Abstract
Aim
To investigate the anti-proliferative effects of Crocus sativus extract and its major constituent, crocin, on three colorectal cancer cell lines (HCT-116, SW-480, and HT-29). The cell growth inhibition effect was compared to that of non-small cell lung cancer (NSCLC) cells. In addition, Crocus sativus' effect on non-cancer cells was evaluated.
Methods
Using high performance liquid chromatography (HPLC), the purity of crocin and the content of crocin extract were determined. Anti-proliferative effects of Crocus sativus extract and crocin on test cells was evaluated by MTS assay.
Results
The purity of crocin was found to be 95.9% and the content of crocin in the extract was 22.9%. Significant concentration-related inhibition effects of the extract on all three colorectal cancer cell lines were observed (P < 0.01). The proliferation was reduced most significantly in HCT-116 cells, to 45.5% at 1.0 mg/ml and to 6.8 % at 3.0 mg/ml. Crocin at 1.0 mM, significantly reduced HCT-116, SW-480, and HT-29 cell proliferation to 2.8%, 52%, and 16.8%, respectively (P < 0.01). Since 3.0 mg/ml Crocus sativus extract contained approximately 0.6 mM crocin, the observed effects suggest that crocin is a major responsible constituent in the extract. Significant anti-proliferative effects were also observed in non-small cell lung cancer cells. However, Crocus sativus extract did not significantly affect the growth of non-cancer young adult mouse colon cells.
Conclusion
Data from this study demonstrated that Crocus sativus extract and its major constituent, crocin, significantly inhibited the growth of colorectal cancer cells while not affecting normal cells. Crocus sativus extract should be investigated further as a viable option in the treatment of colorectal cancer.
Keywords: crocin, Crocus sativus, saffron, high performance liquid chromatography, human colorectal cancer cell lines, anti-proliferation effect
Colorectal cancer is the second leading cause of cancer related-death and the third most commonly diagnosed cancer in the United States, with approximately 100,000 new cases diagnosed per year [1, 2]. If detected very early in the course of the disease, surgical resection may be adequate, but many patients still need supplemental chemotherapy or radiotherapy. In most cases, commonly used chemotherapies are limited by severe side effects and dose-limiting toxicity. Adding more drugs or increasing the dosages increases the probability of adverse effects. The drug-related adverse events not only worsen patients' quality of life, but can also lead to their refusal to continue the potentially curative chemotherapy [3]. Newer therapies involving targeted chemotherapy of metastatic colorectal cancer are also associated with undesirable adverse effects [4]. Considering these facts, significant improvement in treating patients with colorectal cancer is required.
In the last two decades, botanicals with effective anti-cancer activity have been researched. The use of herbal medicines is also on the rise in cancer patients [5, 6]. Long-term consumption of certain botanicals could be associated with reduced cancer incidence and thus has an epidemiological basis for further investigation. Research that explores new botanical candidates with potential anti-cancer effects is imperative, and allows for the development of safe and efficacious anti-cancer therapies [7, 8].
Crocus sativus is a plant of the iris family (Iridaceae) and its flower contains various chemical constituents [9]. Stigmas of the flower (saffron) contain crocin, anthocyanin, carotene and lycopene [10], and these constituents have various pharmacological effects on different illness, including anti-tumor effects by inhibition of cell growth [11]. Recently, Crocus sativus extract (CSE) was found to have anti-cancer activities against leukaemia, osteosarcoma, fibrosarcoma and ovarian carcinoma cells [9]. Crocin (Fig. 1) is a main constituent the CSE. However, effects of CSE and crocin on human colorectal cancer have not been evaluated.
Fig. 1.
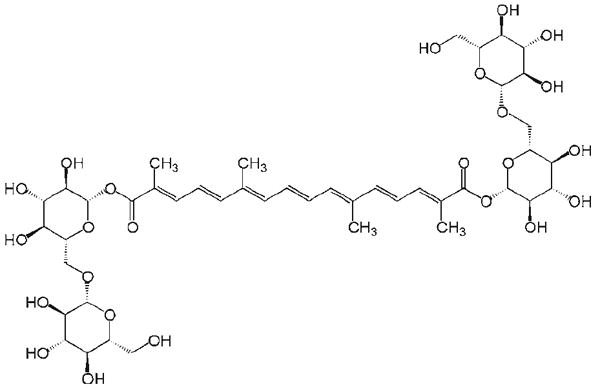
Chemical structure of crocin (MW: 977)
In this study, using high performance liquid chromatography (HPLC), we analyzed CSE and its content of crocin. The anti-proliferation effects of CSE and crocin were evaluated in three human colorectal cancer cell lines, i. e., HT-29, SW-480 and HCT-116. The inhibitory effects on colorectal cancer cell growth were also compared to a different cancer, non-small cell lung cancer (NSCLC) cells. Additionally, we also tested if CSE's anti-proliferative effects can bee seen in non-cancer young adult mouse colon (YAMC) cells.
Materials and Methods
Plant extract, HPLC analysis, and chemicals
CSE and crocin were obtained from Prof. Y. Shoyama's laboratory in the Faculty of Pharmaceutical Sciences, Kyushu University, Japan.
For HPLC analysis, we used Waters 2690 liquid chromatographic system equipped with a 996 photo diode array detector (Milford, MA). The separations were carried out on a Phenomenex Prodigy ODS(2) C18 column (150 × 3.2 mm, 5 μm) (Torrance, CA). The precolumn was a Phenomenex C18 column (30 × 3.2 mm). The diode array detector was set to an acquisition range of 200–700 nm at a spectral acquisition rate of 1.25 scans/s.
For the mobile phase, we used solvent A (methanol) and solvent B (1% [v/v] aqueous acetic acid in water). The mixing of the gradient solvent eluting system was as follows: initial 30% A and 70% B; 0–5 min, linear change to 40% A; 5–10 min, change to 55% A; 10–25 min, change to 68% A; 25–27 min, change to 90% A; 27–30 min, 90% A; 30–33 change to 30% A; 33–40min, 30% A. The flow rate of the mobile phase was 1.0 ml/min and the injection volume was 20 μl. The detection wavelength was set to 442 nm. All solutions were filtered through a 0.2 μm hydrophilic polypropylene membrane (Pall Gelman Laboratory, Ann Arbor, MI) before use. Separation was accomplished at the temperature of 25 °C.
Five different concentrations of crocin solutions were prepared for the determination of the calibration curve. The calibration curve was constructed with crocin content versus peak area (y = 5468.3 + 76637, R2 = 0.9997, linear range: 0.002–0.2 mg/ml). The content of crocin in CSE was calculated using the standard curve of crocin.
All solvents were of HPLC grade from Fisher Scientific (Norcross, GA). Trypsin, McCoy's 5A, Leibovitz's L-15, RPMI 1640 medium, fetal bovine serum (FBS), and penicillin/streptomycin solution (200×) were obtained from Mediatec (Herndon, VA). A CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit was obtained from Promega (Madison, WI).
Cell culture
Human colorectal cancer cells HCT-116 (McCoy's 5A), HT-29 (McCoy's 5A) and SW-480 (Leibovitz's L-15), and human non-small cell lung cancer (NSCLC) cells (RPMI 1640) were obtained from American Type Culture Collection (Manassas, VA). Non-cancer young adult mouse colon (YAMC) cells (RPMI 1640) were obtained from Prof. E.B. Chang in the Digestive Diseases Research Core Center at the University of Chicago. For cancer cell culture, indicated medium containing 10% fetal bovine serum and penicillin/streptomycin was placed in a humidified atmosphere with 5% CO2 at 37 °C. For YAMC cell culture, a humidified atmosphere was maintained with 5% CO2 at 33 °C. Medium was changed every 2–3 days. When the cells reached 80–90% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask [12–14].
Anti-proliferation assay using MTS method
Cells were seeded in 96-well plates (1 × 104 cells/well). After 24 h, the medium was changed and various concentrations of CSE or crocin were added to the wells. All experiments were performed at least in triplicate. After 48 h of treatment, cell growth was evaluated using a modified trichrome stain (MTS) assay according to the manufacturer's requirement. Briefly, at the end of incubation period, the medium was replaced with 100 μl of fresh medium, 20 μl of MTS reagent in each well, and the plate was placed in an incubator for 1–2 h. A 60 μl aliquot of medium from each well was transferred to an ELISA 96-well plate and its absorbance at 490 nm was recorded [15]. Our previous data showed that results obtained from the MTS method are comparable to that obtained from the cell counting method [16].
Statistical analysis. Data are presented as mean ± standard error (SE). Data were analyzed using Student's t-test and analysis of variance (ANOVA) for repeated measures. The level of statistical significance was set at P < 0.05.
Results
HPLC analysis of crocin and CSE. Chromatogram of crocin is shown in Fig. 2, a. Table 1 indicates retention times and peak areas for the crocin and three impurities. Crocin has maximum absorption near 442 nm and we select 442 nm as the assay wavelength. The purity of crocin is 95.9%.
Fig. 2.
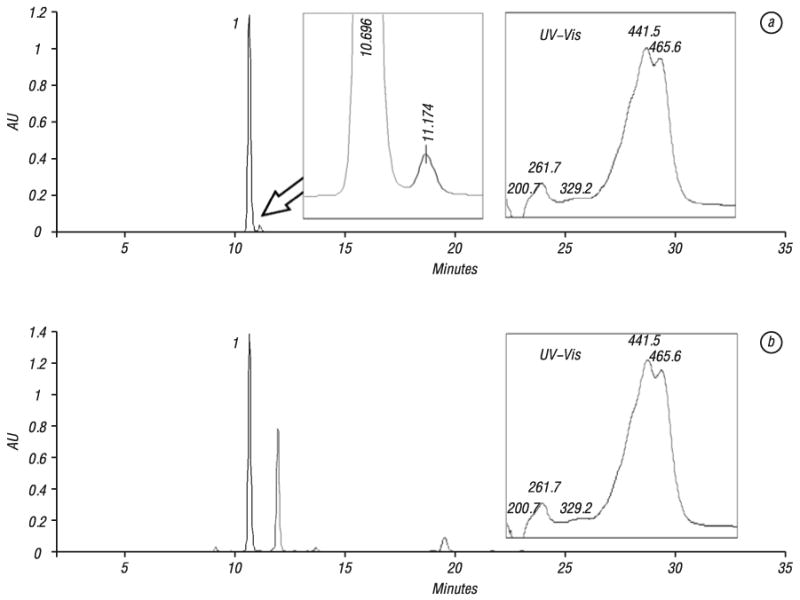
HPLC chromatograms of crocin (a) and Crocus sativus extract (b). HPLC condition is described in Material and Methods section. UV-Vis or ultraviolet-visible spectrum of crocin (200–700 nm) was shown in the right side of the figure. Number “1” indicates the peak of crocin
Table 1.
Purity analysis of crocin
| Crocin | Impurity 1 | Impurity 2 | Impurity 3 | |
|---|---|---|---|---|
| Retention time (min) | 10.70 | 11.17 | 18.92 | 21.82 |
| Peak area | 2.094 × 107 | 8.042 × 105 | 6.303 × 104 | 3.742 × 104 |
| Percent (%) | 95.9 | 3.7 | 0.3 | 0.2 |
Fig. 2B shows the chromatogram of the CSE. The ultraviolet-visible (UV-Vis) spectrum of the crocin peak is also shown. From the chromatogram, crocin was the major compound in the CSE, and it was clearly separated from other components. Determinations were repeated three times and the results are shown in Table 2, in which the content of crocin in the CSE is 22.9%. The CSE at a concentration of 3.0 mg/ml contained approximately 0.6 mM crocin.
Table 2.
Determination of crocin in Crocus sativus extract
| Assay | Peak Area | Content (%) | Average Content (%) | SD | RSD |
|---|---|---|---|---|---|
| 1 | 1.261 × 107 | 22.91 | |||
| 2 | 1.256 × 107 | 22.82 | 22.9 | 0.1 | 0.5% |
| 3 | 1.268 × 107 | 23.03 |
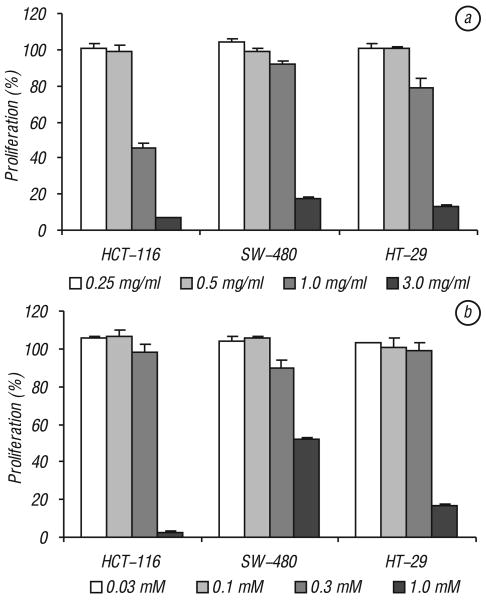
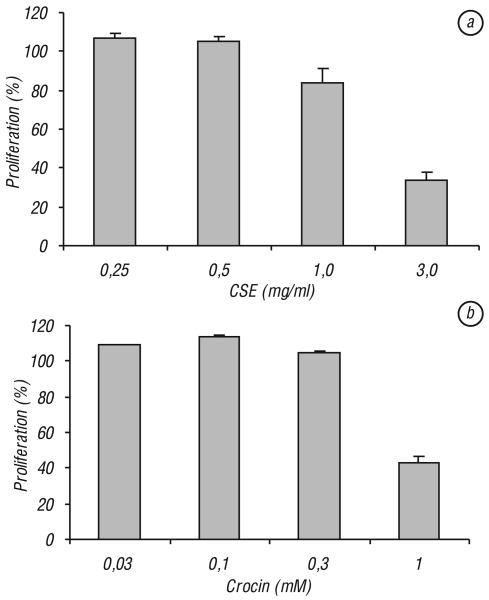
Effects of CSE and crocin on proliferation of colorectal cancer cell lines. Fig. 3, a shows the concentration-related effects of CSE on the three cancer cell lines. CSE has significant anti-proliferative effects on all three tested cell lines, HCT-116, SW-480 and HT-29 (P < 0.01). The proliferation was reduced most significantly in HCT-116 cells, 45.5% proliferation at a concentration of 1.0 mg/ml and 6.8% at a concentration of 3 mg/ml. Although SW-480 and HT-29 cells responded to the extract well, the effects were not as dramatic as that in HCT-116 cells. SW-480 cells showed that proliferation reduced to 91.0% and 17.6% at 1.0 mg/ml and 3.0 mg/ml, respectively. HT-29 cells showed that the proliferation reduced to 79.2% and 12.9% at 1.0 mg/ml and 3.0 mg/ml, respectively.
Fig. 3.
Effects of Crocus sativus extract and crocin on proliferation of three human colorectal cancer cells. Cancer cells were exposed to Crocus sativus extract or CSE (a) and crocin (b) for 48 h and cell proliferation was determined by MTS method. CSE and crocin have the most potent anti-proliferative activities on HCT-116 cells. Control at 48 h is normalized to 100%
Crocin is a major component in the CSE. Fig. 3, b shows the relationship between crocin concentration and the inhibition of proliferation of the three cell lines. At 1.0 mM concentration, HCT-116, SW-480 and HT-29 cells proliferation was significantly reduced to 2.8, 52 and 16.8% proliferation, respectively (P < 0.01). Consistent with the CSE data, crocin has the most significant anti-proliferative effect on HCT-116 cells.
Effects of CSE and crocin on proliferation of NSCLC cells. In addition to colorectal cancer cells, we also evaluated the effects of CSE and crocin on non-digestive system cancer cells, i.e., non-small cell lung cancer (NSCLC) cells. Our data showed that at 1.0 mg/ml and 3.0 mg/ml, CSE reduced the NSCLC proliferation to 83.9% (P < 0.05) and 34.1% (P < 0.01), respectively (Fig. 4, a). At 1.0 mM crocin concentration, the cell proliferation was reduced to 43.3% (P < 0.01; Fig. 4, b).
Fig. 4.
Effects of Crocus sativus extract and crocin on proliferation of non-small cell lung cancer (NSCLC) cells. Cancer cells were exposed to Crocus sativus extract or CSE (a) and crocin (b) for 48 h and cell proliferation was determined by MTS method. Control at 48 h is normalized to 100%
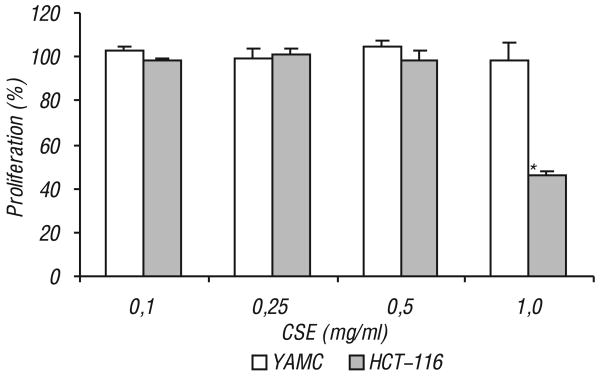
CSE does not affect proliferation of non-cancer YAMC cells. In this part of the study, the effect of CSE on non-cancer young adult mouse colon (YAMC) cells was compared to that of the HCT-116 cells. As shown in Fig. 5, at the tested concentration range, CSE did not show any significant inhibition of the YAMC cells, while cell growth was significantly inhibited in HCT-116 cells at 1.0 mg/ml (P < 0.01).
Fig. 5.
Effects of Crocus sativus extract on proliferation of non-cancer young adult mouse colon (YAMC) cells and colorectal cancer HCT-116 cells. The two different cells were exposed to Crocus sativus extract (CSE) for 48 h and cell proliferation was determined by MTS. Only HCT-116 cell growth was significantly inhibited at 1.0 mg/ml CSE
*P < 0.01 vs. control. Control at 48 h is normalized to 100%.
Discussion
Crocus sativus L. belongs to Iridaceae family, and is cultivated in Asian, Europe and America [17]. Safranal in Crocus sativus is the volatile oil responsible for odor and aroma. Crocus sativus has been used to treat several medical conditions, such as gastrointestinal disorders, urological infections, as well as in treating malignancies [9, 11, 18]. CSE used in this study was prepared from stigmas of Crocus sativus. CSE contains several pharmacologically active constituents. Saffron-colored compounds are unusual water-soluble carotenoids which contain mono and diglycosyl esters of a polyene dicarboxylic acid. The digentiobiosyl ester compound, i. e., alpha crocin, or crocin, is the major component of CSE. Additionally, CSE also contains amino acids, flavonoids, and other chemical compounds [19, 20]. Among these compounds, crocin is the most important since it is the major compound in CSE and has shown significant biological activities [20].
Standardization of active constituents, such as crocin from CSE, is a critical issue for research and potential commercialization of botanicals. Quantitative HPLC can quickly assay CSE from different sources and its results help to determine which sample(s) should be selected [21]. In this study, we developed an HPLC method and the extract used in our study was analyzed. Thus, the measured CSE and crocin can be a reference for future investigations. Our data showed comparable anti-proliferative effects of 3.0 mg/ml CSE vs. 1.0 mM crocin. Since 3.0 mg/ml CSE contained approximately 0.6 mM crocin, the observed effects suggested that crocin is a major responsible constituent in the CSE.
We demonstrated significant anti-proliferative effects of CSE and crocin on three different colorectal cancer cell lines. Each of these cell lines originated from a unique patient tumor biopsy, and has been extensively studied [22, 23]. These cells are immortal, and therefore offer a renewable and endless supply of consistent source material for our investigation. We studied three cell lines to acknowledge and represent the genetic heterogeneity, and consequent variable responsiveness to treatment regimens found in colorectal cancer patients. Importantly, our data also demonstrated that at concentrations with the inhibition of colon cancer cell growth, the extract did not affect non-cancer cells, such as young adult mouse colon cells.
Comparing our data of three studied colorectal cancer cell lines, HCT-116 showed significant higher sensitivity to CSE and crocin than other two cells, SW-480 and HT-29. In cancer chemotherapy, induction of cancer cell apoptosis has been emphasized, and the cell apoptosis is mediated by many factors. Among them, P53 is a transcription factor placed at the nexus of a number of pathways that mediate apoptosis in response to a wide range of cellular stresses [24]. HCT-116 cells are p53 wild-type, while SW-480 and HT-29 cells are mutant in the p53 tumor suppressor gene. Since the effects of CSE and crocin on HCT-116 are stronger than that of HT-29 and SW480, it suggests that some activity of p53 may be linked to the CSE and crocin to express the anti-cancer effects [25].
For comparison purposes, we also evaluated if CSE and crocin could inhibit other cancer cell growth. Since lung cancer is a very common cancer and approximately 85% of all lung cancers are of non-small cell type [26, 27], we selected to test the non-small cell lung cancer cells (NSCLC) cells. Significant growth inhibition of NSCLC cells was observed after CSE and crocin treatments, suggesting the efficacy of Crocus sativus goes beyond colorectal cancer.
Crocin from CSE has anti-tumor effects on cellular DNA and RNA synthesis [18, 28]. Another mechanism for the anti-tumor action of CSE and its constituents is the inhibitory effect on free radical chain reactions [29], because most carotenoids are lipid-soluble and might act as membrane-associated high-efficiency free radical scavengers, connects with their anti-oxidant properties [30, 31].
In summary, data from this study suggest that crocin from CSE may be efficacious in treating certain colorectal cancer. Considering the popularity of botanical use in cancer patients, Crocus sativus should be investigated further as a viable option in the treatment of colorectal cancer.
Acknowledgments
Authors would like to thank Prof. E.B. Chang at the Digestive Diseases Research Core Center at the University of Chicago for providing young adult mouse colon (YAMC) cells. This work was supported in part by the NIH/NCCAM grant AT002445 and AT003255.
Abbreviations used
- CSE
Crocus sativus extract
- FBS
fetal bovine serum
- HPLC
high performance liquid chromatography
- NSCLC
non-small cell lung cancer
- UV-Vis
ultraviolet-visible
- YAMC
non-cancer young adult mouse colon cells
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom PF, Benson AB, 3rd, Chen YJ, Choti MA, Dilawari RA, Enke CA, Fakih MG, Fuchs C, Kiel K, Knol JA, Leong LA, Ludwig KA, Martin EW, Jr, Rao S, Saif MW, Saltz L, Skibber JM, Venook AP, Yeatman TJ, National Comprehensive Cancer Network Colon cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:468–91. doi: 10.6004/jnccn.2005.0024. [DOI] [PubMed] [Google Scholar]
- 3.Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–98. doi: 10.1634/theoncologist.8-2-187. [DOI] [PubMed] [Google Scholar]
- 4.Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–61. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- 5.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 6.Lee TI, Chen HH, Yeh ML. Effects of chan-chuang qigong on improving symptom and psychological distress in chemotherapy patients. Am J Chin Med. 2006;34:37–46. doi: 10.1142/S0192415X06003618. [DOI] [PubMed] [Google Scholar]
- 7.HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20:239–49. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- 8.Bemis DL, Capodice JL, Costello JE, Vorys GC, Katz AE, Buttyan R. The use of herbal and over-the-counter dietary supplements for the prevention of prostate cancer. Curr Urol Rep. 2006;7:166–74. doi: 10.1007/s11934-006-0017-x. [DOI] [PubMed] [Google Scholar]
- 9.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44:155–72. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 11.Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4:83–6. [PubMed] [Google Scholar]
- 12.Choi SH, Im E, Kang HK, Lee JH, Kwak HS, Bae YT, Park HJ, Kim ND. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005;227:153–62. doi: 10.1016/j.canlet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud A, Lavasanifar A. The effect of block copolymer structure on the internalization of polymeric micelles by human breast cancer cells. Colloids Surf B Biointerfaces. 2005;45:82–9. doi: 10.1016/j.colsurfb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Liao PH, Chen SL, Shih HC, Chou MY. Induction of apoptosis in human oral cancer cell lines, OC2 and TSCCa, by chingwaysan. Am J Chin Med. 2005;33:21–7. doi: 10.1142/S0192415X0500262X. [DOI] [PubMed] [Google Scholar]
- 15.Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, Aung HH, Xie JT, Tong R, He TC, Yuan CS. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–42. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 16.Xie JT, Wang CZ, Wicks S, Yin JJ, Kong J, Li J, Li YC, Yuan CS. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp Oncol. 2006;28:25–9. [PubMed] [Google Scholar]
- 17.Xue XH. Cultivation of Crocus sativus. Chung Yao TungPao. 1982;7:3–4. [PubMed] [Google Scholar]
- 18.Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–14. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 19.Rios JL, Recio MC, Giner RM, Mañez S. An update review of saffronand its active compounds. Phytother Res. 1996;10:189–93. [Google Scholar]
- 20.Winterhalter P, Straubinger M. Saffron-renewed interest in an ancient spice. Food Rev Int. 2000;16:39–59. [Google Scholar]
- 21.Liakopoulou-Kyriakides M, Kyriakidis DA. Crocus satvus-biologically active constituents. Stud Nat Prod Chem. 2002;26:293–312. [Google Scholar]
- 22.Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br J Cancer. 2004;91:381–8. doi: 10.1038/sj.bjc.6601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American Ginseng: Ginsenoside Constituents and Antiproliferative Activities of Heat-Processed Panax quinquefolius Roots. Planta Med. 2007;73:669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchynska LG, Nesina IP, Yurchenko NP, Bilyk OO, Grinkevych VN, Svintitsky VS. Expression of p53, p21WAF1/CIP1, p16INK4A and Ki-67 proteins in serous ovarian tumors. Exp Oncol. 2007;29:49–53. [PubMed] [Google Scholar]
- 25.Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, Lacorte JM, Staedel C, Lesuffleur T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 26.Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82:1075–90. doi: 10.1016/s0039-6109(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 27.Saijo N. Recent trends in the treatment of advanced lung cancer. Cancer Sci. 2006;97:448–52. doi: 10.1111/j.1349-7006.2006.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–64. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 29.Nair SC, Varghese CD, Pannikar KR, Kurumboor SK, Parathod RK. Effects of saffron on vitamin A levels and its antitumor activityonthegrowth of solid tumors in mice. Int J Pharmacog. 1994;32:105–14. [Google Scholar]
- 30.Molnar J, Szabo D, Pusztai R, Mucsi I, Berek L, Ocsovszki I, Kawata E, Shoyama Y. Membrane associated antitumor effects of crocin-, ginsenoside- and cannabinoid derivates. Anticancer Res. 2000;20:861–7. [PubMed] [Google Scholar]
- 31.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]