Abstract
Introduction:
The perception of negative health consequences is a common motive for quitting smoking, but specific information on the immediate health effects of occasional smoking among young adults is limited.
Method:
To examine the relationship between cigarette use and symptoms of (a) cough or sore throat and (b) shortness of breath or fatigue after regular activities among young adults, we performed online health screening of a random sample of 25,000 college undergraduates. The screening survey assessed demographic characteristics, smoking and related health behaviors, and respiratory symptoms in the previous 30 days.
Results:
The response rate was 26% (6,492/25,000). Among individuals reporting no smoking in the prior 30 days and smoking on 1–4, 5–10, 11–20, or 21–30 days, the prevalence of one or more days of cough/sore throat increased from 62.5% to 68.3%, 72.0%, 71.4%, and 73.7%, respectively (p < .001). Similarly, the prevalence of shortness of breath/fatigue increased from 42.7% to 47.1%, 56.2%, 59.5%, and 64.6%, respectively (p < .001). After controlling for demographics, other important health behaviors (e.g., days consuming alcohol and getting adequate sleep), and environmental tobacco smoke (ETS) exposure, reporting symptoms of cough/sore throat was associated with smoking on at least 21 days, whereas shortness of breath/fatigue was associated with smoking on 5 or more days. Among those reporting symptoms, increased number of days with respiratory symptoms was associated with smoking on most days as well as ETS exposure.
Discussion:
In conclusion, this cross-sectional study found that occasional smoking and ETS exposure were associated with an increase in the rate of respiratory symptoms (cough/sore throat and shortness of breath/fatigue) among young adults.
Introduction
Young adulthood is a critical transition period in cigarette use (Bachman, Wadsworth, O'Malley, Johnston, & Schulenberg, 1997; Chen & Kandel, 1995). Whereas first experimentation with cigarettes occurs early in life for the majority of individuals, increased frequency of smoking and establishment of regular or heavy cigarette use often occur during the young adult years (Everett, Husten, et al., 1999; Everett, Warren, et al., 1999). Encouraging smoking cessation early in life is crucial to help individuals avoid many of the harms related to smoking (Doll, Peto, Boreham, & Sutherland, 2004; Orleans, 2007). Effective strategies targeting occasional or intermittent young adult smokers are needed to interrupt the progression of smoking behavior and the development of nicotine dependence.
Efforts to encourage cessation often focus on communicating the negative consequences of cigarette use. This approach is based on well-recognized health behavior theories such as the health belief model (Janz & Becker, 1984; Rosenstock, Strecher, & Becker, 1988) and the theory of reasoned action (Ajzen & Fishbein, 1980; Fishbein & Ajzen, 1975). Several studies have identified concerns over health consequences as an important factor in preventing the initiation of smoking (Flay, Phil, Hu, & Richardson, 1998; Leventhal, Glynn, & Fleming, 1987) as well as an important motivator for young adult smokers who are considering quitting (Hansen, Collins, Johnson, & Graham, 1985; McCaul et al., 2006; Milam, Sussman, Ritt-Olson, & Dent, 2000; Riedel, Robinson, Klesges, & McLain-Allen, 2002; Romer & Jamieson, 2001; Rose, Chassin, Presson, & Sherman, 1996). Unfortunately, children and adolescents who smoke tend to discount the health effects of smoking, often believing that they will quit prior to experiencing these consequences (U.S. Department of Health and Human Services [USDHHS], 1994). A report by Moran, Wechsler, and Rigotti (2004) of reduced interest in quitting and less frequent quit attempts among college social smokers, the majority of whom smoke only occasionally, suggests that this discounting also may be an issue for young adult smokers.
One approach to these difficulties is to place a greater emphasis on immediate or short-term risks and symptoms associated with cigarette use that may be more salient for younger smokers (USDHHS, 1994). Unfortunately, specific information regarding the short-term health consequences of occasional or intermittent cigarette use by young adult smokers is lacking. Studies of respiratory effects among younger adolescents have tended to compare the level of symptoms among nonsmokers to those among all smokers as a group or to those among smokers with different levels of daily use (Addington, Carpenter, McCoy, Duncan, & Mogg, 1970; Bewley & Bland, 1976; Bewley, Halil, & Snaith, 1973; Charlton, 1984; Peters & Ferris, 1967; Rush, 1974; Seely, Zuskin, & Bouhuys, 1971). A recent study by Prokhorov et al. (2003) documented increased respiratory symptoms and reduced lung function among college smokers. However, this study involved daily smokers and it is unclear how these findings apply to intermittent or occasional cigarette use. We address this gap in our current knowledge by reporting here on the short-term health effects (e.g., symptoms of cough and shortness of breath) in a sample of young adult college smokers.
Method
Setting
This study was conducted at the University of Minnesota—Twin Cities (fall 2004 undergraduate enrollment = 28,740; 26.6% campus prevalence of past 30-day cigarette use). All study procedures were reviewed and approved by the institutional review board of the University of Minnesota .
Internet health screening
Data for this analysis come from a campus-wide Internet health screening performed in October 2004. The methodology for this screening is described in detail elsewhere (An et al., 2007). A random sample of 25,000 University of Minnesota undergraduates was invited by E-mail to complete a 46-item online health screening survey. The estimated time needed to complete the survey was approximately 5 min. Nonrespondents received up to two reminder E-mails in the first week after the initial invitation. To encourage completion of the survey, all respondents were entered into a lottery for 1 of 10 US$100 prizes. For the purposes of this analysis, we focus on findings for 18- to 24-year-old respondents.
Evaluation and measurement
Participant demographic characteristics assessed include age, year in school, gender, ethnicity, and working status.
Health behavior variables.
Participants were asked, “Think about the past 30 days. On how many days of the past 30 days did you: (1) Drink any alcohol? (2) Exercise for 20 minutes or more? and (3) Get at least 6 hours of sleep?”
Smoking-related variables.
The main item regarding current tobacco use asked, “On how many days in the past 30 days did you smoke cigarettes (even 1 puff)?” To assess smoking level, the survey also asked, “On the days that you smoked, what was the average number of cigarettes you smoked per day?”
Physical symptoms.
Participants were asked, “Think about the past 30 days. On how many days of the past 30 days did you: (1) Have cough or sore throat? and (2) Feel short of breath or tired after regular activities?”
Data analyses
To specifically address the occurrence of physical symptoms among smokers with different levels of occasional use, we categorized the number of days smoking into five categories (i.e., 0, 1–4 , 5–10 , 11–20 , and 21–30 days in the prior 30 days).
We compared level of smoking separately with occurrence of symptoms of cough and of shortness of breath. Pearson's chi-square test was used to examine the association between the number of days smoking (coded categorically as described above) and the proportion of respondents reporting one or more days of symptoms (vs. 0 days). For respondents who reported any symptom days (i.e., one or more days of cough or one or more days of shortness of breath), we performed pairwise comparisons of the mean number of symptom days at different levels of smoking using Tukey's wholly significant difference test.
The occurrence of physical symptoms was highly skewed with a significant proportion of respondents reporting 0 days of symptoms in the prior 30 days. We therefore adopted a two-stage approach to the analysis of independent predictors of these symptoms. In the first stage, binary logistic regression was used to determine the factors that were related to reporting any days (vs. 0 days) of cough/sore throat and shortness of breath/fatigue. In the second stage, ordinary least squares regression modeling was used to determine factors that were significantly associated with the number of symptom days reported by those who experienced one or more symptom days. The main predictor variable of interest was the number of days smoked in the past 30 days.
In these models, the reference group for comparison of smoking behavior comprised those individuals who reported no smoking in the prior 30 days. Comparing each smoking group (i.e., 1–4, 5–10, 11–20, and 21–30 days) to nonsmokers provides a direct assessment of different levels of occasional or intermittent smoking. This assessment of the effect of occasional smoking is stricter than would be a simple test for a significant trend across the total number of days smoked when this information is entered as a continuous variable.
For each symptom (i.e., cough and shortness of breath) for each stage of the analysis (logistic and ordinary least squares models), we constructed three nested models. The first model examined the unadjusted association between the number of days smoking and each symptom. The second model adjusted for demographic characteristics and related health behaviors (i.e., number of days consuming alcohol, exercising at least 20 min, and getting at least 6 hr of sleep). The third model adjusted for these factors as well as exposure to environmental tobacco smoke (ETS). All analyses were performed using STATA version 9.0.
Results
Survey response
The response rate to the Internet health screening survey was 26% (6,492/25,000). Of survey respondents, 419 (6.5% of all respondents) were excluded from this analysis for being less than age 17 or age 25 or older. An additional 20 individuals were excluded due to item nonresponse to the number of days smoked in the prior 30 days. The number of cases included in this analysis is therefore 6,053. This number may vary in specific results described below due to survey item nonresponse.
Baseline characteristics
The baseline characteristics of participants are displayed in Table 1. The average age of participants was 19.84 years (SD = 1.57), and the majority were female (64.5%) and White non-Hispanic (88.1%). In the prior 30 days, the mean number of days drinking alcohol was 5.25 (SD = 5.57). The mean number of days exposed to someone else's cigarette smoke was 7.57 (SD = 9.30). Exercising 20 min or more was common (M = 10.40 days, SD = 8.67, in the prior 30 days); 38% of respondents reported engaging in an average of at least 20 min of exercise three times a week. The mean number of days getting at least 6 hr of sleep was 21.82 (SD = 7.06) in the prior 30 days.
Table 1.
Characteristics of study participants (N = 6,053)
| Characteristics | Number of subjects (%) or mean (SD) |
| Demographics | |
| Mean age, years (SD) | 19.84 (1.57) |
| Gender (%) | |
| Female | 3,922 (64.6) |
| Male | 2,139 (35.2) |
| Year in school (%) | |
| Freshman | 1,967 (32.4) |
| Sophomore | 1,328 (21.9) |
| Junior | 1,288 (21.2) |
| Senior | 1,483 (26.3) |
| White non-Hispanic (%) | 5,691 (88.1) |
| Employment (%) | |
| Not working | 2,214 (36.5) |
| Part time | 3,544 (58.4) |
| Full time | 307 (5.1) |
| Health behaviors (in past 30 days) | |
| Mean days drank alcohol (SD) | 5.25 (5.57) |
| Mean days spent time around others' smoke (SD) | 7.57 (9.30) |
| Mean days exercising ≥20 min (SD) | 10.40 (8.67) |
| Mean days sleeping ≥6 hr (SD) | 21.82 (7.06) |
| Smoking variables | |
| Average days smoked in past 30 days (SD) | 3.61 (8.44) |
| Number of days smoking in past 30 days (%) | |
| 0 | 4,330 (71.5) |
| 1–4 | 701 (11.6) |
| 5–10 | 300 (5.0) |
| 11–20 | 192 (3.2) |
| 21–30 | 530 (8.9) |
| Among smokers | |
| Average days smoked in past 30 days (SD) | 12.68 (11.64) |
| Average number of cigarettes on smoking days (SD) | 3.70 (4.67) |
| Physical symptoms, number of subjects (SD) | |
| One or more days of cough or sore throat | 3,926 (64.91) |
| One or more days of shortness of breath | 2,792 (46.35) |
Most (71.5%; n = 4,330) participants reported not having smoked (even a puff) on any days in the past 30 days; 11.6% (n = 701) reported smoking on 1–4 days, 5.0% (n = 300) on 5–10 days, 3.2% (n = 192) on 11–20 days, and 8.9% (n = 530) on more than 21 of the past 30 days. Among participants who reported any smoking in the past 30 days, the average number of days smoked was 12.69 (SD = 11.64), with a reported average of 3.70 cigarettes/day (SD = 4.67) on these smoking days. Participants smoking on 1–4 days in the prior 30 days reported smoking an average of 1.12 cigarettes/day (SD = 2.46) on these smoking days. Participants smoking on 5–10, 11–20, and 21–30 days reported smoking an average of 2.55 (SD = 2.93), 2.99 (SD = 3.32), and 8.00 (SD = 5.49) cigarettes/day, respectively.
Among survey respondents, 64.9% (n = 3,926) reported one or more days of cough or sore throat in the prior 30 days. Among those reporting any cough or sore throat, the mean number of days of symptoms was 6.19 (SD = 5.63). Among survey respondents, 46.4% (n = 2,972) reported one or more days of shortness of breath or fatigue with regular activities. Among those who reported any shortness of breath, the mean number of days of symptoms was 6.11 (SD = 6.18).
Association between smoking and respiratory symptoms
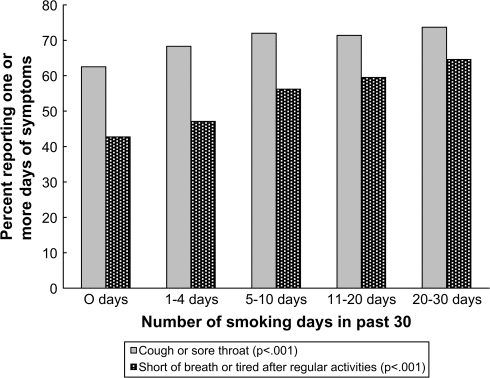
The associations between the number of days smoking and self-reported symptoms of cough/sore throat and shortness of breath/fatigue are shown in Figure 1. Among participants who reported no smoking in the prior 30 days, 62.5% (2,705/4,327) reported one or more days of cough. This value increased to 73.72% (390/529) among participants who reported smoking on 21 or more days in the prior 30 days (χ42 = 42.57, p < .001). Among those individuals reporting any cough, the mean number of days of cough was 5.91, 5.96, 6.04, 6.034, and 8.38 days, respectively, for those reporting no smoking and 1–4, 5–10, 11–20, and 21–30 days of smoking. In all pairwise comparisons among those reporting one or more days of cough, the only group that differed from the others in terms of the mean number of days of cough was the group that smoked on 21–30 days of the past 30 days.
Figure 1.
Percent reporting physical symptoms by number of days smoked. Relationship between number of days smoking and percent reporting any cough/sore throat or shortness of breath/feeling tired after regular activities in the past 30 days.
A similar pattern is evident for symptoms of shortness of breath. Among participants who reported no smoking in the prior 30 days, 42.7% (1,844/4,315) reported one or more days of shortness of breath. This value increased to 64.6% among participants who reported smoking on 21 or more days of the prior 30 days (χ42 = 118.39, p < .001). Among those individuals reporting any shortness of breath, the mean number of days of shortness of breath was 5.76, 5.58, 6.07, 6.04, and 8.58 days, respectively, for those reporting no smoking and 1–4, 5–10, 11–20, and 21–30 days of smoking. In all pairwise comparisons among those reporting one or more days of shortness of breath, the only group that differed from the others in terms of the mean number of days of shortness of breath was the group that smoked on 21–30 days of the past 30 days.
Multivariable models predicting symptoms
Cough or sore throat.
The results of logistic regression models predicting the experience of any cough or sore throat in the prior 30 days are shown in Table 2. Model 1A shows that unadjusted odds of experiencing any cough were related to the number of days smoking. Smokers at all levels (i.e., 1–4, 5–10, 11–20, and 21–30 days in the past 30 days) were more likely to report having any cough or sore throat in the past 30 days than were those reporting no smoking. This finding was less consistent after controlling for demographic characteristics and other health behaviors (Model 1B). Age (p < .001), gender (such that women reported more cough/sore throat than men; p < .001), number of days drinking alcohol (p < .001), and sleeping at least 6 hr (p = .006) contributed significantly to the model. In regard to smoking level, those smoking 5–10 and 21–30 days were still more likely to report cough or sore throat. After adding exposure to ETS to the model (Model 1C), only the group reporting smoking on 21–30 days showed a statistically significant increase in reporting cough or sore throat (p < .05), compared with nonsmokers.
Table 2.
Logistic regression model predicting presence of cough in the past 30 days
| Variable | Model 1A (n = 6,036) |
Model 1B (n = 5,926) |
Model 1C (n = 5,917) |
||||||
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | |
| Smoking level (days smoking at least one puff) | |||||||||
| 0 | Ref | Ref | Ref | ||||||
| 5–10 | 1.54 | 1.19–1.99 | .001 | 1.40 | 1.06–1.84 | .02 | 1.32 | 1.00–1.74 | .05 |
| 11–20 | 1.49 | 1.08–2.06 | .01 | 1.39 | 0.99–1.95 | .06 | 1.27 | 0.89–1.79 | .18 |
| 21–30 | 1.68 | 1.37–2.06 | <.001 | 1.56 | 1.25–1.95 | <.001 | 1.31 | 1.00–1.71 | <.05 |
| Demographics | |||||||||
| Age | 0.86 | 0.83–0.90 | <.001 | 0.87 | 0.84–0.90 | <.001 | |||
| Gender (male vs. female) | 1.46 | 1.30–1.63 | <.001 | 1.45 | 1.29–1.62 | <.001 | |||
| Ethnicity (White vs. others) | 0.88 | 0.75–1.05 | .15 | 0.89 | 0.75–1.05 | .16 | |||
| Health variables (number of days) | |||||||||
| Drank alcohol | 1.03 | 1.02–1.04 | <.001 | 1.02 | 1.01–1.04 | <.001 | |||
| Exercising at least 20 min | 1.00 | 0.99–1.01 | .88 | 1.00 | 0.99–1.01 | .82 | |||
| Sleeping at least 6 hr | 0.99 | 0.98–1.00 | .006 | 0.99 | 0.98–1.00 | .007 | |||
| Environmental tobacco smoke (number of days exposed) | 1.01 | 1.00–1.02 | .02 | ||||||
Note. OR = odds ratio; CI = confidence interval.
For the second stage of this analysis, we conducted an ordinary least squares regression to ascertain what factors were related to the number of days of cough or sore throat among those who reported at least one day of these symptoms. Across the three models examined (unadjusted, adjusted for demographic and health behaviors, and adjusted for demographic and health behaviors and exposure to ETS), only those who smoked on 21–30 days in the prior 30 days had a higher number of days of cough compared with nonsmokers. In the fully adjusted model, the significant predictors were age (β = −0.29, p < .001), number of days drinking alcohol (β = 0.08, p < .001), number of days exercising (β = −0.02, p = .04), number of days spending time around other smokers (β = 0.05, p < .001), and smoking on at least 21 days of the past 30 days (β = 1.05 vs. no smoking, p = .009).
Shortness of breath or fatigue.
The results of logistic regression models predicting the experience of any shortness of breath or fatigue in the prior 30 days are shown in Table 3. Model 2A shows that unadjusted odds of experiencing any shortness of breath were related to the number of days smoking. Smokers at all levels (i.e., 1–4, 5–10, 11–20, and 21–30 days in the past 30 days) were more likely to report experiencing shortness of breath with regular activities in the past 30 days than those reporting no smoking. This finding persisted for those who smoked on at least 5 days in the prior 30 days after controlling for demographic characteristics and other health behaviors (Model 2B). Age (p < .001), gender (such that women reported more shortness of breath or feeling tired than men; p < .001), ethnicity (such that non-White people reported more shortness of breath or feeling tired than White people, p = .01), and number of days exercising (p < .001) and sleeping at least 6 hr (p = .003) also contributed significantly to the model. The increased odds of experiencing any shortness of breath persisted for those who smoked on at least 5 days in the prior 30 days even after adding exposure to ETS (Model 2C).
Table 3.
Logistic regression model for presence of shortness of breath after regular activities in the past 30 days
| Variable | Model 2A (n = 6,024) |
Model 2B (n = 6,012) |
Model 2C (n = 5,902) |
||||||
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | |
| Smoking level (days smoking at least one puff) | |||||||||
| 0 | Ref | Ref | Ref | ||||||
| 1–4 | 1.19 | 1.04–1.42 | .03 | 1.15 | 1.00–1.40 | .11 | 1.12 | 0.97–1.36 | .21 |
| 5–10 | 1.72 | 1.37–2.16 | <.001 | 1.70 | 1.37–2.24 | <.001 | 1.55 | 1.24–2.04 | .001 |
| 11–20 | 1.97 | 1.39–2.47 | <.001 | 1.89 | 1.47–2.73 | <.001 | 1.61 | 1.23–2.33 | .004 |
| 21–30 | 2.45 | 2.03–2.96 | <.001 | 2.31 | 1.99–3.00 | <.001 | 1.71 | 1.38–2.26 | <.001 |
| Demographics | |||||||||
| Age | 0.93 | 0.90–0.96 | <.001 | 0.93 | 0.90–0.96 | <.001 | |||
| Gender (male vs. female) | 1.91 | 1.68–2.10 | <.001 | 1.89 | 1.67–2.09 | <.001 | |||
| Ethnicity (White vs. others) | 1.24 | 1.04–1.45 | .01 | 1.24 | 1.04–1.46 | .01 | |||
| Health variables (number of days) | |||||||||
| Drank alcohol | 1.01 | 0.99–1.02 | .08 | 1.01 | 0.98–1.01 | .40 | |||
| Exercising at least 20 min | 0.97 | 0.97–0.98 | <.001 | 0.97 | 0.97–0.98 | <.001 | |||
| Sleeping at least 6 hr | 0.99 | 0.98–1.00 | .003 | 0.99 | 0.98–1.00 | .004 | |||
| Environmental tobacco smoke (number of days exposed) | 1.02 | 1.01–1.03 | <.001 | ||||||
Note. OR = odds ratio; CI = confidence interval.
Across the three models predicting the number of days of shortness of breath for those experiencing any symptoms (unadjusted, adjusted for demographic and health behaviors, and adjusted for demographic and health behaviors and exposure to ETS), only those who smoked on 21–30 days in the prior 30 days had a higher number of days of shortness of breath compared with nonsmokers. In the fully adjusted model, the significant predictors were gender (women reporting more days of shortness of breath than men; β = 0.57, p = .03), ethnicity (non-Whites reporting more days of shortness of breath than Whites; β = 0.92, p = .009), number of days drinking alcohol (β = −0.07, p = .04), number of days exercising (β = −0.05, p = .001), number of days around other people's cigarette smoke (β = 0.09, p < .001), and smoking on at least 21 days of the past 30 days (β = 1.32 vs. nonsmokers, p = .007).
Discussion
The findings of this study demonstrate an association between occasional and intermittent smoking and self-reported symptoms of cough and shortness of breath among young adult smokers. One strength of the present study is our ability to control for the influence of related health behaviors and current exposure to ETS. However, the findings differ somewhat for symptoms of cough compared with symptoms of shortness of breath. The apparent association between the occurrence of cough and occasional smoking (i.e., smoking on 1–4, 5–10, and 11–20 days in the prior 30 days) appears to be due in large part to related health behaviors (i.e., alcohol consumption and getting adequate sleep) and particularly to exposure to ETS. In contrast, we found a more robust association between the occurrence of shortness of breath and occasional smoking. Smoking on at least 5 days in the prior 30 days increased the odds of experiencing shortness of breath or fatigue with regular activities even after accounting for these factors.
The physiological mechanisms underlying this apparent association are not clear. Our results are consistent with a body of literature on the effects of smoking on respiratory symptoms among children and adolescents. Bewley et al. (1973) reported increased respiratory symptoms among children smoking as little as 1 cigarette/week. Findings from the Monitoring the Future project demonstrate increased symptoms of shortness of breath, cough, and wheezing among high school seniors who reported occasional but not regular smoking (USDHHS, 1994). In a cohort study of nearly 12,000 children, Woolcock, Peat, Leeder, and Blackburn (1984) found that reductions in maximal expiratory flow rate developed as soon as 1 year after smoking reached a level of at least 10 cigarettes/week. In their study of college smokers, Lipkus and Prokhorov (2007) documented a 15-year increase in “lung age” (i.e., mean lung age of 35 years vs. chronological age of 20 years) related to daily cigarette use. Unfortunately, we did not assess lung function directly (i.e., spirometry) in the present study and cannot speak to how occasional smoking might influence lung age.
The higher rate of reported health symptoms among occasional smokers could be due to an increase in the rate of acute respiratory illness. Cigarette smoking is well recognized to increase the susceptibility to bacterial and viral infections (Arcavi & Benowitz, 2004). Exposure to cigarette smoking damages the lining of the respiratory tract, reduces mucociliary clearance, and impairs both humoral and cellular immune responses (Dye & Adler, 1994). Smoking increases the odds of developing pneumococcal pneumonia (odds ratio = 2.6) in otherwise healthy adults (i.e., with no chronic lung disease; Pastor, Medley, & Murphy, 1998). Smokers also may be at increased risk of viral respiratory infections such as the common cold and influenza. For example, a study of U.S. Army recruits found that smokers had a 50% higher risk of upper respiratory infections compared with nonsmokers (Blake, Abell, & Stanley, 1988). Similar studies have found increased rates of influenza and an increased rate of complications from influenza related to cigarette smoking among otherwise healthy young adults (Finklea, 1969; Kark & Lebiush, 1981; Kark, Lebiush, & Rannon, 1982).
The finding that related health behaviors (drinking alcohol, sleep, exercise) and exposure to ETS account for some of the apparent association between respiratory symptoms and occasional smoking should not be considered an endorsement of a “safe” level of smoking (i.e., smoking 1–4 days/month). Instead, this finding lends additional support to the general approach of encouraging “healthier lifestyles” for young adults that include abstinence from cigarette use. Both our own prior work (An et al., 2006) and the work of others (Griffin, Botvin, Nichols, & Doyle, 2003; Perry et al., 2002) have reported positive effects when using a more lifestyle-oriented approach to behavior change.
The finding of the association between symptoms of shortness of breath and exposure to ETS deserves additional comment. This result is consistent with the well-documented effects of cigarette smoke exposure on respiratory health (Moritsugu, 2007). The findings of increased symptoms of shortness of breath among young adults exposed to ETS may be incorporated into efforts to build consumer demand for smoke-free public policies that include bars and restaurants and private restrictions on smoking at parties, in homes, and in cars.
Several limitations should be considered when interpreting the results of the present study. First, the low response rate to the Internet health screening raises concerns that response bias may have influenced the observed associations. For example, occasional smokers with physical symptoms and health concerns may have been more likely to respond to the survey than occasional smokers who were not experiencing symptoms. Future studies of more representative samples of young adult smokers are needed to confirm the findings reported here. Consideration also should be given to assessing perceived risk or vulnerability for health effects to determine how these perceptions may differ for nonsmokers, occasional smokers, and daily smokers.
Second, this single cross-sectional survey cannot be used to make definitive statements regarding the causal relationship between occasional smoking and the reported symptoms. Reporting bias may have accounted for the observed associations if health concerns about cigarette use led smokers in this sample to respond more positively to questions regarding physical symptoms. Unmeasured factors also could have contributed to the observed associations. For example, parental smoking and early childhood exposure to ETS could increase the risk of both cigarette use and respiratory symptoms among young adults. Unfortunately, we did not assess either of these factors and were not able to control for them in our multivariate models. Prospective studies including more detailed assessment of smoking and ETS exposure histories are needed to better assess the causal relationship between occasional smoking and physical symptoms among young adults.
Third, our assessment of symptoms was limited (i.e., cough/sore throat and shortness of breath/fatigue). Future studies should consider assessing symptoms separately (i.e., symptoms of shortness of breath separate from symptoms of fatigue) as well as include some measure of the level of exertion or specific activities that trigger these symptoms. Consideration should be given to future assessment of additional respiratory symptoms such as wheezing, phlegm, or morning cough that others have found to be associated with cigarette use among young adults (Prokhorov et al., 2003). Additional physiological assessments (e.g., spirometry or exercise testing) would also help to improve understanding of the nature of the reported symptoms.
Finally, it is important to acknowledge the complex relationship between perceived risk and behavior change. Slovic, Finucane, Peters, and MacGregor (2004) argued that perceived health risks (either long term or short term) are but one part of a network of factors that influence behavior change. Additional study of affective factors (i.e., emotional response to the smoking experience) that influence risk perception and a broader range of salient risks (e.g., risk of reduced social acceptability and risk of addiction) is needed.
Despite these limitations, the findings reported here provide new information regarding the potential health effects of occasional or intermittent smoking among young adults. The findings suggest that occasional smoking may increase the odds of experiencing nearly 1 week of impaired health per month compared with total abstinence from cigarette use. This information, if replicated in future studies, may be useful as part of behavior change interventions targeting young adult smokers.
Funding
ClearWay Minnesota (RC 2002-0025); University of Minnesota Transdisciplinary Tobacco Research Center (NIH P50 013333).
Declaration of Interests
None declared.
Supplementary Material
References
- Addington WW, Carpenter RL, McCoy JF, Duncan KA, Mogg K. The association of cigarette smoking with respiratory symptoms and pulmonary function in a group of high school students. Journal of the Oklahoma State Medical Association. 1970;63:525–529. [PubMed] [Google Scholar]
- Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Eaglewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- An LC, Hennrikus DJ, Perry CL, Lein EB, Klatt C, Farley DM, et al. Feasibility of Internet health screening to recruit college students to an online smoking cessation intervention. Nicotine & Tobacco Research. 2007;9(Suppl. 1):S11–S18. doi: 10.1080/14622200601083418. [DOI] [PubMed] [Google Scholar]
- An LC, Perry CL, Lein EB, Klatt C, Farley DM, Bliss RL, et al. Strategies for increasing adherence to an online smoking cessation intervention for college students. Nicotine & Tobacco Research. 2006;8(Suppl. 1):S7–S12. doi: 10.1080/14622200601039881. [DOI] [PubMed] [Google Scholar]
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Archives of Internal Medicine. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- Bachman JG, Wadsworth KN, O'Malley PM, Johnston LD, Schulenberg J. Smoking, drinking, and drug use in young adulthood: The impacts of new freedoms and new responsibilities. Hillsdale, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Bewley BR, Bland JM. Smoking and respiratory symptoms in two groups of schoolchildren. Preventive Medicine. 1976;5:63–69. doi: 10.1016/0091-7435(76)90009-8. [DOI] [PubMed] [Google Scholar]
- Bewley BR, Halil T, Snaith AH. Smoking by primary schoolchildren: Prevalence and associated respiratory symptoms. British Journal of Preventive and Social Medicine. 1973;27:150–153. doi: 10.1136/jech.27.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GH, Abell TD, Stanley WG. Cigarette smoking and upper respiratory infection among recruits in basic combat training. Annals of Internal Medicine. 1988;109:198–202. doi: 10.7326/0003-4819-109-3-198. [DOI] [PubMed] [Google Scholar]
- Charlton A. Children's coughs related to parental smoking. British Medical Journal. 1984;288:1647–1649. doi: 10.1136/bmj.288.6431.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescent to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. British Medical Journal. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye JA, Adler KB. Effects of cigarette smoking on epithelial cells of the respiratory tract. Thorax. 1994;49:825–834. doi: 10.1136/thx.49.8.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett SA, Husten CG, Kann L, Warren CW, Sharp D, Crossett L. Smoking initiation and smoking patterns among U.S. college students. Journal of American College Health. 1999;48:55–60. doi: 10.1080/07448489909595674. [DOI] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Preventive Medicine. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Finklea JF. Cigarette smoking and epidemic influenza. American Journal of Epidemiology. 1969;90:390–399. doi: 10.1093/oxfordjournals.aje.a121084. [DOI] [PubMed] [Google Scholar]
- Fishbein M, Ajzen I. Belief, attitude, intention and behavior: An introduction to theory and research. Boston: Addison-Wesley; 1975. [Google Scholar]
- Flay BR, Phil D, Hu FB, Richardson J. Psychosocial predictors of different stages of cigarette smoking among high school students. Preventive Medicine. 1998;27:A9–A18. doi: 10.1006/pmed.1998.0380. [DOI] [PubMed] [Google Scholar]
- Griffin KW, Botvin GJ, Nichols TR, Doyle MM. Effectiveness of a universal drug abuse prevention approach for youth at high risk for substance use initiation. Preventive Medicine. 2003;36:1–7. doi: 10.1006/pmed.2002.1133. [DOI] [PubMed] [Google Scholar]
- Hansen WB, Collins LM, Johnson CA, Graham JW. Self-initiated smoking cessation among high school students. Addictive Behaviors. 1985;10:265–271. doi: 10.1016/0306-4603(85)90007-3. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The health belief model: A decade later. Health Education Quaterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Kark JD, Lebiush M. Smoking and epidemic influenza-like illness in female military recruits: A brief survey. American Journal of Public Health. 1981;71:530–532. doi: 10.2105/ajph.71.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark JD, Lebiush M, Rannon L. Cigarette smoking as a risk factor for epidemic A(H1N1) influenza in young men. The New England Journal of Medicine. 1982;307:1042–1046. doi: 10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Glynn K, Fleming R. Is the smoking decision an ‘informed choice’? Effect of smoking risk factors on smoking beliefs. The Journal of the American Medical Association. 1987;257:3373–3376. [PubMed] [Google Scholar]
- Lipkus IM, Prokhorov AV. The effects of providing lung age and respiratory symptoms feedback on community college smokers’ perceived smoking-related health risks, worries and desire to quit. Addictive Behaviors. 2007;32:516–532. doi: 10.1016/j.addbeh.2006.05.018. [DOI] [PubMed] [Google Scholar]
- McCaul KD, Hockemeyer JR, Johnson RJ, Zetocha K, Quinlan K, Glasgow RE. Motivation to quit using cigarettes: A review. Addictive Behaviors. 2006;31:42–56. doi: 10.1016/j.addbeh.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Milam JE, Sussman S, Ritt-Olson A, Dent CW. Perceived invulnerability and cigarette smoking among adolescents. Addictive Behaviors. 2000;25:71–80. doi: 10.1016/s0306-4603(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Moran S, Wechsler H, Rigotti NA. Social smoking among US college students. Pediatrics. 2004;114:1028–1034. doi: 10.1542/peds.2003-0558-L. [DOI] [PubMed] [Google Scholar]
- Moritsugu KP. The 2006 report of the surgeon general: The health consequences of involuntary exposure to tobacco smoke. American Journal of Preventive Medicine. 2007;32:542–543. doi: 10.1016/j.amepre.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Orleans TC. Helping young adult smokers quit: The time is now. American Journal of Public Health. 2007;97:1353. [Google Scholar]
- Pastor P, Medley F, Murphy TV. Invasive pneumococcal disease in Dallas County, Texas: Results from population-based surveillance in 1995. Clinical Infectious Diseases. 1998;26:590–595. doi: 10.1086/514589. [DOI] [PubMed] [Google Scholar]
- Perry CL, Williams CL, Komro KA, Veblen-Mortenson S, Stigler MH, Munson KA, et al. Project Northland: Long-term outcomes of community action to reduce adolescent alcohol use. Health Education and Research. 2002;17:117–132. doi: 10.1093/her/17.1.117. [DOI] [PubMed] [Google Scholar]
- Peters JM, Ferris BG. Smoking, pulmonary function and respiratory symptoms in a college-age group. American Review of Respiratory Disease. 1967;95:774–782. doi: 10.1164/arrd.1967.95.5.774. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Warneke C, de Moor C, Emmons KM, Mullin Jones M, Rosenblum C, et al. Self-reported health status, health vulnerability, and smoking behavior in college students: Implications for intervention. Nicotine & Tobacco Research. 2003;5:545–552. doi: 10.1080/1462220031000118649. [DOI] [PubMed] [Google Scholar]
- Riedel BW, Robinson LA, Klesges RC, McLain-Allen B. What motivates adolescent smokers to make a quit attempt? Drug and Alcohol Dependence. 2002;68:167–174. doi: 10.1016/s0376-8716(02)00191-6. [DOI] [PubMed] [Google Scholar]
- Romer D, Jamieson P. Do adolescents appreciate the risks of smoking? Evidence from a national survey. Journal of Adolescent Health. 2001;29:12–21. doi: 10.1016/s1054-139x(01)00209-9. [DOI] [PubMed] [Google Scholar]
- Rose JS, Chassin L, Presson CC, Sherman SJ. Prospective predictors of quit attempts and smoking cessation in young adults. Health Psychology. 1996;15:261–268. doi: 10.1037//0278-6133.15.4.261. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Education Quarterly. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Rush D. Respiratory symptoms in a group of American secondary school students: The overwhelming association with cigarette smoking. International Journal of Epidemiology. 1974;3:153–165. doi: 10.1093/ije/3.2.153. [DOI] [PubMed] [Google Scholar]
- Seely JE, Zuskin E, Bouhuys A. Cigarette smoking: Objective evidence for lung damage in teenagers. Science. 1971;172:741–743. doi: 10.1126/science.172.3984.741. [DOI] [PubMed] [Google Scholar]
- Slovic P, Finucane ML, Peters E, MacGregor DG. Risk as analysis and risk as feelings: Some thoughts about affect, reason, risk, and rationality. Risk Annals. 2004;24:311–322. doi: 10.1111/j.0272-4332.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Preventing tobacco use among young people: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1994. [Google Scholar]
- Woolcock AJ, Peat JK, Leeder SR, Blackburn CRB. The development of lung function in Sydney children: Effects of respiratory illness and smoking. A ten year study. European Journal of Respiratory Diseases. 1984;65(Suppl.):1–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.