Abstract
Objectives: To determine the effectiveness of eccentric exercise (EE) programmes in the treatment of common tendinopathies.
Data sources: Relevant randomised controlled trials (RCTs) were sourced using the OVID website databases: MEDLINE (1966–Jan 2006), CINAHL (1982–Jan 2006), AMED (1985–Jan 2006), EMBASE (1988–Jan 2006), and all EBM reviews – Cochrane DSR, ACP Journal Club, DARE, and CCTR (Jan 2006). The Physiotherapy Evidence Database (PEDro) was also searched using the keyword: eccentric.
Review methods: The PEDro and van Tulder scales were employed to assess methodological quality. Levels of evidence were then obtained according to predefined thresholds: Strong–consistent findings among multiple high‐quality RCTs. Moderate–consistent findings among multiple low‐quality RCTs and/or clinically controlled trials (CCTs) and/or one high‐quality RCT. Limited–one low‐quality RCT and/or CCT. Conflicting–inconsistent findings among multiple trials (RCTs and/or CCTs). No evidence–no RCTs or CCTs.
Results: Twenty relevant studies were sourced, 11 of which met the inclusion criteria. These included studies of Achilles tendinopathy (AT), patella tendinopathy (PT) and tendinopathy of the common wrist extensor tendon of the lateral elbow (LET). Limited levels of evidence exist to suggest that EE has a positive effect on clinical outcomes such as pain, function and patient satisfaction/return to work when compared to various control interventions such as concentric exercise (CE), stretching, splinting, frictions and ultrasound. Levels of evidence were found to be variable across the tendinopathies investigated.
Conclusions: This review demonstrates the dearth of high‐quality research in support of the clinical effectiveness of EE over other treatments in the management of tendinopathies. Further adequately powered studies that include appropriate randomisation procedures, standardised outcome measures and long‐term follow‐up are required.
Keywords: tendinopathy, eccentric, systematic review
Tendinopathy is the preferred term used to describe various tendon pathologies, including paratendinitis, tendinitis and tendinosis in the absence of biopsy‐proven histopathologic evidence.1 Tendinopathy of the Achilles tendon (AT) alone has been reported to constitute 7–9% of total injuries in top‐level runners.2 Other tendinopathies are also prevalent:1–2% of the general population have been reported as experiencing tendinopathy in the common wrist extensor origin of the lateral elbow (LET),3 and 20% of all knee injuries (n = 266) assessed in a sports clinic setting over six months were diagnosed as patella tendinopathy (PT).4 Other common sites of tendinopathy include the proximal hamstring insertion, the rotator cuff tendons, and the wrist flexor tendon insertion at the medial elbow.5 Considering the prevalence of these tendinopathies, the determination of modalities effective in treating tendon pathology remains important.3,6
Various modalities have been recommended as appropriate treatment options for tendinopathy, depending upon the phase of presentation. In the acute phase of treatment, reduction of risk factors such as training errors,6 flexibility issues6,7,8,9,10 and biomechanical abnormalities,6,8,11along with symptom reduction using relative rest,9,10,12 ice,7,8,10 and physical modalities such as ultrasound and laser have been suggested.6,7,8,9,10 In chronic, long‐standing cases, a complete rehabilitation programme incorporating strengthening,6,7,9,11,12,13,14,15,16,17,18,19 flexibility,6 proprioception,6 massage9,11,12 and endurance6 has been recommended. Eccentric exercise (EE) strengthening programmes have been emphasised recently as a key element of strength training in rehabilitation,6,7,8,9,11,12,13,15,17,18,19,20,21,22,23,24 in part due to literature supporting their use in the treatment of AT.25,26,27 In more recent, non‐systematic reviews, EE has been recommended as a treatment modality for other tendinopathies such as PT and LET.6,19,28
It has been proposed that EE may counteract the failed healing response which apparently underlies tendinopathy, by promoting collagen fibre cross‐linkage formation within the tendon, thereby facilitating tendon remodelling.19,21 However, as the basic pathophysiology of tendinopathy is still poorly understood, the mechanisms by which EE may help resolve tendinopathy remain hard to determine. Beyond this, it is essential that the clinical effectiveness of physical modalities such as EE is established as a matter of priority.
To date no systematic review has investigated EE and its effectiveness in tendinopathy rehabilitation. Therefore, this systematic review was undertaken to evaluate the current evidence for the effectiveness of EE programmes in the treatment of common tendinopathies.
Methodology
Aim
The aim of this systematic review was to evaluate the evidence for the effectiveness of EE programmes in the treatment of common tendinopathies.
Search strategy
The following databases were searched using the OVID website:29 MEDLINE (1966–Jan 2006), CINAHL (1982–Jan 2006), AMED (1985–Jan 2006), EMBASE (1988–Jan 2006), and all EBM reviews – Cochrane DSR, ACP Journal Club, DARE, and CCTR (Jan 2006). A defined search strategy was implemented incorporating the first two phases of a highly sensitive search strategy for OVID MEDLINE.30 Keywords used in the initial phase of the search included: tend*, eccentric*, Achill*, patell*, epicondyl*, tennis elbow, and rotator cuff (table 1). The Physiotherapy Evidence Database (PEDro)31 was also searched using the keyword: eccentric; and a hand search of three prominent sports medicine journals was undertaken: the British Journal of Sports Medicine (1995–Jan 2006), the American Journal of Sports Medicine (1995–Jan 2006) and the online resource of the Scandinavian Journal of Medicine and Science in Sports (2000–Jan 2006).
Table 1 Predefined search strategy used for OVID databases.
| Phase 1 | Phase 2 | Phase 3 |
|---|---|---|
| 1. Tend$.mp. | 15. randomized controlled trial.pt. | 24. clinical trial.pt. |
| 2. Soft Tissue injuries/ | 16. controlled clinical trial.pt. | 25. exp Clinical Trials/ |
| 3. Tendon Injuries/ | 17. Randomized Controlled Trials/ | 26. (clinic adj25 trial$).tw. |
| 4. Achill$.mp. | 18. Random Allocation/ | 27. ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw. |
| 5. Patell$.mp. | 19. Double‐Blind Method/ | 28. Placebos/ |
| 6. epicondyl$.mp. | 20. Single‐Blind Method/ | 29. placebo$.tw. |
| 7. tennis elbow.mp. | 21. or/15–20 | 30. random$.tw. |
| 8. rotator cuff.mp. | 22. Animal/ not Human/ | 31. Research Design/ |
| 9. (jumper$ adj knee).mp. | 23. 21 not 22 | 32. (latin adj square).tw. |
| 10. or/1–9 | 33. or/24–32 | |
| 11. exercise programme.mp | 34. 33 not 22 | |
| 12. eccentric$.mp. | 35. 34 not 23 | |
| 13. or/11–12 | 36. and/14,23 | |
| 14. and/10,13 | 37. and/14,35 | |
| 38. or/36–37 |
Inclusion and exclusion criteria
Randomised controlled trials investigating the use of EE to treat tendinopathy of the Achilles tendon, patella tendon, common wrist extensor tendon origin, or rotator cuff tendons were included. Other less common tendinopathies fell outside the scope of this review. Studies where at least one treatment group received an EE programme as the mainstay of treatment were included. Study participants must have been diagnosed clinically with tendinopathy of the studied tendon. Both mid‐portion and insertional tendinopathies were included.
There were no restrictions placed on age, gender or activity level of the study participants, and non‐English studies were eligible for inclusion. Co‐interventions were allowed alongside EE in the intervention group as long as they were standardised. Studies that included participants who had previously ruptured, or had undergone surgery of, the involved tendon were excluded as were studies that compared two different types of EE programmes without a control group.
Outcome measures
The clinical outcome measures of interest in this review were pain, function and patient satisfaction/return to activity.
Pain has been defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage and described in terms of such damage”.32 All pain scales were included in the review, but with conclusions based on the reliability and validity of pain scale used. A recent review of pain measurement methods recommended the visual analogue scale (VAS)33 and the McGill Pain Questionnaire34 as the most reliable and sensitive tools for pain measurement.35
Function may be defined as an activity that is natural to, or the purpose of, a person or thing.36 Functional assessment has also been defined as “any systematic attempt to measure objectively the level at which a person is functioning, in any of a variety of areas such as physical health, quality of self‐maintenance, quality of role activity, intellectual status, social activity, attitude towards the world and self, and emotional status”.37
Therefore, outcomes that objectively measured any part of function were included in the review: e.g. strength, or range of movement.
The third outcome measure of interest was patient satisfaction/return to sport/return to activity, which are now accepted widely as a necessary part of outcome assessment;38 for the purposes of this review these were analysed together as one dichotomous outcome.
Studies that reported at least one of these three clinically orientated outcomes were included in the systematic review.
Quality assessment
The PEDro31 and van Tulder39 scales were used to assess methodological quality.
The PEDro scale is based on the Delphi list,40 and its reliability has been reported as being “fair” to “good” in a recent assessment;41 Maher et al (2003) therefore concluded that the PEDro scale had sufficient reliability for its use in systematic reviews of physiotherapy randomised controlled trials (RCTs). The PEDro scale consists of 11 criteria, of which the first is not included in the final internal validity score. The answer to each criterion is a simple yes/no and the score is expressed as a mark out of 10. To achieve a ‘yes', it must be explicitly clear upon reading the article that the criterion has been satisfied.
The van Tulder scale is the methodological quality scale utilised in the updated guidelines for systematic reviews of the Cochrane Collaboration back review group.39 The internal validity portion of this scale consists of 11 criteria and the answer to each may be yes/no/don't know. In the case of a ‘don't know', the authors were contacted to help clarify the answer. If there was no reply or it remained unclear, the answer stayed as a ‘don't' know'.
Three assessors (BW, DB and RNW) independently reviewed the included articles and a consensus was reached to determine the final quality scores. The PEDro scores for articles found in the PEDro database were subsequently compared to those available on the website.
Methodological scores were calculated for each study and the two scales were then compared to determine whether these were consistent measures of quality. The studies were then rated as high or low quality based on definitions used in a previous systematic review using the van Tulder scale.42 A high‐quality study was defined as satisfying six or more of the 11 quality criteria in the van Tulder scale, and six or more of the 10 criteria in the PEDro scale. Studies that did not meet this level were rated as low quality.
Levels of evidence were then determined using the following criteria:39Strong–consistent findings among multiple high‐quality RCTs. Moderate–consistent findings among multiple low‐quality RCTs and/or clinically controlled trials (CCTs) and/or one high‐quality RCT. Limited–one low‐quality RCT and/or CCT. Conflicting–inconsistent findings among multiple trials (RCTs and/or CCTs). No evidence from trials–no RCTs or CCTs.
Data management and statistical analysis
Where possible, the mean differences between pre‐treatment scores and post‐treatment scores were calculated for continuous data sets: a standard deviation was then obtained for the mean differences assuming a covariance of zero. For dichotomous data, the numbers of events in each group were extracted along with the groups' sample sizes. If a study reported data that were not adequate for inclusion in the analysis, all efforts were made to obtain it from the relevant author. Values were then entered for analysis into Review Manager 4.2.8 software,43 which is commonly used for meta‐analysis of data in Cochrane Collaboration systematic reviews.
The results are expressed as a weighted mean difference (WMD) or relative risk (RR) and 95% confidence intervals (CI), depending on the type of data entered, and are based on the random effects model. The statistical significance level was set at p = 0.05 for all results. Sample size calculations were obtained using an online calculator,44 in collaboration with a biostatistician.
Results
Selection of studies
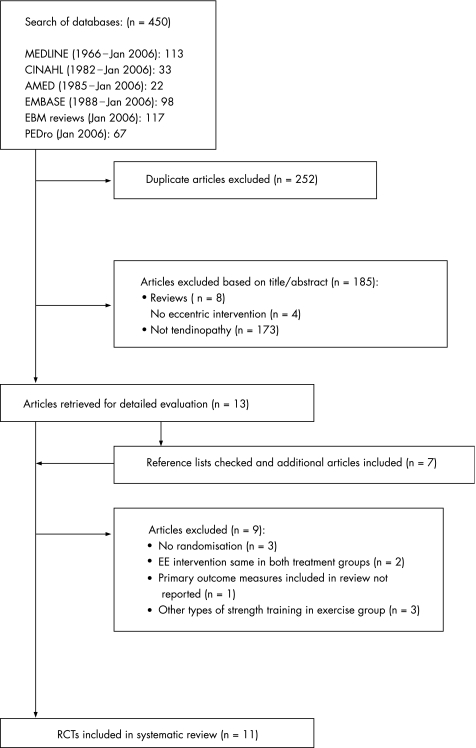
The initial search resulted in 450 titles from the included databases (fig 1); of these, 252 were discarded as duplicate references, leaving a total of 198. The hand search of three sports journals resulted in no further inclusions. Of these 198 articles, 185 were excluded based on the title and/or abstract: eight were reviews, four did not have EE as a treatment group and 173 did not investigate tendinopathy, leaving 13 potential articles.27,45,46,47,48,49,50,51,52,53,54,55,56 The articles' reference lists were then checked for additional studies and a further seven potential articles were found.57,58,59,60,61,62,63 At this stage a second eligibility screening was done on the 20 articles and a further nine were excluded: three were not randomised,47,48,64 two included treatment and control groups that both received EE programmes,49,56 one did not report any of the included primary outcome measures adequately58 and three investigated strength programmes other that eccentric training.58,60,61,62 Thus, a total of 11 articles were included in the current review.
Figure 1 Study selection process. Flow‐diagram based on the QUOROM guidelines.65 RCT, randomised controlled trial; EE, eccentric exercise.
Description of studies
The characteristics of each included study can be found in table 2. In total, 443 tendons from 250 males and 172 females were included in the 11 trials. Seven studies reported drop outs,45,46,50,52,54,55,63 with a mean percentage drop out rate of 12.5% (range 6.5–17.4%). All studies gave descriptive statistics on age of subjects with an overall mean of 36.5 years. The duration of symptoms were reported in nine studies with a mean of 19.7 months. Four studies investigated subjects diagnosed with AT,27,50,52,59 four investigated subjects with PT45,53,55,57 and the remaining three investigated subjects with LET.46,54,63 Randomised studies investigating the effect of EE on rotator cuff or other less common tendinopathies were not found.
Table 2 Characteristics of included studies.
| Study ID | Type of study | Participants' characteristics | Interventions | Outcomes |
|---|---|---|---|---|
| Cannell 200157 | RCT | Participants: 19 subjects involved in various sports. | Ice, AIs and rest for the first two weeks of the study; then 12‐week intervention. | Length of follow‐up: no follow‐up past intervention |
| Method of randomisation: sealed envelope draw. | EE = 10: 7 male, 3 female, Age = 26 (23–29) years, DOS = 3.1 (1.6–4.6) mths. | EE group: 3 sets of 20 drop‐squats 1/day, 5 days/week. Load increase over 4 levels and dependent on body weight. Activity level could increase over this period. | Outcomes assessed: | |
| 2 groups: drop‐squats (EE) or leg extension/curl exercises (CE). | CE = 9: 6 male, 3 female, Age = 26 (19–33) years, DOS = 4.2 (2.3–6.1) mths. | CE group: 3 sets of 10 lifts of each exercise, 1/day, 5 days/week. Weights increased as per table over 4 levels. Activity increased over this period as able. | Pain: VAS | |
| Return to sport: reported at the 12‐week stage –y/n | ||||
| Muscle strength: quads and hamstrings moments of force 30°/sec on both legs. | ||||
| Jonsson 200545 | RCT | Participants: 15 patients active in various sports. | 12‐week intervention. | Length of follow‐up: 32.6 months. |
| Method of randomisation: not stated. | EE = 10: 7 male, 1 female. Age = 25.7±9.9 years, DOS = 15.4±6 mths. | No sports‐specific training for 6 weeks. Given by same physiotherapist. Both groups performed exercise on decline board. Training was meant to be painful. Load increased to attain this. | Outcomes assessed: | |
| 2 groups: EE and CE. | CE = 9: 6 male, 1 female, Age = 24.1±6.1 years, DOS = 19.6±20.3 mths. | EE group: exercises done 2 times a day, 7 days/week. 3×15 reps. Concentric activity done by uninjured leg. | Pain: VAS | |
| CE group: a/a, eccentric activity avoided as much as possible. | Function: VISA score for knee function. | |||
| Patient satisfaction: satisfied/not satisfied. | ||||
| Mafi 200127 | RCT | Participants: 44 patients referred as potential surgical candidates: 24 male, 20 female. | 12‐week intervention. | Length of follow‐up: no follow‐up past intervention. |
| Method of randomisation: envelope. | EE = 22, Age = 48.1±9.5 years, DOS = 18 (3–120) mths. | EE group: exercises done 2 times a day, 7 days/week. Two exercises used short and long calf muscle loading. Each 3×15 reps. Concentric activity done by uninjured leg. Increased load when exercise became pain free. | Outcomes assessed: | |
| 2 groups: EE and CE. | CE = 22, Age = 48.4±8.3 years, DOS = 23 (5–120) mths. | CE group: various concentric exercises used, from calf raises to side jumps. | Pain: VAS | |
| Patient satisfaction: satisfied/not satisfied. | ||||
| Martinez‐Silvestrini 200546 | RCT | Participants: 94 subjects: 50 male, 44 female. | 6‐week intervention. | Length of follow‐up: no follow‐up past intervention. |
| Method of randomisation: not stated. | DOS: >3 mths. | Stretching group: 2 times/day 3 repetitions held for 30 secs, 30 sec rest between. | Outcomes assessed: | |
| 3 groups: stretching, EE + stretching, CE + stretching. | Age: | EE group: eccentric resistance band exercises, avoiding concentric activity. 3 sets of 10 reps 1 time/day 2, 5 minutes of rest between sets. | Grip strength: pain free. | |
| St = 43.1 years. | CE group: a/a but eccentric load avoided during exercise. | Patient‐rated forearm evaluation questionnaire. | ||
| EE + St = 46.6 years. | Advice on ice massage and strap use was also given to all patients. | DASH | ||
| CE + St = 47.0 years. | SF–36. | |||
| Pain: VAS | ||||
| Patient satisfaction: 5 point scale. | ||||
| Neisen‐Vertommen 199259 | RCT. | Participants: 17 non‐competitive recreational athletes | 12‐week intervention. | Length of follow‐up: no follow‐up past intervention. |
| Method of randomisation: not stated. | EE = 8 | 5 sets of 10 reps, in a pain free ROM, 1 time/day 6/week. | Outcomes assessed: | |
| 2 groups: EE and CE. | 4 male, Age = 39.5±3.2 years, DOS: 3.7±1.1 mths. | |||
| 4 female, Age = 31±2.6 years, DOS: 3.7±0.9 mths. | EE group: protocol outlined in another journal article, raised step exercise, eccentric only. | Concentric and eccentric plantarflexor average and peak torque, 30°/sec and 50°/sec. | ||
| CE = 9 | CE group: progressive concentric exercise programme on universal gym. | Pain: scale from 1–10. | ||
| 6 male, Age = 37.33±1.7 | Each group progressed weight as able. | |||
| 3 female, Age = 28.66±3.2 years. | Return to activity: scale from 1–10; 10 denoted full activity of pre‐injured level. | |||
| Roos 200450 | RCT. | Participants: 44: 21 male, 23 female. Age = mean 45 years. DOS: 5.5 (1–180) mths. | 12‐week intervention. | Length of follow‐up: 1 year. |
| Method of randomisation: envelope. | ||||
| EE = 16 | EE group: as described by Alfredson et al 1998. Straight and bend knee exercises. Day 1–2 1×15; day 3–4 2×15; day 5–7 3×15; then 3×15 from then on. Load added as tolerated. | Outcomes assessed: | ||
| 3 groups: EE, night splint or combination of both. | EE + Sp = 15 | Night splint group: anterior night splint, night‐time use only. | FAOS | |
| Sp = 13 | Combination group: a/a. | Return to sport: y/n. | ||
| Selvanetti 200363 | RCT | Participants: 60 patients. | 20–30 sessions. | Length of follow‐up: mean 11 months. |
| Method of randomisation: envelope numbered and sealed. | EE = 31: 17 males, 14 female, Age = 41.3 (33–54) years, DOS = 6.6 (2–10) mths. | EE group: 3 mins warm‐up, 4 PNF contract relax (10 sec contract, 2 sec rest, 30 sec stretch), 3 sets of 10 reps ecc. Exercises with theraband, 30 secs rest between sets, PNF stretches times 4 again then ice for 15 minutes. | Outcomes assessed: | |
| 2 groups: sham ultrasound and counselling, and EE, contract‐relax stretching and counselling. | US = 29: 15 males, 14 female, Age = 40.5 (32–52) years, DOS = 6.8 (3–11) mths. | US group: placebo US (20 sessions, 5/week). | Pain: VAS scale, but 0 = severe, 10 = no pain. | |
| Patient satisfaction: subjective general enhancement; 0–100% | ||||
| Silbernagel 200152 | RCT. | Participants: 40 (57 involved tendons) patients. | 12‐week intervention. | Length of follow‐up: 6 months. |
| EE = 30 tendons, 17 male, 5 female, Age = 47±14.7 years, DOS = 20±25.4 mths. | One‐year follow‐up for summary of questions to patient. | |||
| Method of randomisation: not stated. | EE group: extensive exercise programme split into 3 phases, including ROM exercises, concentric exercises and eccentric exercises. Pain allowed to reach 5 on VAS, no morning stiffness following, and decrease in VAS pain by morning. | |||
| 2 groups: EE and CE. | CE = 27 tendons, 14 male, 4 female, Age = 41±10.2 years, DOS = 41±55.9 mths. | CE group: a/a minus the eccentric exercises. Frequency of all exercises in all groups varied from week to week. | Outcomes assessed: | |
| Pain: VAS | ||||
| Function: plantarflexion, jumping test, toe raising test. | ||||
| Patient satisfaction: y/n. | ||||
| Stasinopoulos 200453 | RCT. | Participants: 30 patients, DOS = minimum 3 mths. | 4‐week intervention. | Length of follow‐up: 3 months. |
| Method of randomisation: drawing lots. | EE = 10: 7 male, 3 female, Age = 28.12±2.03 years. | All patients received 3 treatments per week. | Outcomes assessed: | |
| 3 groups: EE, pulsed US, and frictions (F). | US = 10: 6 males, 4 female, Age = 29.17±3.76 years. | EE group: static stretching exercises, 3 sets of 15 unilateral eccentric squats, load increased as able, 2 minute rest between sets. | Pain status: worse, no change, somewhat better, much better, no pain. | |
| F = 10: 5 male, 5 female, Age = 26.24±4.17 years. | US group: local pulsed US 0.4–0.8 W/cm2 ratio1:4, 2 ms pulse duration, frequency 1 MHz. ‐10 minutes. | |||
| Friction group: Cyriax and Cyriax technique for 10 minutes. | ||||
| Svernlov 200154 | RCT. | Participants: pilot study: 30 patients. | 12‐week intervention. | Length of follow‐up: 12 months for pilot, after 3 months training in clinical study. |
| Method of randomisation: not stated. | EE = 15: 13 male, 2 female, Age = 42.1 years, DOS = 10.7 (3–24) mths. | EE group: warm‐up ex. 2–3 mins, static stretch 3–5 times (15–30 secs), eccentric exercises, 3 sets of 5 with dumb‐bell 10 sec duration, static stretch as before, performed 1 time/day. | Outcomes assessed: | |
| 2 groups: EE and St. Both with use of brace. | St = 15: 6 male, 9 female, Age = 43 years, DOS = 8.4 (3–20) mths. | Stretching group: 10 secs of contractions of muscle, relaxation 2 secs, stretching 15–20 secs, repeated 3–5 times twice daily. | Pain: VAS | |
| Strength testing: using strain gauge device. | ||||
| Patient satisfaction: y/n. | ||||
| Visnes 200555 | RCT. | Participants: 29 male and female elite volleyball players (12 with bilateral symptoms) in Norway. | 12‐week intervention. | Length of Follow‐up: 6 months after end of intervention. |
| Method of randomisation: by statistician who was blinded to player identity. | EE = 13: 8 male, 5 female, Age = 26.8±4.6 years, DOS = 67±44 mths | EE group: twice daily, 3 sets of 15 reps, done without warming up. Decline squat exercise, eccentric loading only on affected leg, recommended to have 5/10 pain upon exercising. Load was increased as pain decreased. | Outcomes assessed: | |
| 2 groups: EE and control. | C = 16: 11 male, 5 female, Age = 26.4±3.4 years, DOS = 79±75 mths. | Control group: no intervention, trained as usual. | Function: VISA scores for knee function. | |
| Global evaluation score (pain and function) and jumping performance. |
AI, anti‐inflammatories; a/a, as above; C, control; CE, concentric exercise; DASH, disabilities of the arm, shoulder and hand; DOS, duration of symptoms; EE, eccentric exercise; F, frictions; FAOS, foot and ankle outcome score; RCT, randomised controlled trial; Sp, splint; St, stretching; US, ultrasound; VAS, visual analogue scale; VISA, Victorian Institute of Sport Assessment.
Studies included interventions of EE programmes of varying lengths: a 12‐week exercise programme was undertaken in eight studies,27,45,50,52,54,55,57,59 while two other studies implemented a four‐week programme53,63 and one study implemented a six‐week programme.46 Comparison groups included concentric exercise (CE) programmes,27,45,46,52,57,59 stretching,46,54 ultrasound,53,63 frictions,53 splints,50 or normal training.55 The length of follow‐up varied from six weeks46 to one year.50,52,54 The most common follow‐up time point was immediately after the conclusion of treatment at 12 weeks.27,45,55,57,59
Various outcome measures were used in the included studies. The most common was patient satisfaction or return to activity outcomes, with seven of the 11 studies reporting this outcome.27,45,52,54,57,59,63 Six studies used a pain VAS as their pain outcome measure;27,45,46,52,54,57 however, three of these studies27,54,57 failed to report the VAS data in sufficient detail to be used in statistical analysis. Other studies utilised either a ten‐point numerical rating scale59 or a five‐point ordinal rating scale.53 Functional outcome measurement varied significantly amongst the trials with no consistency between studies: measures included Victorian Institute of Sport Assessment (VISA)66,67 scores,45,55 muscle strength,54 disabilities of the arm, shoulder and hand (DASH)68 scores,46 Foot and Ankle Outcome (FAOS)69 scores,50 Ko70 scores,63 and peak muscle torque values.57
Methodological quality
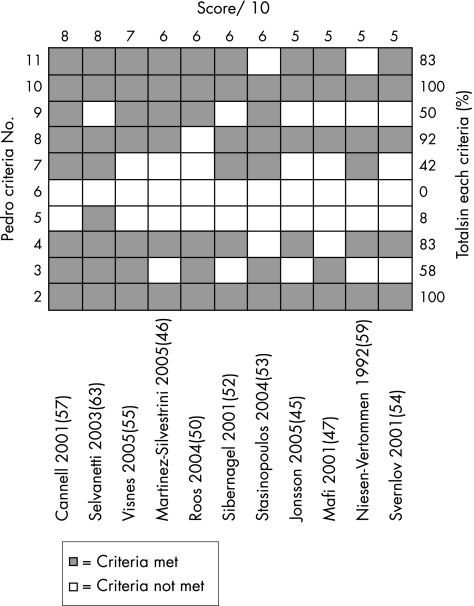
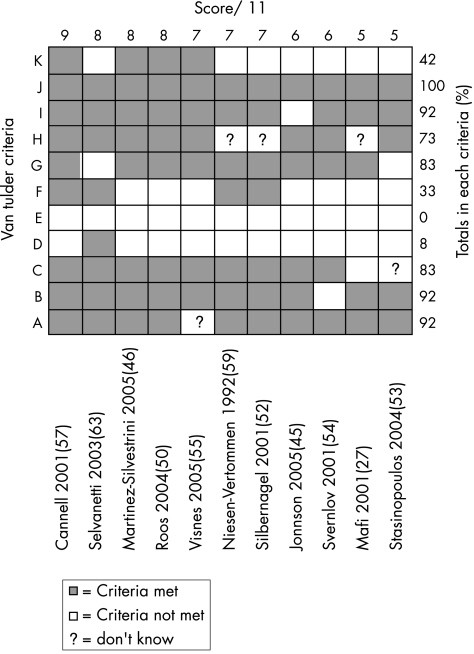
The internal validity scores for the included studies are shown in figs 2 and 3. The median score for methodological quality of the included studies was 7 out of 11 for the van Tulder score (range of 5 to 9), and 6 out of 10 for the PEDro score (range of 5 to 8). Using the cut‐off point of 6 in both scales for high‐quality studies, six studies attained a high‐quality rating.46,50,52,55,57,63
Figure 2 PEDro31 scores for included studies. Studies are ordered by PEDro score. Criteria 1 omitted as not included in internal validity scores. ▪ = criteria met; □ = criteria not met.
Figure 3 van Tulder39 scores for included studies. Studies are ordered by van Tulder score. ▪ = criteria met; □ = criteria not met; ? = don't know.
The PEDro scale results showed that in all studies subjects were randomly allocated to groups (criteria 2), and between group statistical comparisons were reported for at least one key outcome (criteria 10). The van Tulder scale scores showed the timing of outcome assessment was identical for all intervention groups for all important outcomes (criteria J), and high levels of allocation concealment (criteria B) and drop‐out rate description (criteria I) were achieved throughout the studies (92%).
The most common methodological failings of the studies under the van Tulder rating were inadequate therapist (100%), subject (92%) and assessor (67%) blinding, and a lack of intention‐to‐treat analysis (58%). The PEDro scale showed a similar pattern with inadequate therapist (100%), subject (92%) and assessor (58%) blinding, and a lack of intention‐to‐treat analysis (50%) being commonly reported problems.
The PEDro values were compared to the online scores available and were found to fall within one point of each other. The quality rating of only one study would have changed from high to low quality if online scores were used.52 Upon further deliberation it was decided that the score for this study should remain at 6/10 as we felt the measurement of at least one key outcome was obtained from more than 85% of the subjects initially allocated to groups.
Outcome measures
Summary statistics for the included studies can be found in table 3. Comparisons between tendinopathies for the outcome measures of pain and satisfaction/return to activity are shown in figs 4 and 5.
Table 3 Results of included studies for clinical outcome measures. Incomplete data were not included.
| Outcome measure | Study ID | Dx | Intervention | Wk | WMD (95% CI) | RR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| Pain | |||||||
| Pain VAS (100 mm) | Silbernagel 200152 | AT | EE vs CE | 6 | 2.00 (−18.90, 22.90) | 0.85 | |
| 12 | 18.00 (−3.68, 39.68) | 0.10 | |||||
| 26 | 10.00 (−8.81, 28.81) | 0.30 | |||||
| Jonsson 200545 | PT | EE vs CE | 12 | 44.80 (20.09, 69.51) | 0.00004 | ||
| Martinez‐Silvestrini 200546 | LET | EE+St vs CE+St | 6 | 8.00 (−8.02, 24.02) | 0.33 | ||
| EE+St vs St | |||||||
| 6 | −1.00 (−16.46, 14.46) | 0.90 | |||||
| Decrease in pain (Yes/No)* | Stasinopoulos 200453 | PT | EE vs US | 4 | 8.00 (1.21, 52.69) | 0.03 | |
| 8 | 21.00 (1.40, 315.98) | 0.03 | |||||
| 16 | 21.00 (1.40, 315.98) | 0.03 | |||||
| EE vs F | 4 | 4.00 (1.11, 14.35) | 0.03 | ||||
| 8 | 5.00 (1.45, 17.27) | 0.01 | |||||
| 16 | 5.00 (1.45, 17.27) | 0.01 | |||||
| FAOS Pain Score (/100) | Roos 200450 | AT | EE vs EE+Sp | 12 | 1.40 (−19.16, 21.96) | 0.89 | |
| 52 | 4.00 (−15.01, 23.01) | 0.68 | |||||
| EE vs Sp | 12 | 14.00 (−6.56, 37.94) | 0.18 | ||||
| 52 | 4.00 (−14.11, 22.11) | 0.67 | |||||
| Function | |||||||
| FAOS Sport/Rec score (/100) | Roos 200450 | AT | EE vs EE+Sp | 12 | 17.00 (−8.87, 42.87) | 0.20 | |
| 52 | 10.00 (−17.94, 37.94) | 0.48 | |||||
| EE vs Sp | 12 | 20.00 (−8.87, 45.87) | 0.13 | ||||
| 52 | 8.00 (−17.89, 33.89) | 0.54 | |||||
| Plantarflexion (°) | Silbernagel 200152 | AT | EE vs CE | 12 | 2.00 (3.36, 7.36) | 0.46 | |
| Jumping test (cm) | Silbernagel 200152 | AT | EE vs CE | 12 | 0.00 (−4.89, 4.89) | 1.00 | |
| Toe raise Test (n) | Silbernagel 200152 | AT | EE vs CE | 12 | −2.00 (−14.55, 10.55) | 0.75 | |
| VISA scores (/100) | Jonsson 200545 | PT | EE vs CE | 12 | 45.90 (24.54, 67.26) | <0.0001 | |
| Visnes 200555 | PT | EE vs C | 12 | 0.10 (−14.38, 14.58) | 0.99 | ||
| Hamstring moment (Nm) | Cannell 200157 | PT | EE vs CE | 12 | 5.00 (−133.98, 143.98) | 0.94 | |
| Quadriceps moment (Nm) | Cannell 200157 | PT | EE vs CE | 12 | 106.00 (−73.74, 285.74) | 0.25 | |
| DASH (/100) | Martinez‐Silvestrini 200546 | LET | EE vs CE | 6 | 0.00 (−9.76, 9.76) | 1.00 | |
| EE+St vs St | 6 | −3.00 (−12.71, 6.71) | 0.54 | ||||
| Ko scores (/100) | Selvanetti 200363 | LET | EE vs US | 4 | 38.70 (29.75, 47.65) | <0.00001 | |
| 48 | 39.20 (30.32, 48.08) | <0.00001 | |||||
| Grip strength (mmHg) | Martinez‐Silvestrini 200546 | LET | EE vs CE | 6 | −3.00 (−13.10, 7.10) | 0.56 | |
| EE+St vs St | 6 | −4.00 (−12.50, 4.50) | 0.36 | ||||
| Satisfaction/ Return to activity | |||||||
| Neisen‐Vertommen 199259 | AT | EE vs CE | 12 | 4.50 (0.63, 32.38) | 0.14 | ||
| Mafi 200127 | AT | EE vs CE | 12 | 2.25 (1.25, 4.05) | 0.007 | ||
| Silbernagel 200152 | AT | EE vs CE | >26 | 1.56 (0.73, 3.32) | 0.25 | ||
| Cannell 200157 | PT | EE vs CE | 12 | 1.35 (0.81, 2.24) | 0.25 | ||
| Jonsson 200545 | PT | EE vs CE | 12 | 17.27 (1.15, 260.07) | 0.04 | ||
| >26 | 17.27 (1.15, 260.07) | 0.04 | |||||
| Svernlov 200154 | LET | EE vs St | >26 | 1.06 (0.81, 1.39) | 0.68 | ||
| Selvanetti 200363 | LET | EE vs US | >26 | 21.97 (3.17, 152.20) | 0.002 |
AT, Achilles tendinopathy; C, control; CE, concentric exercise; CI, confidence interval; DASH, disabilities of the arm, shoulder and hand; DOS, duration of symptoms; Dx, diagnosis; F, frictions; FAOS, foot and ankle outcome score; EE, eccentric exercise; LET, lateral elbow tendinopathy; p, statistical significance; PT, patellar tendinopathy; RCT, randomised controlled trial; RR, relative risk; S, splint; St, stretching; US, ultrasound; VAS, visual analogue scale; VISA, Victorian Institute of Sport Assessment; WMD, weighted mean difference; Wk, week of data collection.
*Yes, much better /no pain; No, worse/no change/slightly better
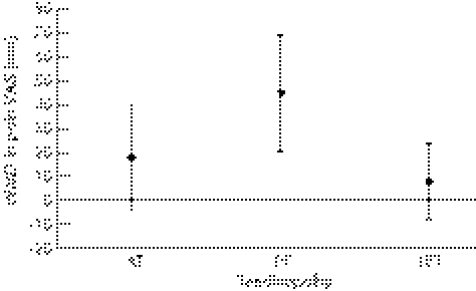
Figure 4 Summary of findings. Pain VAS changes in the treatment of three tendinopathies, EE compared to CE (WMD±95% CI are shown). AT, Achilles tendinopathy; CE, concentric exercise; CI, confidence interval; EE, eccentric exercise; LET, lateral elbow tendinopathy; mm, millimetres; PT, patellar tendinopathy; VAS, visual analogue scale; WMD, weighted mean difference.
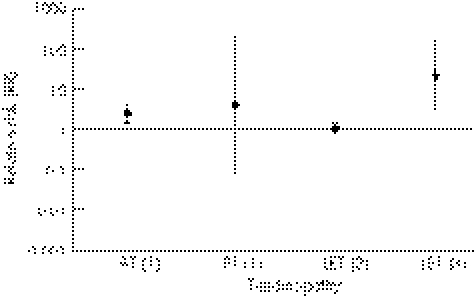
Figure 5 Summary of findings. RR of Satisfaction/Return to Activity in EE groups compared to controls (±95% CI): (1) eccentric vs concentric ‐12 weeks; (2) eccentric vs st. >6 months; (3) eccentric vs US >6 months. AT, Achilles tendinopathy; CI, confidence interval; EE, eccentric exercise; LET, lateral elbow tendinopathy; mm, millimetres; PT, patellar tendinopathy; RR, relative risk; st, stretching; US, ultrasound; VAS, visual analogue scale.
Pain
Only three studies using the pain VAS as an outcome measure were able to be included in data analysis;45,46,52 no pooling of data was achievable due to the low number of studies.
Silbernagel et al52 found an 18 mm (95% CI −3.68 to 39.68) difference in VAS favouring EE to CE intervention at the 12‐week stage of rehabilitation in AT; however, this was not statistically significant (p = 0.10). In PT, Jonsson et al45 found a WMD of 44.8 mm (95% CI 20.09 to 69.51) between the eccentric and concentric exercise groups at 12 weeks. This result was statistically significant (p = 0.0004) but the study was found to be of low methodological quality. Martinez‐Silvestrini et al46 studied three different interventions for LET: eccentric, concentric and stretching groups. The WMD for pain VAS comparing eccentric to concentric was 8.00 (95% CI −8.02, to 24.02), and for eccentric compared to a stretching group was −1.00 (95% CI −16.46 to 14.46). Overall, this study showed no statistically significant differences between EE and any comparison group. Stasinopoulos et al53 investigated PT and used a scale dividing subjects into two groups, either ‘much better/no pain' or ‘worse/no change/slightly better';53 they found a RR of 21 (95% CI 1.40 to 315.98) at 16 weeks comparing EE to ultrasound, and a RR of 5 (95% CI 1.45 to 17.27) at 16 weeks comparing EE to frictions. Although these results were statistically significant in favour of EE (p = 0.03 and 0.01 respectively), the pain scale had not been validated and the study was of low methodological quality. Roos et al50 used the FAOS69 pain sub‐score to determine a change in pain in AT and found no statistically significant differences at 12 weeks comparing EE to a control group (WMD 1.40; 95% CI −19.16 to 21.96) or EE to a splint group (WMD 14.00; 95% CI −6.56 to 34.56).
In summary, only two of the 11 included studies, both investigating PT, reported statistically significant results using a validated outcome measure of pain.45,53 Due to the low methodological quality and heterogeneity of these two studies, only a limited level of evidence exists to suggest that EE is clinically effective in reducing pain in PT. Owing to small trial numbers and very large confidence intervals, there is no evidence available to suggest whether EE is effective in reducing pain in either AT or LET.
Function
Various measures of functional outcome were used to determine whether EE was effective in increasing function in tendinopathy, and due to this variation, pooling of data was not possible.
In AT, Roos et al50 reported function at 12 weeks using the FAOS subscale of sport and recreation. The study compared EE to a control group (WMD 17.00; 95% CI −8.87 to 42.87) and EE to a splint group (WMD 20.00; 95% CI −5.87 to 45.87); both of these differences were not statistically significant. Other outcomes, including plantarflexion range of motion (WMD 2.00; 95% CI −3.36 to 7.36), a jumping test (WMD 0.00; 95% CI −4.89 to 4.89), and a toe raise test (WMD −2.00; 95% CI, −14.55 to 10.55), also failed to show statistically significant differences between EE and comparison groups.52
Two studies examined function in PT using the VISA66,67 scale, a functional pain‐rating scale specifically designed for AT and PT, and found quite different results.45,55 Jonsson et al45 showed that EE intervention increased VISA scores significantly compared to a CE programme (WMD 45.90; 95% CI 24.54 to 67.26); in contrast, Visnes et al55 found a WMD of 0.10 (95% CI −14.38 to 14.58) comparing EE to a control of normal training in elite volleyball players. Cannell et al57 also investigated PT, and measured hamstring and quadriceps moments as a functional outcome. No statistically significant difference was found between the two intervention groups for either hamstrings moment (WMD 5.00; 95% CI −133.98 to 143.98) (p = 0.94), or for quadriceps moment (WMD 106; 95% CI −73.74 to 285.74) (p = 0.25). Martinez‐Silvestrini et al46 used the DASH68 functional outcome measure for LET and found no significant difference in function comparing EE to CE (WMD 0.00; 95% CI −9.76 to 9.76) (p = 1.00), and comparing EE to stretching (WMD −3.00; 95% CI −12.71 to 6.71) (p = 0.54). The same study also assessed grip strength as a functional measure, but again there were no statistically significant results. Selvanetti et al63 also studied the common extensor tendon origin to determine the effect of EE compared to ultrasound, and found a significant difference in Ko70 scores at 4 weeks (WMD 38.70; 95% CI 29.75 to 47.65; p<0.00001) and at 11 months (WMD 39.20; 95% CI 30.32 to 48.08; p<0.00001). This scale was taken from a previous study that investigated another type of treatment for LET, but had not been validated.70
No firm conclusions can be derived from these studies regarding the effect of EE on function due to the variety of outcome measurement used and the small number of available studies. The only two statistically significant results reported were either from a study of low quality,45 or were obtained using a non‐validated functional outcome measure.63
Patient satisfaction/return to activity
Patient satisfaction/return to activity was significantly different for AT (p = 0.003) when 12‐week data were pooled from two studies (RR 2.38; 95% CI 1.36 to 4.18).27,59 These studies, however, were both of low methodological quality. In contrast, a significant risk ratio in favour of EE was not found in a high‐quality study measuring satisfaction after 12 months (RR 1.56; 95% CI 0.73 to 3.32) (p = 0.25).52 Pooling of data was completed for satisfaction/return to activity at the 12‐week stage of rehabilitation in PT for EE compared to CE (RR 4.17; 95% CI 0.08 to 206.41).45,57 This result was more pronounced and statistically significant at a follow‐up point of 32.6 months (mean) in another study (RR 17.27; 95% CI 1.15 to 260.07; p = 0.04), although this was of low quality.45 In LET, satisfaction/return to activity for EE intervention compared to ultrasound was statistically significant at six months post‐treatment (RR 21.97; 95% CI 3.17 to 152.20; p = 0.002),63 whereas the RR for EE compared to stretching at the same time point was 1.06 (95% CI 0.81 to 1.39).54
In summary, moderate evidence exists to suggest satisfaction/return to activity is more likely with 12 weeks of EE therapy compared to CE intervention in AT, but this is based on small study numbers. There is also moderate evidence to suggest that EE therapy is associated with increased satisfaction/return to activity at six months post‐treatment in LET when compared to ultrasound therapy. Only limited evidence exists to support the effectiveness of EE on satisfaction/return to activity in PT.
Discussion
This systematic review was undertaken with the aim of determining the effectiveness of EE in the treatment of various common tendinopathies. Eleven RCTs met the inclusion criteria; they included studies of AT, PT and LET. RCTs investigating the effect of EE on rotator cuff tendinopathy were not found using the defined search strategy. Since the time of data analysis, a small non‐randomised pilot study has been published investigating rotator cuff tendinopathy and the effect of EE therapy; however, it would not have satisfied the inclusion criteria used here, and would not, therefore, have affected the results of this review.71
Due to the lack of high‐quality studies with clinically significant results, no strong conclusions could be made regarding the effectiveness of EE (compared to control interventions) in relieving pain, improving function or achieving patient satisfaction. A limited level of evidence exists suggesting EE reduces pain in PT at the 12‐week stage of treatment when compared to CE. There is also limited evidence suggestive of an increase in function using EE compared to ultrasound in the treatment of LET, although the validity of the outcome measure used in this study is unclear.63 Patient satisfaction/return to activity results were more positive for EE, with moderate evidence suggesting EE intervention may increase patient satisfaction/return to activity compared to CE in AT, as well as to support EE compared to ultrasound in LET; limited evidence exists supporting EE compared to CE in PT. Another systematic review known to have investigated the effect of different physical interventions in the treatment of LET came to similar conclusions regarding the effectiveness of exercise therapy.72 This group found insufficient evidence to determine the effectiveness of exercise therapy in the treatment of LET but concluded that results from preliminary studies warranted further evaluation in this area. Other systematic reviews investigating exercise therapy in PT or AT were not found.
Overall, the methodological quality was considered high in only six studies of the 11 included in this review. It was assumed that the risk of misclassification was low, as reliable and valid measures of methodological quality were used.39,41 The most common deficits in methodology were the lack of blinding of subjects, assessors and therapists. The blinding of subjects and therapists will always remain difficult when implementing exercise therapy interventions in research.73 Liddle et al (2004) omitted the van Tulder item of ‘blinding of care provider' in a systematic review of exercise and chronic low back pain as they felt the item was inapplicable to exercise interventions. If this item was deleted in both methodological scores used in this review, quality ratings of all included studies would not have changed.
Treatment effects are found to be overestimated in low‐quality systematic reviews where non‐randomised studies such as prospective cohorts are included.74,75 This may be due to non‐randomisation and inadequate allocation concealment methods contained within these prospective studies.74 For this reason, prospective, non‐randomised studies were not included in this systematic review. However, many non‐randomised trials have been undertaken to investigate the effectiveness of EE in common tendinopathies.25,26,47,48,64,76,77 One non‐randomised prospective study investigating AT compared EE to a surgical intervention and found a larger, significant treatment effect on pain VAS than reported here (WMD 25.8; 95% CI 11.36 to 40.24).25 This result should be viewed with caution due to the low methodological quality of the study concerned.
Findings from this review are limited by the fact that included trials were based upon small sample sizes, and these numbers were often too small to reach adequate statistical power. Heterogeneity of the studies included in the review was considered another problem, with differences in study population, interventions, controls and outcome measures. Only a few studies reported similar outcomes, making the pooling of data impossible for the majority of outcome measures. The lack of long‐term follow‐up in research in this area is also an issue, as only three studies included a one‐year outcome measurement.50,52,54 This makes any potential longer‐term clinical benefit of EE hard to determine.
The treatment regime most commonly used in the included studies was derived from an initial study of AT.25 This comprised three sets of 15 repetitions, done twice daily, seven days a week for 12 weeks. Upon correspondence with one of the authors of this study, it was found that the regime was based on clinical experience, rather than derived from any empirical evidence; e.g. data from ‘dose response'‐type studies. However, the lack of understanding about the basic pathophysiology of tendinopathy makes determining the optimal dosage of intervention difficult. A recent review of EE in AT tried to address the issue of treatment dosage21 and concluded that because the studies in this area have not used an underlying rationale to determine loading parameters, progressions and frequency of treatment, further research needs to be undertaken before an optimal dosage can be determined.
In summary, there is a dearth of high‐quality research available to establish the effectiveness of EE therapy in the treatment of three common tendinopathies. Due to low sample numbers, large confidence intervals were present in many studies, making the majority of results inconclusive. Limited to moderate levels of evidence exist in a number of areas, thus warranting further research in this field. Although clinical benefits of EE could not be fully determined due to the lack of quality research with adequate follow‐up, the overall trend suggested a positive effect of EE, with no study reporting adverse effects. However, a recent study suggests that sedentary subjects with AT may show less promising results with EE therapy compared to athletic subjects.78 Further determination of variations between population sub‐groups such as these will also require high‐quality RCTs to be undertaken before any firm conclusions can be made.
Randomised controlled trials are commonly accepted as the ‘gold standard' way to investigate the effectiveness of a particular healthcare intervention.79 Thus, further RCTs in this area must be adequately powered and include appropriate randomisation procedures, standardised outcome measures and long‐term follow‐up. As a precursor to this, RCTs should be undertaken to determine the ‘dose‐response' effect of various EE programme durations and intensities. It is only then that the clinical benefit of EE in the treatment of tendinopathy may be fully elucidated compared to other treatment modalities. While clinicians may opt to continue to utilise EE programmes in the treatment of common tendinopathies, they should be aware of the lack of evidence for the superior effectiveness of this approach in comparison with other active modalities such as concentric exercise and stretching.
What is already known on this topic
Tendinopathy is a non‐inflammatory degenerative condition that may take 6–12 months to resolve with conservative intervention.
The pathogenesis of tendinopathy remains unclear.
EE programmes have been emphasised in recent literature as a key part of tendinopathy rehabilitation.
What this study adds
There is a lack of evidence supporting the effectiveness of EE programmes over other active modalities such as CE and stretching.
Further adequately powered RCTs with standardised outcome measures and long‐term follow‐up are required to ascertain the clinical benefits of EE programmes in the treatment of tendinopathy.
Acknowledgements
Dr Melanie Bell from the Department of Preventative and Social Medicine, University of Otago, Dunedin, New Zealand for statistical support, and the administration staff at the School of Physiotherapy, University of Otago, Dunedin, New Zealand.
Abbreviations
AT - achilles tendinopathy
CCT - clinically controlled trial
CE - concentric exercise
EE - eccentric exercise
FAOS - foot and ankle outcome score
LET - lateral elbow
PED - physiotherapy evidence database
PT - patella tendinopathy
RCT - randomised controlled trial
RR - relative risk
VAS - visual analogue scale WMD, weighted mean difference
Footnotes
Competing interests: None identified.
References
- 1.Maffulli N, Kahn K M, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy 199814840–843. [DOI] [PubMed] [Google Scholar]
- 2.Jarvinen T A H, Kannus P, Paavola M.et al Achilles tendon injuries. Cur Opin Rheumatol 200113150–155. [DOI] [PubMed] [Google Scholar]
- 3.Gabel G T. Acute and chronic tendinopathies at the elbow. Cur Opin Rheumatol 199911138–143. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Aho H, Jarvinen M.et al Computerized recording of visits to an outpatient sports clinic. Am J Sports Med 19871579–85. [DOI] [PubMed] [Google Scholar]
- 5.Maffulli N, Wong J, Almekinders L C. Types and epidemiology of tendinopathy. Clin Sports Med 200322675–692. [DOI] [PubMed] [Google Scholar]
- 6.Wang J H, Iosifidis M I, Fu F H. Biomechanical basis for tendinopathy. Clin Orthop Relat Res 2006443320–332. [DOI] [PubMed] [Google Scholar]
- 7.Whaley A L, Baker C L. Lateral epicondylitis. Clin Sports Med 200423677–691. [DOI] [PubMed] [Google Scholar]
- 8.Ashe M C, McCauley T, Khan K M. Tendinopathies in the upper extremity: a paradigm shift. J Hand Ther 200417329–334. [DOI] [PubMed] [Google Scholar]
- 9.Cook J L, Khan K M, Purdam C R. Masterclass. Conservative treatment of patellar tendinopathy. Physical Therapy in Sport 2001254–65. [Google Scholar]
- 10.Kader D, Saxena A, Movin T.et al Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med 200236239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter G. Master class. The conservative management of Achilles tendinopathy. Physical Therapy in Sport 200016–14. [Google Scholar]
- 12.Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin Sports Med 200322727–741. [DOI] [PubMed] [Google Scholar]
- 13.Alfredson H, Lorentzon R. Chronic Achilles tendinosis: recommendations for treatment and prevention. Sports Med 200029135–146. [DOI] [PubMed] [Google Scholar]
- 14.Cook J L, Khan K M. What is the most appropriate treatment for patellar tendinopathy? Br J Sports Med 200135291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans P J, Miniaci A. Rotator cuff tendinopathy: many causes, many solutions… part 2 conservative and surgical management. Journal of Musculoskeletal Medicine 19981532–34. [Google Scholar]
- 16.Fyfe I, Stanish W D. The use of eccentric training and stretching in the treatment and prevention of tendon injuries. Clin Sports Med 199211601–624. [PubMed] [Google Scholar]
- 17.Khan K M, Cook J L, Taunton J E.et al Overuse tendinosis, not tendinitis. Part 1: a new paradigm for a difficult clinical problem. Phys Sportsmed 2000538–43. [DOI] [PubMed] [Google Scholar]
- 18.LaStayo P C, Woolf J M, Lewek M D.et al Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther 200333557–571. [DOI] [PubMed] [Google Scholar]
- 19.Peers K H E, Lysens R J J. Patellar tendinopathy in athletes: Current diagnostic and therapeutic recommendations. Sports Med 20053571–87. [DOI] [PubMed] [Google Scholar]
- 20.Alfredson H. The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scand J Med Sci Sports 200515252–259. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery R, Cronin J, Bressel E. Eccentric strengthening: Clinical applications to Achilles tendinopathy. New Zealand Journal of Sports Medicine 20053322–30. [Google Scholar]
- 22.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 200443131–142. [DOI] [PubMed] [Google Scholar]
- 23.Sandrey M A. Using Eccentric Exercise in the Treatment of Lower Extremity Tendinopathies. Athletic Therapy Today 2004958–59. [Google Scholar]
- 24.Stanish W D, Rubinovich R M, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res 198620865–68. [PubMed] [Google Scholar]
- 25.Alfredson H, Pietila T, Jonsson P.et al Heavy‐load eccentric calf muscle training for the treatment of chronic achilles tendinosis. Am J Sports Med 199826360–366. [DOI] [PubMed] [Google Scholar]
- 26.Fahlstrom M, Jonsson P, Lorentzon R.et al Chronic Achilles tendon pain treated with eccentric calf‐muscle training. Knee Surg Sports Traumatol Arthrosc 200311327–333. [DOI] [PubMed] [Google Scholar]
- 27.Mafi N, Lorentzon R, Alfredson H. Superior short‐term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc 2001942–47. [DOI] [PubMed] [Google Scholar]
- 28.Khan K M, Cook P T. Overuse Tendon Injuries: Where does the pain come from? Sports Medicine and Arthroscopic Review 2000817–31. [Google Scholar]
- 29.Ovid Technologies Inc N.Y. Ovid web gateway. http://gateway.ut.ovid.com/gw1/ovidweb.cgi (accessed 21 January 2006)
- 30.Robinson K A, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol 200231150–153. [DOI] [PubMed] [Google Scholar]
- 31.Centre for Evidence‐Based Physiotherapy, Sydney PEDro. Physiotherapy Evidence Database. http://www.pedro.fhs.usyd.edu.au (accessed 21 January 2006)
- 32.Merskey H, Bogduk N.Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd edn. Seattle: IASP Press, 1994211
- 33.Miller M D, Ferris D G. Measurement of subjective phenomena in primary care research: the visual analogue scale. Fam Pract Res J 19931315–24. [PubMed] [Google Scholar]
- 34.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 19751277–299. [DOI] [PubMed] [Google Scholar]
- 35.Ong K S, Seymour R A. Pain measurement in humans. Surgeon 2004215–27. [DOI] [PubMed] [Google Scholar]
- 36.Allen R E, Fowler H W, Fowler F G.The Concise Oxford Dictionary of Current English. Oxford: Clarendon Press; New York, Oxford University Press 1990
- 37.Lawton M P. The functional assessment of elderly people. J Am Geriatr Soc 197119465–481. [DOI] [PubMed] [Google Scholar]
- 38.Deyo R A, Battie M, Beurskens A J.et al Outcome measures for low back pain research. A proposal for standardized use. Spine 1998232003–2013. [DOI] [PubMed] [Google Scholar]
- 39.van Tulder M, Furlan A, Bombardier C.et al Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 2003281290–1299. [DOI] [PubMed] [Google Scholar]
- 40.Verhagen A P, de Vet H C, de Bie R A.et al The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998511235–1241. [DOI] [PubMed] [Google Scholar]
- 41.Maher C G, Sherrington C, Herbert R D.et al Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 200383713–721. [PubMed] [Google Scholar]
- 42.van Tulder M W, Touray T, Furlan A D.et al Muscle relaxants for non‐specific low back pain: a systematic review within the framework of the Cochrane collaboration. Spine 2003281978–1992. [DOI] [PubMed] [Google Scholar]
- 43.Cochrane Collaboration, The Oxford. Review Manager (RevMan). http://www.cc‐ims.net/RevMan (accessed January 2006)
- 44.UCLA Department of Statistics, L A. Power Calculator. http://calculators.stat.ucla.edu/powercalc (accessed 30 March 2006)
- 45.Jonsson P, Alfredson H. Superior results with eccentric compared to concentric quadriceps training in patients with jumper's knee: A prospective randomised study. Br J Sports Med 200539847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez‐Silvestrini J A, Newcomer K L, Gay R E.et al Chronic lateral epicondylitis: Comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther 200518411–420. [DOI] [PubMed] [Google Scholar]
- 47.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: Normalised tendon structure and decreased thickness at follow up. Br J Sports Med 2004388–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purdam C R, Johnsson P, Alfredson H.et al A pilot study of the eccentric decline squat in the management of painful chronic patellar tendinopathy. Br J Sports Med 200438395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roe C, Odegaard T T, Hilde F.et al Effect of supplement of essential fatty acids on lateral epicondylitis: A randomised trial. (In Norwegian.) Tidsskr Nor Laegeforen 20051252615–2618. [PubMed] [Google Scholar]
- 50.Roos E M, Engstrom M, Lagerquist A.et al Clinical improvement after 6 weeks of eccentric exercise in patients with mid‐portion Achilles tendinopathy – a randomized trial with 1‐year follow‐up. Scand J Med Sci Sports 20041286–295. [DOI] [PubMed] [Google Scholar]
- 51.Shalabi A, Kristoffersen‐Wiberg M, Aspelin P.et al Immediate Achilles tendon response after strength training evaluated by MRI. Med Sci Sports Exerc 2004361841–1846. [DOI] [PubMed] [Google Scholar]
- 52.Silbernagel K G, Thomee R, Thomee P.et al Eccentric overload training for patients with chronic Achilles tendon pain – a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports 200111197–206. [DOI] [PubMed] [Google Scholar]
- 53.Stasinopoulos D, Stasinopoulos I. Comparison of effects of exercise programme, pulsed ultrasound and transverse friction in the treatment of chronic patellar tendinopathy. Clin Rehabil 200418347–352. [DOI] [PubMed] [Google Scholar]
- 54.Svernlov B, Adolfsson L. Non‐operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports 200111328–334. [DOI] [PubMed] [Google Scholar]
- 55.Visnes H, Hoksrud A, Cook J.et al No effect of eccentric training on jumper's knee in volleyball players during the competitive season: A randomized clinical trial. Clin J Sport Med 200515225–232. [DOI] [PubMed] [Google Scholar]
- 56.Young M A, Cook J L, Purdam C R.et al Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med 200539102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannell L J, Taunton J E, Clement D B.et al A randomised clinical trial of the efficacy of drop squats or leg extension/leg curl exercises to treat clinically diagnosed jumper's knee in athletes: pilot study. Br J Sports Med 20013560–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen K, Di Fabio R P. Evaluation of eccentric exercise in treatment of patellar tendinitis. Phys Ther 198969211–216. [DOI] [PubMed] [Google Scholar]
- 59.Niesen‐Vertommen S L, Taunton J E, Clement D B.et al The effect of eccentric versus concentric exercise in the management of Achilles tendonitis. Clin J Sport Med 19922109–113. [Google Scholar]
- 60.Pienimaki T, Karinen P, Kemila T.et al Long‐term follow‐up of conservatively treated chronic tennis elbow patients. A prospective and retrospective analysis. Scand J Rehabil Med 199830159–166. [DOI] [PubMed] [Google Scholar]
- 61.Pienimaki T T, Tarvainen T K, Siira P T.et al Progressive strengthening and stretching exercises and ultrasound for chronic lateral epicondylitis. Physiotherapy 199682522–530. [Google Scholar]
- 62.Smidt N, van der Windt D A, Assendelft W J.et al Corticosteroid injections, physiotherapy, or a wait‐and‐see policy for lateral epicondylitis: a randomised controlled trial. Lancet 2002359657–662. [DOI] [PubMed] [Google Scholar]
- 63.Selvanetti A, Barrucci A, Antonaci A.et al The role of eccentric exercise in the functional re‐education of lateral epicondylitis: A randomised controlled clinical trial. (In Italian. ) Med Sport (Roma) 200356103–113. [Google Scholar]
- 64.Shalabi A, Kristoffersen‐Wilberg M, Svensson L.et al Eccentric training of the gastrocnemius‐soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med 2004321286–1296. [DOI] [PubMed] [Google Scholar]
- 65.Moher D, Cook D J, Eastwood S.et al Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 19993541896–1900. [DOI] [PubMed] [Google Scholar]
- 66.Visentini P J, Khan K M, Cook J L.et al The VISA score: an index of severity of symptoms in patients with jumper's knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport 1998122–28. [DOI] [PubMed] [Google Scholar]
- 67.Robinson J M, Cook J L, Purdam C.et al The VISA‐A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med 200135335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudak P L, Amadio P C, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). TheUpper Extremity Collaborative Group (UECG). Am J Ind Med 199629602–608. [DOI] [PubMed] [Google Scholar]
- 69.Roos E M. A User's Guide to: Foot and Ankle Outcome Score (FAOS) http://www.koos.nu/FAOSGuide2003.pdf (accessed 20 April 2006)
- 70.Ko J Y, Chen H S, Chen L M. Treatment of lateral epicondylitis of the elbow with shock waves. Clin Orthop Relat Res 200138760–67. [DOI] [PubMed] [Google Scholar]
- 71.Jonnson P, Wahlstrom P, Ohberg L.et al Eccentric training in chronic painful impingement syndrome of the shoulder: results of a pilot study. Knee Surg Sports Traumatol Arthrosc 20061476–81. [DOI] [PubMed] [Google Scholar]
- 72.Bisset L, Paungmali A, Vicenzino B.et al A systematic review and meta‐analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med 200539411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liddle S D, Baxter G D, Gracey J H. Exercise and chronic low back pain: what works? Pain 2004107176–190. [DOI] [PubMed] [Google Scholar]
- 74.Schulz K F, Chalmers I, Hayes R J.et al Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995273408–412. [DOI] [PubMed] [Google Scholar]
- 75.Moher D, Pham B, Jones A.et al Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses? Lancet 1998352609–613. [DOI] [PubMed] [Google Scholar]
- 76.Alfredson H, Nordstrom P, Pietila T.et al Bone mass in the calcaneus after heavy loaded eccentric calf‐muscle training in recreational athletes with chronic achilles tendinosis. Calcif Tissue Int 199964450–455. [DOI] [PubMed] [Google Scholar]
- 77.Alfredson H, Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc 200311196–199. [DOI] [PubMed] [Google Scholar]
- 78.Sayana M K, Maffulli N. Eccentric calf muscle training in non‐athletic patients with Achilles tendinopathy. J Sci Med Sport 20071052–58. [DOI] [PubMed] [Google Scholar]
- 79.Reeves B C, MacLehose R R, Harvey I M.et al Comparisons of effect sizes derived from randomised and non‐randomised studies. In: Black N, Reeves B, Brazier J, et al eds. Health Services Research Methods: A Guide to Best Practice London: BMJ Books, 199873–85.