded to the survey had a high overall prevalence of pelvic organ prolapse (78%, n = 42) with 62% having both a cystocele and rectocele (n = 14), 33% having only a rectocele (n = 14), and 5% with only a cystocele (n = 2). Greater than two-thirds of the women (70%) had an anal sphincter contraction on rectal examination that was fair or strong. No differences (P = .42) were seen in the anal sphincter contraction assessments according to the three providers who completed baseline clinical examinations. All women were taught pelvic floor muscle exercises with digital rectal examination and 93% (5/54) also received medications for their FI. Women had an average of 2.6 visits (range 1 to 10) over 7 months (range 1 to 12 months). Women who received only pelvic floor muscle exercises (n = 5) had a median number of 2 visits (range 1–3) over 8 months (range 2–10 months).
In Table 2, outcome measures for the combination treatment of FI were compared from the baseline assessment to the time of the mailed questionnaire (mean of 6 months, range 1 to 12 months). Complete data for the FISI and the MMHQ were available for only 45 women. FISI scores (P <.001) and MMHQ scores (P = .02) improved significantly with combination treatment. Six percent of women (n = 3) were completely continent after treatment (FISI score of 0). When excluding women with flatus incontinence only after treatment, 15% of women (n= 8) were completely continent. Although the total MMHQ score improved after treatment, not all subscales improved. Significant improvement was found in 50% of the MMHQ subscales: overall impact (P = .009), role impact (P= .01), physical impact (P = .001), and impact on sleep/energy (P= .005). MMHQ subscales that did not improve significantly (P > .05) included: social interactions, relationships (including sexual relationships), emotional impact, and severity/coping mechanisms. In response to the patient satisfaction questionnaire (PSQ), 83% of women were either completely or somewhat satisfied with their treatment (Table 2). Overall, 60% of the women felt as though they were better or much better after receiving combination treatment for FI (GPI Score) and women estimated that they had improved by a mean of 49% (EPI score).
Table 2.
Outcomes of Combination Treatments in Women with Fecal Incontinence
| Questionnaires | Post-treatment |
|---|---|
| Change in FISI, n = 49 | 7.0 ± 11.1* |
| Change in MMHQ, n = 39 | 7.6 ± 23.7† |
| Patient satisfaction questionnaire (PSQ) | |
| Completely satisfied | 18 (33) |
| Somewhat satisfied | 27 (50) |
| Not at all | 9 (17) |
| Global perception of improvement (GPI) | |
| Much Better/Better | 32 (59) |
| About the same | 16 (30) |
| Worse/Much Worse | 6 (11) |
| Estimated percent improvement (EPI) | 48.7 ± 36.1 |
KEY: FISI = Fecal Incontinence Severity Index; MMHQ = Modified Manchester Health Questionnaire
All data presented as n (%) or mean ± standard deviation
P < .001,
P = .02 for improvement in scores from baseline assessment
In multivariable models controlling for age (Table 3), a positive change in FISI score (13 points) was associated with a fair or strong external anal sphincter contraction on physical examination.. The number of visits, Brink’s assessment, and having prior rectal surgery were not associated with improvement in FISI scores after treatment. In addition, anorectal manometry measures and endoanal ultrasound findings were not associated with improvements in FISI scores in multivariable models. Aftercontrolling for age, a positive change in MMHQ score (22 points) was associated with a fair or strong external anal sphincter contraction.
Table 3.
Multivariable Analysis for Factors Associated with Improved FI Severity and HR-QOL
| FISI score, n = 49a |
MMHQ score, n = 39b |
|||||
|---|---|---|---|---|---|---|
| Variables | Change | 95% CI | P value | Change | 95% CI | P value |
| Age | -0.1 | -0.3 to 0.1 | .669 | 0.1 | -0.5 to 0.8 | .425 |
| Fair/strong external anal sphincter contraction | 13.0 | 7.0-18.9 | .006 | 22.2 | 6.7-37.7 | < .001 |
HR-QOL, health-related quality-of-life.
A 13-point decrease in Fecal Incontinence Severity Index (FISI) score (range, 0-61) corresponds to a decrease in fecal incontinence (FI) symptom frequency from daily to weekly or weekly to monthly.
A Modified Manchester health Questionnaire (MMHQ) score (range, 0-100) decrease of 22 points represents symptoms occuring "always" to "often."
COMMENT
Combination FI treatment therapy that included medications and multi-component behavioral therapy improved both FI severity and HR-QOL in women.at a specialty-care center. Greater improvements in FI severity and HRQOL were associated with having a fair or strong external anal sphincter contraction on physical examination after controlling for age. Overall, women were satisfied with the treatment provided. This study provides data to suggest that women have improvements in fecal incontinence severity, with the frequency of fecal incontinence improving from daily to weekly or weekly to monthly. Although HR-QOL improved with this treatment, the degree of improvement may not be clinically significant.
This study adds to the existing literature by reporting outcomes of combination treatment for FI with validated measures for both FI severity and HR-QOL. In a recent review article on questionnaires for urinary and anal incontinence, the authors mention that few validated questionnaires exist for fecal or anal incontinence symptoms and/or quality of life.17 Although not included in that review article, other validated questionnaires have been used to evaluate bowel symptoms.18, 19 Others have reported significant improvements in the treatment of FI with pelvic floor muscle exercises with or without the use of medications.8, 20 However, few studies examined treatment outcomes for combination therapy.10, 21 In one trial that reported changes in FI symptom severity and QOL after anal sphincteroplasty, 75% of the women continued to have FI at 5 years after surgery.11 Few studies examine differences in outcomes with or without the addition of behavioral therapy or medications prior to or after surgical interventions for FI.
Although this study is a clinical, prospective cohort study, this information is important in designing and implementing future trials evaluating combination treatments for FI. Specifically, this trial reports that women with fair or strong baseline external anal sphincter contraction on digital rectal examination do better with combination therapy than women who have either a weak or absent voluntary sphincter contraction. Since age has been found to be associated with weaker resting and squeeze pressures on anorectal manometry studies, it may also affect anal sphincter contraction on physical exam.22 We found that when controlling for age in the multivariable model, a stronger external anal sphincter contraction was still predictive of a modest improvement in FI severity and HR-QOL.
The utility of specialized testing with anal rectal manometry and endoanal ultrasound, and possibly MRI, has not been rigorously tested and continues to be debated in the literature.23, 24 Other studies have shown that internal and external anal sphincter damage diagnosed by endoanal ultrasound after vaginal delivery in women who sustained vaginal tears is associated with more severe FI symptoms.25, 26 In this study, the results of anorectal manometry testing (resting and squeeze pressures) and endoanal ultrasound (external and internal anal sphincter tears) prior to combination treatment did not predict FI severity or HR-QOL outcomes. It is possible that a larger sample size would have provided the appropriate power to detect such a difference and more data are needed to evaluate the utility of these studies fortreatment outcomes. Also, women with sphincter defects identified on ultrasound testing may have been under-represented in this cohort, because they would have been more likely to have undergone surgical treatment.
Other limitations of this study involve the collection of additional data that could elucidate differences in treatment outcomes. For example, more information is needed on obstetrical factors, such as delivery type and complications. Other studies have found that women who had instrumented deliveries and episiotomies are at a higher risk for FI.27 The measure for HR-QOL, the Modified Manchester Health Questionnaire, is a lengthy questionnaire with 31 items and 8 subscales, but it comparable to other HR-QOL measures for FI, such as the FIQOL.12 Having incomplete data on the MMHQ (n = 39, 72% of responders) is a limitation of this study and decreases the ability to interpret HR-QOL outcomes. Another limitation is the subjective nature of the rectal examination and assessment of the strength of the sphincter contraction. However, no significant differences were found in the numbers of women with impaired or normal sphincter contractions when analyzing the data according to the three different providers. The types of pelvic floor muscle exercises taught and the medications utilized may vary by providers in the community. Given that this was a single site study, all providers taught patients pelvic floor muscle exercises by digital palpation while providing written and oral instructions for progressive home exercises. Lastly, significant differences were seen between responders and non-responders to the survey that may have impacted the improvements seen on the FISI and the MMHQ after combination treatment. Since non-responders had more severe FI symptoms and HR-QOL on baseline questionnaires compared to responders, they may have had less improvement and been less likely to respond to the survey. However, since 100% of the non-responders had a fair or strong external anal sphincter contraction on physical examination, which was the only predictor of successful treatment in our study, this suggests that they might have been even more likely to improve with combination therapy. It is possible that women with weaker external sphincter contractions may have been more motivated to participate in a program of pelvic floor muscle exercises and complete the surveys than were women who had stronger external sphincter strength at baseline.
The types of medications utilized varied according to individual complaints, but usually involved the use of fiber and/or loperamide prescribed at the initial visit. No known algorithms exist for these types of therapies for FI, but some recent guidelines have been published.28, 29 Our study evaluated the results of treatment within a year of initiation of treatment. More data from a larger sample size are also needed to make evidence-based recommendations for FI treatment. However, our results suggest that referring women with FI for combination therapy many improve FI severity and HR-QOL. Future studies are needed that evaluate and compare the individual components of combination FI treatments, as well as their long-term durability.
Acknowledgments
(1) K24DK068389-03 to Dr. Richter
(2) Supported in part by GCRC Grant MO1 RR-00032 From the National Center for Research Resources
The authors would like to acknowledge the efforts by summer students, Matthew P. Malone and Kym Do, who provided additional support for this study.
Footnotes
Presented: Society of Gynecological Surgeons, Annual Meeting, Savannah, Georgia, April 14-16, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norton C, Whitehead WE, Bliss DZ, Metsole P, Tries J. Conservative and pharmacologic management of fecal incontinence in adults. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence, 3rd International Consultation on Incontinence. Paris, France: Health Publications Ltd; 2005. pp. 1521–64. [Google Scholar]
- 2.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: a population-based study. Am J Obstet Gynecol. 2005;193:2071–6. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005;53(4):629–635. doi: 10.1111/j.1532-5415.2005.53211.x. [DOI] [PubMed] [Google Scholar]
- 4.Varma M, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK, Beattie MS, Subak LL. Fecal Incontinence in Females Older Than Aged 40 Years: Who is at Risk? Dis Colon Rectum. 2006;49:841–51. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004–9. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Macmillan AK, Merrie AEH, Marshall RJ, Parry BR. The Prevalence of Fecal Incontinence in Community-Dwelling Adults: A Systematic Review of the Literature. Dis Colon Rectum. 2004;47:1341. doi: 10.1007/s10350-004-0593-0. [DOI] [PubMed] [Google Scholar]
- 7.Sailer M, Bussen D, Debus ES, Fuchs KH, Thiede A. Quality of life in patients with benign anorectal disorders. Br J Surg. 1998;85:1716–9. doi: 10.1046/j.1365-2168.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- 8.Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–9. doi: 10.1016/j.gastro.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Boreham MK, Richter HE, Kenton KS, et al. Anal incontinence in women presenting for gynecologic care: prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol. 2005;192:1637–42. doi: 10.1016/j.ajog.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Cheetham M, Brazzelli M, Norton C, Glazener CM. Drug treatment for faecal incontinence in adults. Cochrane Database of Systematic Reviews. 2003:CD002116. doi: 10.1002/14651858.CD002116. [DOI] [PubMed] [Google Scholar]
- 11.Trowbridge ER, Morgan D, Trowbridge MJ, Delancey JOL, Fenner DE. Sexual function, quality of life, and severity of anal incontinence after anal sphincteroplasty. Am J Obstet Gynecol. 2006;195:1753. doi: 10.1016/j.ajog.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Kwon S, Visco AG, Fitzgerald MP, Ye W, Whitehead WE Pelvic Floor Disorders Network. Validity and reliability of the Modified Manchester Health Questionnaire in assessing patients with fecal incontinence. Dis Colon Rectum. 2005;48:323–31. doi: 10.1007/s10350-004-0899-y. [DOI] [PubMed] [Google Scholar]
- 13.Herzog A, Diokno A, Grown M. Two year incidence, remission, and change in patterns of urinary incontinence in non institutionalized older adults. Jnl of Gerontology: Medical Sciences. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 14.Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R. A digital test for pelvic muscle strength in women with urinary incontinence. Nursing Research. 1994;43:352–6. [PubMed] [Google Scholar]
- 15.Remes-Troche J, Ozturk R, Philips C, Stessman M, Rao S. Cholestyramine—a useful adjunct for the treatment of patients with fecal incontinence. Int J Colorectal Dis. 2008;23:189. doi: 10.1007/s00384-007-0391-y. [DOI] [PubMed] [Google Scholar]
- 16.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urodyn. 2006;25:411–7. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 17.Avery KN, Bosch JL, Gotoh M, et al. Questionnaires to assess urinary and anal incontinence: review and recommendations. J Urol. 2007;177:39–49. doi: 10.1016/j.juro.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Locke GR, Seide BM, Zinsmeister AR. A new questionnaire for constipation and faecal incontinence. Aliment Pharmacol Ther. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 20.Norton C, Kamm MA. Anal sphincter biofeedback and pelvic floor exercises for faecal incontinence in adults--a systematic review. Aliment Pharmacol Ther. 2001;15:1147–54. doi: 10.1046/j.1365-2036.2001.01039.x. [DOI] [PubMed] [Google Scholar]
- 21.Wald A. Fecal Incontinence in Adults. N Engl J Med. 2007;356:1648–55. doi: 10.1056/NEJMcp067041. [DOI] [PubMed] [Google Scholar]
- 22.Fox J, Fletcher J, Zinsmeister A, Seide B, Riederer S, Bharucha A. Effect of Aging on Anorectal and Pelvic Floor Functions in Females. Dis Colon Rectum. 2006;49:1726. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 23.Bharucha AE. PRO: Anorectal Testing Is Useful in Fecal Incontinence. Am J Gastroenterol. 2006;101:2679–81. doi: 10.1111/j.1572-0241.2006.00900_1.x. [DOI] [PubMed] [Google Scholar]
- 24.Wald A. CON: Anorectal Manometry and Imaging Are Not Necessary in Patients with Fecal Incontinence. Am J Gastroenterol. 2006;101:2681–83. doi: 10.1111/j.1572-0241.2006.00900_2.x. [DOI] [PubMed] [Google Scholar]
- 25.Richter HE, Fielding JR, Bradley CS, et al. Endoanal Ultrasound Findings and Fecal Incontinence Symptoms in Women With and Without Recognized Anal Sphincter Tears. Obstet Gynecol. 2006;108:1394–1401. doi: 10.1097/01.AOG.0000246799.53458.bc. [DOI] [PubMed] [Google Scholar]
- 26.Rhona M, Michael B, Leslie D, Catriona K, Colm OH, Connell PRO. Internal anal sphincter defect influences continence outcome following obstetric anal sphincter injury. Am J Obstetr Gynecol. 2007;196:217.e1. doi: 10.1016/j.ajog.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler TL, II, Richter HE. Delivery method, anal sphincter tears and fecal incontinence: new information on a persistent problem. Curr Opin Obstet Gynecol. 2007 Oct;19(5):474–9. doi: 10.1097/GCO.0b013e3282ef4142. [DOI] [PubMed] [Google Scholar]
- 28.Rao SS American College of Gastroenterology Practice Parameters C. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99:1585–604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- 29.Norton C, Thomas L, Hill J. Guideline Development: Management of faecal incontinence in adults: summary of NICE guidance. BMJ. 2007;334:1370–1371. doi: 10.1136/bmj.39231.633275.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]


