Abstract
Tendons and ligaments within the upper and lower limbs are some of the more common sites of musculoskeletal injuries during physical activity. Several extrinsic and intrinsic factors have been shown to be associated with these injuries. More recently, studies have suggested that there is also, at least in part, a genetic component to the Achilles tendon, rotator cuff and anterior cruciate ligament injuries. However, specific genes have not been suggested to be associated with rotator cuff or anterior cruciate ligament injuries. Sequence variants of the tenascin C (TNC) gene, on the other hand, have been shown to be associated with Achilles tendinopathies and Achilles tendon ruptures, whereas a variant of the collagen V α 1 (COL5A1) gene has also been shown to be associated with Achilles tendinopathies. Both genes encode for important structural components of tendons and ligaments. The COL5A1 gene encodes for a component of type V collagen, which has an important role in regulating collagen fibre assembly and fibre diameters. The TNC gene, on the other hand, encodes for TNC, which regulates the tissue's response to mechanical load. To date, only variants in two genes have been shown to be associated with Achilles tendon injuries. In addition, although specific genes have not been identified, investigators have suggested that there is also a genetic component to both rotator cuff and anterior cruciate ligament injuries. In future, specific genotypes associated with increased risk of injury to specific tendons and ligaments can prevent these injuries by identifying individuals at higher risk.
Tendon and/or ligaments such as the rotator cuff tendons (shoulder), the anterior cruciate ligament (ACL; knee), and the Achilles tendon (ankle) are some of the more common sites of musculoskeletal injuries during both competitive and recreational sporting activities.1,2,3 It has been reported that tendon injuries account for approximately 30–50% of all sporting injuries,4 of which Achilles tendon injuries account for between 6% and 18%.5 Partial or full‐thickness tears of the rotator cuff are reported to be the cause of most of the pain and dysfunction associated with the shoulder,1,3 whereas the vast majority of knee ligament injuries occur to the ACL.6 The focus of this review is to highlight the current evidence for a genetic component of (1) tendon injuries, with reference to Achilles tendon and rotator cuff injuries, and (2) ligament injuries, using ACL injuries as the example. It should be noted that there are, besides Achilles tendon, rotator cuff and ACL injuries, other common ligament and tendon injuries. As genetic contributions, if any, for these other tendon and ligament injuries have not been investigated, they have not been discussed in this review.
Spectrum of injuries
It is well recognised that there is a spectrum of injuries that can affect the Achilles tendon and surrounding structures,7 the rotator cuff 8,9,10 and ACL. With respect to the Achilles tendon, partial or complete ruptures and overuse injuries (commonly referred to as either “tendinopathy” or “tendinosis”) are the most common injuries. For the purpose of this review, Achilles tendon injuries will refer to these common injuries, and include both acute‐onset (spontaneous ruptures) and repetitive‐strain overuse injuries (chronic tendinopathies). The term tendinopathy rather than tendinosis was chosen on the basis that (1) some authors11 prefer to use the term tendinopathy, which is a non‐encompassing term implying that there is an underlying pathology in and around the tendon, and (2) several pathological conditions may coexist in the tendon,12 which often justifies the use of the term tendinopathy, rather than tendinosis.
Injuries of the rotator cuff are also classified into a variety of conditions, and are frequently collectively referred to as rotator cuff disorders10 or rotator cuff disease.8,9 These disorders can range from tendinosis to partial or complete tears of the rotator cuff tendons.8 Only rotator cuff tears will be considered here. The genetic evidence, admittedly limited, is best developed for these types of injuries.
The spectrum of injuries that are described in the ACL is narrower, and, in most instances, ACL injuries are referred to as either partial or complete ruptures or tears. For the purpose of this review, only the possible genetic components related to complete ACL tears are discussed.
Genetic risk factors associated with tendon and ligament injuries
The exact aetiology underlying Achilles tendon,3,4,13,14 rotator cuff3 and ACL15,16 injuries remains undefined. Several intrinsic and extrinsic risk factors have nevertheless been shown to be implicated in all three types of injuries.8,10,14,16,17,18 Some studies have also suggested a genetic predisposition to both Achilles tendon ruptures and chronic Achilles tendinopathy,19 as well as, more recently, to tears of the rotator cuff1 and ACL.20
It needs to be emphasised that there is probably a spectrum of connective tissue disorders with a genetic component. At one end of the spectrum are the classical Mendelian disorders, wherein the genetic factors are the major determinants of the severity and prognosis of the disorder; these have major implications for the health status of the respective family members. Examples of the classical Mendelian connective tissue disorders are, among others, osteogenesis imperfecta (OI),21 Ehlers–Danlos syndrome (EDS)22 and Marfan's syndrome.23 At the other end of the spectrum are the complex, multifactorial conditions, wherein the development of the condition is determined by the complex interactions of multiple gene products (ie, proteins) and the environment; these are referred to as gene–gene and gene–environment interactions. The identification of the genetic components underlying Mendelian disorders is usually achieved using linkage analysis or direct candidate gene sequencing.24 However, the identification of the genes predisposing individuals (from a population and not from a family) to an increased risk of developing a multifactorial condition is more difficult. This search is further complicated by the likelihood that a number of genes are involved, each having a small contribution, and by the gene–environment interactions. Lumbar disc disease is an example of a multifactorial musculoskeletal condition for which both genetic and non‐genetic factors have been identified, and interactions between the genetic and non‐genetic factors have also been described.25,26,27,28,29
The identification of several intrinsic and extrinsic risk factors associated with Achilles tendon injuries, as well as rotator cuff and ACL tears, suggests that these conditions are complex, and that both gene–gene and gene–environment interactions are probably involved in the aetiology of these conditions.
Achilles tendon injuries
Over the past two decades, there have been several publications investigating the ABO blood group as a biochemical marker for injuries to the Achilles tendon, and to a lesser extent other tendons and ligaments.19,30,31 Some of these studies found that blood group O or the A/O ratio was associated with Achilles tendon ruptures,19,30,31 other tendon ruptures19,30 including the long head of the biceps, extensor pollicis longus and the quadriceps, and Achilles peritendinitis.31 The ABO blood group has also been reported to be associated with patients with multiple tendon ruptures or re‐ruptures.30,32 In contrast, several other studies have not found any associations between the ABO blood group and either Achilles tendon ruptures33,34,35,36 or Achilles tendinopathy in other populations.36
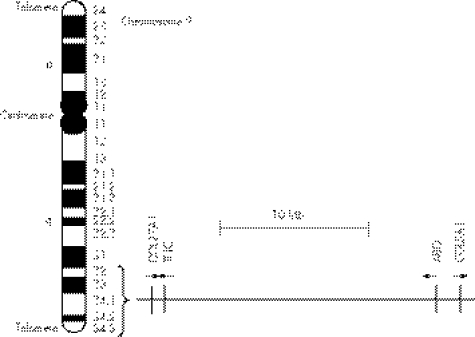
The ABO gene, located on the telomeric end of the long arm of human chromosome 9 (9q34), encodes for enzymes that produce the major antigens in the ABO blood group system (fig 1).37 The fact that some studies found an association between the ABO blood groups, a biochemical marker for variants of the ABO gene, and Achilles tendon pathology justifies investigating this region of chromosome 9 for potential candidate genes for Achilles tendon injuries.19,31,38 It should be noted that the lack of association (ie, a negative result) of the ABO blood group with Achilles tendon injuries in some studies does not imply that one can exclude the existence of a causal variant either at the ABO locus or in close proximity (adjacent gene/s). The explanation for this is beyond the scope of this review.
Figure 1 Schematic representation of human chromosome 9 (ideogram). The centromere is the structural region of the chromosome that binds with the nuclear spindle at mitosis and meiosis. The telomere refers to each end region of the chromosome, which consists of tandem repeats of simple DNA sequences. The chromosome is comprised of a short arm (p) and a long arm (q). The dark and light areas reflect the unique banding pattern of chromosome 9 when stained using cytogenetic techniques. The nomenclature of each band is indicated on the right side of the chromosome. A segment corresponding to the telomeric end of the long arm of chromosome 9 (9q32–q34), which encompasses the 155 kb COL27A1, 20 kb tenascin C (TNC), 20 kb ABO and 201 kb COL5A1 genes, is also shown. The vertical lines represent the relative position of each gene, while the arrows indicate the direction of their transcription. COL27A1 encodes type XXVII collagen; TNC encodes TNC; ABO encodes the ABO transferases; and COL5A1 encodes the pro‐α1(V) chain of type V collagen. Mb, megabase.
As previously reviewed by September et al,39 genes that encode structural proteins, which include a variety of collagens, proteoglycans and glycoproteins, are ideal candidate genes for Achilles tendon injuries. In support of this, the expression of a number of these genes is either upregulated or downregulated during Achilles tendon pathology.40,41 Two of these genes, namely tenascin C (TNC) and COL5A1, together with COL27A1, have both been localised to the same chromosomal region as the ABO gene on the telomeric end of the long arm of chromosome 9 (fig 1).
Recently, two studies following a candidate gene approach, starting at the ABO locus, identified polymorphisms within the TNC and COL5A1 genes to be associated with Achilles tendon injuries in a physically active Caucasian population from South Africa.42,43 However, no association was observed between the ABO blood groups and Achilles tendon injuries within these South African subjects.36 We are also unaware of any published investigations detailing the functional effects of these two polymorphisms within TNC and COL5A1 on gene expression or protein function. The function of the large majority of sequence variants (polymorphisms) is not known and many of these lie outside of genes. For this reason, it is common in genetic association studies to find a statistical correlation of a specific disease trait with polymorphisms of unknown function, or even with polymorphisms specifically believed to be non‐functional.44 This phenomenon of linkage disequilibrium is observed because, even though the specific polymorphism has no functional effect on the observed trait, it is inherited together with a nearby unknown, causal variant. Furthermore, the biological marker (the polymorphism shown to be associated with the trait) and the causal variant do not have to be within the same gene.
Both studies that have reported an association with a specific polymorphic marker and Achilles tendon injuries followed a genetic association approach. The genetic association approach is currently one of the more common methods used to identify regions of the human genome that contain genes that predispose individuals to disease. Although the principles of genetic association studies have been reviewed previously,39 the basic principles are summarised below. The genome of unrelated individuals is highly homologous, with only approximately 0.1% of the nucleotides within the DNA sequences differing. These differences or polymorphisms are found over the entire human genome and can produce different variants or alleles of the same gene. These alleles can, in some instances, affect the expression of the gene and/or the function of its protein product. Individuals can be genotyped for these polymorphisms, preferably within a gene (intragenic), and the association of the specific variants with a particular trait can be determined. The two most important types of polymorphisms are microsatellites and single‐nucleotide polymorphisms (SNPs). Microsatellites are loci containing short stretches of DNA sequences that are repeated in a tandem array (fig 2A). SNPs, on the other hand, refer to a DNA sequence variant that involves the substitution of a single base at a particular position (fig 2B). One can discriminate between the different bases of a particular SNP using a number of methods, one of which is the restriction endonuclease method that produces restriction fragment length polymorphisms (RFLPs). A restriction endonuclease is an enzyme that is able to cut DNA at a specific sequence, generating DNA fragments of known sizes. Often individuals will be genotyped for a number of SNPs to form a haplotype; a haplotype consists of a number of closely linked markers present on a single chromosome, which tend to be inherited together. Haplotype analyses are often more informative than single polymorphisms in the identification of disease‐susceptible genomic regions. Other polymorphisms, including copy number variants (CNVs), have also been associated with diseases and phenotypic traits.45 CNVs refer to duplications or deletions of several kilobase stretches of genomic DNA, and studies have shown that variation in the copy number of certain CNVs correlates with changes in the specific gene expression levels.46,47,48
Figure 2 Nucleotide sequences of polymorphic regions within the human (A) TNC and (B) COL5A1 genes. (A) The nucleotide sequence, GenBank accession number Z11654, corresponds to part of intron 17 of the TNC gene and contains the GT dinucleotide repeat polymorphism (shown in capital letters). This sequence contains 17 GT dinucleotide repeats. (B) The nucleotide sequence, GenBank accession number AL603650, corresponds to part of the 3′ end (exon 67) of the COL5A1 gene. The positions of two single‐nucleotide polymorphisms (SNPs), together with the restriction enzyme used to genotype individuals for these SNPs, are shown. The alternative nucleotide is given under the wild‐type sequence. The stop codon, as well as the DpnII (GA/TC; rs13946) and BstUI (CG/CG; rs12722) restriction sequences, both located in the 3′‐untranslated region of the gene, are underlined.
The first study investigated a GT dinucleotide repeat polymorphism (a microsatellite) within the TNC gene (fig 2A). This case–control study included 114 physically active Caucasian subjects, of whom 72 were clinically diagnosed with chronic Achilles tendinopathy and 42 with spontaneous Achilles tendon ruptures, and 127 asymptomatic physically active Caucasian control subjects.42 The number of GT dinucleotide repeats within each copy of the TNC gene was determined in each subject. A significant difference was noted in the allele (each allele had a different number of GT repeats) frequencies of this polymorphism between the injured and control groups (p = 0.001). More specifically, the alleles containing 12 and 14 GT repeats were over‐represented in the injured group, while the frequencies of the alleles containing 13 and 17 GT repeats were under‐represented. Furthermore, the authors predict that it is likely that individuals who were homozygous or heterozygous for the under‐represented alleles (13 and 17 GT repeats) may have a lower risk of developing Achilles tendon injuries (odds ratio = 0.2; 95% confidence interval (CI) 0.1 to 0.3, p<0.001). Interestingly, there were no differences in the frequency of the GT dinucleotide repeat polymorphism between the Achilles tendon rupture and Achilles tendinopathy groups.
The second study also followed a case–control genetic association approach, but used two SNPs within the COL5A1 gene (fig 2B). As previously mentioned, in the case of SNPs, alleles are discriminated by the particular base at a specific single‐nucleotide position. The BstUI and DpnII RFLPs were used to genotype 111 physically active Caucasian subjects, of whom 72 were clinically diagnosed with chronic Achilles tendinopathy and 39 with spontaneous Achilles tendon ruptures, and 129 asymptomatic physically active Caucasian control subjects.43 This study suggested that the BstUI RFLP, but not the DpnII RFLP, was strongly associated with chronic Achilles tendinopathy but not with Achilles tendon rupture. This contrasted with the TNC study in which no differences were observed between these two pathology groups. As mentioned previously, owing to inheritance patterns (linkage disequilibrium), it is common for only a subset and not all of the polymorphisms within a locus to be associated with a trait.
TNC gene
The TNC gene encodes for TNC, which is a glycoprotein abundantly found in tissues such as tendons subjected to high tensile and compressive stress.49 The TNC gene expression is regulated in a dose–response manner by mechanical loading in tendons.50,51 The protein is able to bind various cell surface receptors, such as integrins, and other components of the extracellular matrix, and has therefore been implicated in the regulation of cell–matrix interactions.52 Moreover, TNC expression has been shown to be upregulated in Achilles tendinopathy,50 providing evidence that TNC is involved in tendinopathy. It is therefore reasonable to identify the TNC gene as an ideal candidate gene for Achilles tendon injuries.
COL5A1 gene
The COL5A1 gene encodes the α1 chain (pro‐α1(V) chain) of the low‐abundance heterotrimeric type V fibrillar collagen.53,54 The pro‐α1(V) chain is found in most of the isoforms of type V collagen.55 Type V collagen is found in tendons and other connective tissues where it regulates the assembly (fibrillogenesis) of collagen fibres.56 The COL5A1 gene is also an ideal candidate gene for Achilles tendon injuries.
If the TNC and/or COL5A1 genes are directly involved in the aetiology of Achilles tendinopathy, the causal variants within these genes need to be identified. Recently, Matsuda et al57 have identified a SNP located in exon 17 of TNC, which causes an amino acid substitution at position 1677 of a leucine by an isoleucine. Interestingly, this SNP is adjacent to the GT dinucleotide microsatellite within intron 17 of this gene. This amino acid substitution has been predicted to change the structural stability of the fibronectin III domain contained in the TNC protein, and has recently been shown to be associated with adult asthma in a Japanese population.57 The association of this functional SNP with Achilles tendon injury needs to be investigated, preferably prospectively. In addition, the findings of the association studies investigating TNC and COL5A1 need to be repeated in an independent population. Preliminary data have, however, shown that the BstUI RFLP within the COL5A1 gene is also associated with Achilles tendinopathies in a second Caucasian population from Australia (unpublished data).
The association of polymorphisms within the TNC and COL5A1 genes with symptoms of Achilles tendon injury does not prove that either type V collagen or TNC proteins are directly involved in a cause–effect relationship. It is therefore still possible that any other gene/s in close proximity to the aforementioned genes (chromosome 9) may be coding for the causal protein. COL27A1, which encodes for the homotrimeric type XXVII collagen, is a recently identified fibrillar collagen that has also been mapped to the same region as TNC and COL5A1 (fig 1).58,59 Two enhancer elements that bind SOX 9 have been identified within the COL27A1 gene.60 A non‐coding promoter variant could decrease COL27A1 protein expression levels without affecting the gene sequence and therefore could be a potential risk factor for tendinopathy. COL27A1 is expressed in cartilage, eye, ear, lung and colon of the mouse, but the precise function of this protein in these tissues is unknown. It may also be expressed in tendons and should be investigated.
There are a host of other proteins, besides type V collagen and TNC, which are involved in the structure and/or function of tendons. Therefore, the possible association of polymorphisms in these genes with Achilles tendon injuries should be investigated.39,61 For example, both type XII and XIV collagens respond to mechanical loading and are involved in fibrillogenesis, processes with which both COL5A1 and TNC are associated.62 Furthermore, in a rat model of rotator cuff tendinopathy, it has been shown that type XII collagen is upregulated during tendon healing.63 Therefore, it would be interesting to investigate the association of genes encoding other collagenous and non‐collagenous proteins expressed in tendons.
It is important to note that, although connective tissues such as tendons are predominantly made up of the extracellular matrix (ECM), the synthesis, degradation and maintenance of the ECM depend on the presence of cells within it. The cell‐mediated remodelling and healing of tendons is also a vital process to maintain healthy tendons. Although the focus of this and another39 review has been on genes that encode the structural proteins of tendons, other genes encoding proteins involved in other biological processes within tendons could also be considered as candidate genes for Achilles tendon injuries. The regulation of different biological processes within tendons, such as the remodelling, degradation and healing processes, are all vital and are performed by specific proteinases, the proteinase inhibitors, growth factors, cytokines and regulators of apoptosis. The proteinases found in tendons such as the matrix metalloproteinases and the ADAM‐TS (A disintegrin and metalloproteinase‐thrombospondin) family,61,64 as well as the tissue inhibitors of metalloproteinases,64 can all be considered as candidate genes for Achilles tendon injuries. Detailed reviews on regulatory enzymes found in the ECM have been published.61,64,65 However, none of the genes encoding these ECM regulatory proteins have been mapped to the telomeric end of the long arm of chromosome 9, as evident from the various databases on NCBI.36,39
Apoptosis has received some attention as one possible mechanism associated with mechanical loading‐induced tendinopathy.66 Any gene encoding for proteins involved in these, or any other biological process in tendons, could be a potential genetic risk factor for Achilles tendon injuries. Several genes involved in apoptosis have also been mapped to the telomeric region of chromosome 9q.36 Owing to the nature of genetic association studies, the association of polymorphisms within the TNC and COL5A1 genes with Achilles tendon injuries could be attributed to the causal variants within any one of these genes.
The aetiology of Achilles tendon injuries is multifactorial, and therefore it is important that future studies investigate the interactions of the different intrinsic and extrinsic risk factors. The interaction of COL5A1 and TNC with environmental factors, such as body weight and exposure to physical activity, could not be excluded from the previous studies and therefore needs to be investigated.42,43 An interaction of increased body weight with COL9A3 has been reported in lumbar disc degeneration.29
It is interesting to note that mutations in COL5A1 and tenascin X (TNX) have been associated with both EDS and benign joint hypermobility syndrome.67,68 Since TNC belongs to the same family as TNX, it will therefore be biologically plausible to investigate the function of TNX in tendons. In addition, there have been case reports in which the spontaneous rupture of the Achilles tendon or patellar tendon has been noted in individuals diagnosed with EDS type II, an autosomal dominant condition for which mutations within the COL3A1 gene have been identified.69,70,71COL3A1 may therefore be a good candidate gene for spontaneous Achilles tendon ruptures.
Tears of the rotator cuff and ACL
In recent years, two studies have reported the investigation of the genetic susceptibility to tears of the rotator cuff and tearing of the ACL.1,20 Both studies provided evidence relating to the genetic contribution of injuries to the rotator cuff and ACL, which were based on clinical information collected from affected individuals, their siblings and matched controls.
The study by Harvie et al1 investigated a prospective, cross‐sectional study of individuals with full‐thickness tears of the rotator cuff. They evaluated 213 patients, 150 spouses and 129 siblings. The participants completed the Short Form‐36 Health Survey Questionnaire, the Oxford Shoulder Score, and the Score of Constant and Murley for each shoulder, and, in addition, all underwent ultrasound examinations of both shoulders. The study showed that siblings had more than twice the risk of developing tears of the rotator cuff (relative to a control group) (p<0.001) and nearly five times the risk of experiencing symptoms (p<0.001). This illustrates that there is a significant genetic susceptibility towards the development of full‐thickness tears of the rotator cuff and the associated symptoms.
The case–control study by Flynn et al20 was the first to investigate the familial predisposition to tears of ACL. This particular study was a retrospective, questionnaire‐based, case–control study, and therefore provides weak evidence of a genetic contribution. A total of 348 affected and 384 control subjects completed the questionnaire. The study reported that a greater proportion of subjects with an ACL tear had at least one relative with an ACL tear in comparison to the matched controls (p = 0.013). More specifically, individuals with an ACL tear are twice as likely to have a relative with an ACL tear and more than twice as likely to have a first‐degree relative with an ACL tear, suggesting that there may be a genetic contribution to tears of the ACL.
The authors1,20 provide a detailed description of the limitations of the two respective studies. However, the findings from these studies suggest that there is a genetic risk to developing full‐thickness tears of the rotator cuff and ACL tears. Interestingly, no associations were found between the ABO blood groups and rotator cuff impingement31 or ACL ruptures.31 However, as previously discussed, this does not imply that the ABO locus or, more specifically, genes within this region can be excluded.
The functions of tendons and ligaments are very different: (1) ligaments connect two articulating bones across a joint and are responsible for maintaining joint congruency and guiding joint movement; (2) tendons, on the other hand, connect muscle to bone and convert muscle contractions to joint motion. However, normal ligaments and tendons are very similar in their compositions, with only certain minor variations.72 Although these injuries have different pathologies, they do share a few extrinsic and intrinsic risk factors. It is therefore conceivable that they may also share, at least some, similar genetic risk factors. For this reason, it is feasible that TNC and COL5A1, as well as other structural candidate genes, should be investigated for associations with both rotator cuff and ACL injuries.
Conclusion
The aetiologies of spontaneous Achilles tendon ruptures, chronic Achilles tendinopathies, rotator cuff injuries and ACL injuries are complex and result from a combination of intrinsic and extrinsic risk factors. Polymorphisms within the COL5A1 and TNC genes, which are both located in close proximity with the ABO gene, have been shown to be associated with Achilles tendon injury in a physically active South African Caucasian population.42,43 The functions of these polymorphisms are currently unknown, and it remains to be investigated whether these polymorphisms are also associated with either rotator cuff or ACL injuries.
What is already known on this topic
Several studies have suggested a genetic component to Achilles tendon, rotator cuff tendon and anterior cruciate ligament injuries.
Sequence variants within two genes (TNC and COL5A1) have recently been found to be associated with Achilles tendon injuries.
What this study adds
This review summarises the genetic evidence associated with tendon and ligament injuries, and highlights different approaches to identify genes predisposing individuals to complex conditions.
Plausible candidate genes for future studies are also summarised.
It is important that the COL5A1 and TNC association studies are repeated in another population, as this will provide added strength and confidence that the associations identified are reflecting biological processes, which are important in comprehending the disease aetiology. The sequence variants within the TNC and COL5A1 genes associated with Achilles tendon pathology may in future be used as potential genetic markers to identify individuals with an increased genetic risk for developing Achilles tendon injuries. However, it must be stated that these markers cannot be used as a diagnostic tool for Achilles tendon pathology, and that much research is still required before any genetic service can be offered.
Acknowledgements
AVS was supported by the post‐doctoral innovation award from the National Research Foundation of South Africa.
Abbreviations
ACL - anterior cruciate ligament
CNV - copy number variant
ECD - Ehlers–Danlos syndrome
ECM - extracellular matrix
RFLP - restriction fragment length polymorphism
SNP - single‐nucleotide polymorphism
TNC - tenascin C
TNX - tenascin X
Footnotes
Competing interests: None.
References
- 1.Harvie P, Ostlere S J, Teh J.et al Genetic influences in the aetiology of tears of the rotator cuff. Sibling risk of a full‐thickness tear. J Bone Joint Surg Br 200486696–700. [DOI] [PubMed] [Google Scholar]
- 2.Woo S L, Abramowitch S D, Kilger R.et al Biomechanics of knee ligaments: injury, healing, and repair. J Biomech 2006391–20. [DOI] [PubMed] [Google Scholar]
- 3.Rees J D, Wilson A M, Wolman R L. Current concepts in the management of tendon disorders. Rheumatology 200645508–521. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen T A, Kannus P, Maffulli N.et al Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin 200510255–266. [DOI] [PubMed] [Google Scholar]
- 5.Mazzone M F, McCue T. Common conditions of the Achilles tendon. Am Fam Physician 2002651805–1810. [PubMed] [Google Scholar]
- 6.Beynnon B D, Johnson R J, Abate J A.et al Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med 2005331579–1602. [DOI] [PubMed] [Google Scholar]
- 7.Puddu G, Ippolito E, Postacchini F. A classification of Achilles tendon disease. Am J Sports Med 19764145–150. [DOI] [PubMed] [Google Scholar]
- 8.Barr K P. Rotator cuff disease. Phys Med Rehabil Clin N Am 200415475–491. [DOI] [PubMed] [Google Scholar]
- 9.Tytherleigh‐Strong G, Hirahara A, Miniaci A. Rotator cuff disease. Curr Opin Rheumatol 200113135–145. [DOI] [PubMed] [Google Scholar]
- 10.Gomoll A H, Katz J N, Warner J J.et al Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis Rheum 2004503751–3761. [DOI] [PubMed] [Google Scholar]
- 11.Almekinders L C. Tendinitis and other chronic tendinopathies. J Am Acad Orthop Surg 19986157–164. [DOI] [PubMed] [Google Scholar]
- 12.Schepsis A A, Jones H, Haas A L. Achilles tendon disorders in athletes. Am J Sports Med 200230287–305. [DOI] [PubMed] [Google Scholar]
- 13.Kannus P, Jarvinen T L, Jarvinen T A.et al Painful Achilles tendon and its treatment. Scand J Med Sci Sports 20041469–71. [DOI] [PubMed] [Google Scholar]
- 14.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 200443131–142. [DOI] [PubMed] [Google Scholar]
- 15.Childs S G. Pathogenesis of anterior cruciate ligament injury. Orthop Nurs 20022135–40. [DOI] [PubMed] [Google Scholar]
- 16.Bahr R, Krosshaug T. Understanding injury mechanisms: a key component of preventing injuries in sport. Br J Sports Med 200539324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewett T E, Myer G D, Ford K R. Anterior cruciate ligament injuries in female athletes: Part 1, Mechanisms and risk factors. Am J Sports Med 200634299–311. [DOI] [PubMed] [Google Scholar]
- 18.Matava M J, Purcell D B, Rudzki J R. Partial‐thickness rotator cuff tears. Am J Sports Med 2005331405–1417. [DOI] [PubMed] [Google Scholar]
- 19.Kannus P, Natri A. Etiology and pathophysiology of tendon ruptures in sports. Scand J Med Sci Sports 19977107–112. [DOI] [PubMed] [Google Scholar]
- 20.Flynn R K, Pedersen C L, Birmingham T B.et al The familial predisposition toward tearing the anterior cruciate ligament: a case control study. Am J Sports Med 20053323–28. [DOI] [PubMed] [Google Scholar]
- 21.Prockop D J, Chu M L, de Wet W.et al Mutations in osteogenesis imperfecta leading to the synthesis of abnormal type I procollagens. Ann N Y Acad Sci 1985460289–297. [DOI] [PubMed] [Google Scholar]
- 22.De Paepe A, Nuytinck L, Hausser I.et al Mutations in the COL5A1 gene are causal in the Ehlers‐Danlos Syndromes I and II. Am J Hum Genet 199760547–554. [PMC free article] [PubMed] [Google Scholar]
- 23.Judge D P, Dietz H C. Marfan's syndrome. Lancet 20053661965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawn T M, Barrett J H. Genetic linkage studies. Lancet 20053661036–1044. [DOI] [PubMed] [Google Scholar]
- 25.Ala‐Kokko L. Genetic risk factors for lumbar disc disease. Ann Med 20023442–47. [DOI] [PubMed] [Google Scholar]
- 26.Tilkeridis C, Bei T, Garantziotis S.et al Association of a COL1A1 polymorphism with lumbar disc disease in young military recruits. J Med Genet 200542e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noponen‐Hietala N, Kyllonen E, Mannikko M.et al Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis 2003621208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solovieva S, Kouhia S, Leino‐Arjas P.et al Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology 200415626–633. [DOI] [PubMed] [Google Scholar]
- 29.Solovieva S, Lohiniva J, Leino‐Arjas P.et al COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene‐environment interaction. Spine 2002272691–2696. [DOI] [PubMed] [Google Scholar]
- 30.Jozsa L, Balint J B, Kannus P.et al Distribution of blood groups in patients with tendon rupture. J Bone Joint Surg Br 198971‐B272–274. [DOI] [PubMed] [Google Scholar]
- 31.Kujala U M, Jarvinen M, Natri A.et al ABO blood groups and musculoskeletal injuries. Injury 199223131–133. [DOI] [PubMed] [Google Scholar]
- 32.Aroen A, Helgo D, Granlund O G.et al Contralateral tendon rupture risk is increased in individuals with a previous Achilles tendon rupture. Scand J Med Sci Sports 20041430–33. [DOI] [PubMed] [Google Scholar]
- 33.Mahrlein R, Schmelzeisen H, Papathanassopoulos A. Achillessehnenrupturen und blutgruppenzugehorigkeit. Aktuelle Traumatol 19952513–15. [Google Scholar]
- 34.Leppilahti J, Puranen J, Orava S. ABO blood group and Achilles tendon rupture. Ann Chir Gynaecol 199685369–371. [PubMed] [Google Scholar]
- 35.Maffulli N, Reaper J A, Waterston S W.et al ABO blood groups and Achilles tendon rupture in the Grampian region of Scotland. Clin J Sport Med 200010269–271. [DOI] [PubMed] [Google Scholar]
- 36.Mokone G G.Risk factors for Achilles tendon injuries: an emphasis on the identification of specific genetic factors. South Africa: University of Cape Town, 2006
- 37.Bennett E P, Steffensen R, Clausen H.et al Genomic cloning of the human histo‐blood group ABO locus. Biochem Biophys Res Commun 199520618–25. [DOI] [PubMed] [Google Scholar]
- 38.Maffulli N, Kader D. Tendinopathy of tendo Achillis. J Bone Joint Surg Br 2002841–8. [DOI] [PubMed] [Google Scholar]
- 39.September A V, Mokone G G, Schwellnus M P.et al Genetic risk factors for Achilles tendon injuries. Int Sports Med J 20067201–215. [Google Scholar]
- 40.Ireland D, Harrall R, Curry V.et al Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol 200120159–169. [DOI] [PubMed] [Google Scholar]
- 41.Alfredson H, Lorentzon M, Backman S.et al cDNA‐arrays and real‐time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res 200321970–975. [DOI] [PubMed] [Google Scholar]
- 42.Mokone G G, Gajjar M, September A V.et al The Guanine‐thymine dinucleotide repeat polymorphism within the tenascin‐C gene is associated with Achilles tendon injuries. Am J Sports Med 2005331016–1021. [DOI] [PubMed] [Google Scholar]
- 43.Mokone G G, Schwellnus M P, Noakes T D.et al The COL5A1 gene and Achilles tendon pathology. Scand J Med Sci Sports 20061619–26. [DOI] [PubMed] [Google Scholar]
- 44.Palmer L J, Cardon L R. Shaking the tree: mapping complex disease genes with linkage disequilibrium. Lancet 20053661223–1234. [DOI] [PubMed] [Google Scholar]
- 45.Feuk L, Carson A R, Scherer S W. Structural variation in the human genome. Nat Rev Genet 2006785–97. [DOI] [PubMed] [Google Scholar]
- 46.Aldred P M, Hollox E J, Armour J A. Copy number polymorphism and expression level variation of the human alpha‐defensin genes DEFA1 and DEFA3. Hum Mol Genet 2005142045–2052. [DOI] [PubMed] [Google Scholar]
- 47.Linzmeier R M, Ganz T. Copy number polymorphisms are not a common feature of innate immune genes. Genomics 200688122–126. [DOI] [PubMed] [Google Scholar]
- 48.Freeman J L, Perry G H, Feuk L.et al Copy number variation: new insights in genome diversity. Genome Res 200616949–961. [DOI] [PubMed] [Google Scholar]
- 49.Jarvinen T A, Kannus P, Jarvinen T L.et al Tenascin‐C in the pathobiology and healing process of musculoskeletal tissue injury. Scand J Med Sci Sports 200010376–382. [DOI] [PubMed] [Google Scholar]
- 50.Jarvinen T A, Jozsa L, Kannus P.et al Mechanical loading regulates tenascin‐C expression in the osteotendinous junction. J Cell Sci 19991123157–3166. [DOI] [PubMed] [Google Scholar]
- 51.Jarvinen T A, Jozsa L, Kannus P.et al Mechanical loading regulates the expression of tenascin‐C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci 2003116857–866. [DOI] [PubMed] [Google Scholar]
- 52.Jones F S, Jones P L. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 2000218235–259. [DOI] [PubMed] [Google Scholar]
- 53.Smith M, Simpson N E. Report of the committee on the genetic constitution of chromosomes 9 and 10. Cytogenet Cell Genet 198951202–225. [DOI] [PubMed] [Google Scholar]
- 54.Greenspan D S, Byers M G, Eddy R L.et al Human collagen gene COL5A1 maps to the q34.2–q34.3 region of chromosome 9, near the locus for Nail‐Patella syndrome. Genomics 199212836–837. [DOI] [PubMed] [Google Scholar]
- 55.Birk D E. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 200132223–237. [DOI] [PubMed] [Google Scholar]
- 56.Birk D E, Fitch J M, Babiarz J P.et al Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci 199095649–657. [DOI] [PubMed] [Google Scholar]
- 57.Matsuda A, Hirota T, Akahoshi M.et al Coding SNP in tenascin‐C Fn‐III‐D domain associates with adult asthma. Hum Mol Genet 2005142779–2786. [DOI] [PubMed] [Google Scholar]
- 58.Boot‐Handford R P, Tuckwell D S, Plumb D A.et al A novel and highly conserved collagen (pro(alpha)1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J Biol Chem 200327831067–31077. [DOI] [PubMed] [Google Scholar]
- 59.Pace J M, Corrado M, Missero C.et al Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol 2003223–14. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins E, Moss J B, Pace J M.et al The new collagen gene COL27A1 contains SOX9‐responsive enhancer elements. Matrix Biol 200524177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev Mol Med 200571–25. [DOI] [PubMed] [Google Scholar]
- 62.Young B B, Zhang G, Koch M.et al The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem 200287208–220. [DOI] [PubMed] [Google Scholar]
- 63.Thomopoulos S, Hattersley G, Rosen V.et al The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res 200220454–463. [DOI] [PubMed] [Google Scholar]
- 64.Riley G P. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports 200515241–251. [DOI] [PubMed] [Google Scholar]
- 65.Kaushal G P, Shah S V. The new kids on the block: ADAMTSs, potentially multifunctional metalloproteinases of the ADAM family. J Clin Invest 20001051335–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murrell G A. Understanding tendinopathies. Br J Sports Med 200236392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giunta C, Nuytinck L, Raghunath M.et al Homozygous Gly530Ser substitution in COL5A1 causes mild classical Ehlers‐Danlos syndrome. Am J Med Genet 2002109284–290. [DOI] [PubMed] [Google Scholar]
- 68.Schalkwijk J, Zweers M C, Steijlen P M.et al A recessive form of the Ehlers‐Danlos syndrome caused by tenascin‐X deficiency. N Engl J Med 20013451167–1175. [DOI] [PubMed] [Google Scholar]
- 69.Matziolis G, Drahn T, Perka C. Spontaneous patellar tendon rupture in a patient with Ehlers‐Danlos syndrome). Unfallchirurg 20031061051–1053. [DOI] [PubMed] [Google Scholar]
- 70.Palmeri S, Mari F, Meloni I.et al Neurological presentation of Ehlers‐Danlos syndrome type IV in a family with parental mosaicism. Clin Genet 200363510–515. [DOI] [PubMed] [Google Scholar]
- 71.Palvolgyi R, Balint B J, Jozsa L. The Ehlers‐Danlos syndrome causing lacerations in tendons and muscles. Arch Orthop Trauma Surg 197995173–176. [DOI] [PubMed] [Google Scholar]
- 72.Hildebrand K A, Frank C B, Hart D A. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther 200411368–378. [DOI] [PubMed] [Google Scholar]