Abstract
Objective
Abnormal imaging in the patellar tendon reveals pathology that is often associated with knee pain. Anthropometric measures of body size and mass, such as height, weight and waist‐to‐hip ratio (WHR), have been individually associated with abnormal imaging. The aim of this study was to investigate the anthropometric factors that have the strongest relationship with abnormal imaging in volleyball players.
Methods
Height, weight, body mass index (BMI), waist girth, hip girth and WHR were measured in a cohort of 113 competitive volleyball players (73 men, 40 women). The univariate (ANOVA) and multivariable (discriminant function analysis) association between abnormal imaging and these anthropometric factors were investigated.
Results
No significant association was found in the female volleyball players. A significant univariate association was observed between abnormal imaging and heavier weight, greater BMI, larger waist and hip girth and larger WHR in the male volleyball players. Waist girth was the only factor that retained this association in a multivariable model (p<0.05).
Conclusions
Men with a waist girth greater than 83 cm seem to be at greater risk of developing patellar tendon pathology. There may be both mechanical and biochemical reasons for this increased risk.
Patellar tendon pathology detected by abnormal imaging1 may be present in 41% of competitive volleyball players.2,3 In more than two thirds of these players abnormal imaging was associated with pain.2,3 Players with patellar tendon pain and abnormal imaging may require several months away from sport or they may never return to their pre‐injury level of competition.4,5 However, few studies have investigated the treatment of this injury6,7 and clinicians have limited management strategies.8 Identification of risk factors for patellar tendon pathology may lead to preventive management or the early identification of injury.
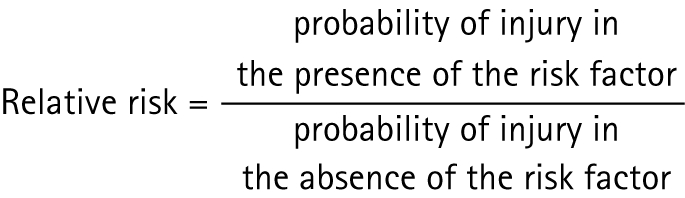
There are likely to be some anthropometric risk factors for patellar tendon pathology. Anthropometric factors including taller height,9 heavier weight10 and a larger waist‐to‐hip ratio (WHR)11 have been shown in the literature to be associated with abnormal patellar tendon imaging. These factors indicate that larger body size and greater body mass may increase the risk of developing patellar tendon pathology.
Conversely, waist and hip girth were not found to be associated with abnormal imaging, but this was in a single study that only investigated female athletes.11 Additionally, body mass index (BMI) is related to weight and height, but there is a paucity of evidence concerning the association between this factor and abnormal imaging.
Anthropometric factors related to body size and mass, such as weight and waist girth, are likely to be correlated. Some factors may only be associated with abnormal patellar tendon imaging by virtue of their association with another factor. For this reason, determining the factors most strongly associated with abnormal patellar tendon imaging may identify the most clinically‐useful risk factors.
Therefore, our main aim was to identify the anthropometric factors (height, weight, BMI, waist girth, hip girth and WHR) that have the strongest multivariable relationship with abnormal patellar tendon imaging among male and female volleyball players. We also investigated factors associated with this injury at the univariate level.
Materials and methods
Subjects
Male and female volleyball players were recruited from the Victorian State League competition in Australia. To minimise selection bias all players from all teams were invited to participate in the study. Players under 18 years of age were excluded because juvenile injuries, such as Osgood–Schlatter disease and Sinding‐Larsen–Johansson syndrome, are difficult to differentiate from patellar tendon injury.12
Procedures
Potential anthropometric risk factors (weight, height and waist and hip girth) were assessed at the start of the volleyball season using protocols with acceptable intra‐ and inter‐tester reliability.13,14 The assessors were blind to the players' imaging and pain status.
An experienced musculoskeletal ultrasonographer performed bilateral patellar tendon ultrasound scans with a high resolution 12 MHz transducer (Siemens Accuson, Medical Solutions Inc., Malvern, Philadelphia, USA). The ultrasonographer was blind to the factor scores and current pain findings. Tendons were defined as abnormal if they contained a focal hypoechoic region evident in both the longitudinal and transverse images and/or appeared diffusely hypoechoic and thickened in the proximal tendon. Ultrasound imaging has been shown to be reliable in detecting abnormality within the patellar tendon.15
Data analysis
The outcome variable consisted of three groups: (1) normal imaging, (2) abnormal imaging unilaterally and (3) abnormal imaging bilaterally. Men and women were separated in all analyses because they differed significantly (p<0.05) among all anthropometric factors except for hip girth.
Descriptive data (mean, SD) were produced for each factor across the three groups for men and women. Analysis of variance determined whether there were significant differences between the group means for each factor. The α‐level was Bonferroni‐adjusted (α/c, where α = 0.05 and c = the number of comparisons) to accommodate for multiple comparisons (adjusted α = 0.01).16 A trend was defined as being between 0.10 and the adjusted α‐level (0.01).16 Post‐hoc tests (Student Newman‐Keuls) were performed among significant univariate factors. Factors that were significant or showed a trend towards significance were entered into a forward‐stepwise‐discriminate function analysis.
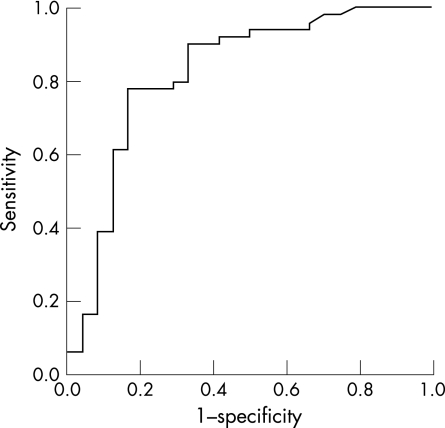
Among factors that were significant in the discriminant model, receiver operator characteristic (ROC) curves were calculated in order to investigate the risk‐factor score that most accurately discriminated between the two groups. A ROC curve plots the true‐positive rate (sensitivity) against the false‐positive rate (one minus the specificity) for each possible cut‐off score. The area under the ROC curve can be interpreted as the probability of the presence of a particular risk factor, correctly identifying a player in one of two groups from a pair of players, one randomly selected from each group. The area under the curve can range from 0.5 (no diagnostic accuracy) to 1.0 (perfect diagnostic accuracy).17
The prevalence of abnormal imaging in the sample was noted and used to determine the pre‐test odds of abnormal imaging unilaterally and bilaterally. For each significant risk factor, the risk‐factor score derived from the ROC curve was used to calculate the relative risk of being in a particular group compared with another. For each of these risk factors, the pre‐test and post‐test probability of being in a particular group were also calculated and the absolute risk increase explained by the risk factor was determined.
 |
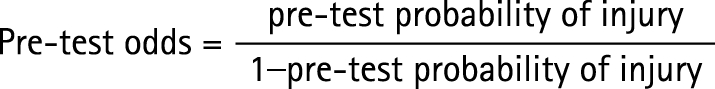 |
 |
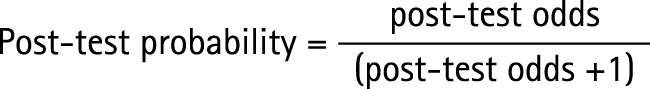 |
Results
There were 113 volleyball players (73 men, 40 women) in the cohort. Descriptive data for each factor across each of the three groups are shown in table 1.
Table 1 Descriptive data and significance tests among men and women.
| Normal imaging | Abnormal imaging unilaterally | Abnormal imaging bilaterally | p Value | ||
|---|---|---|---|---|---|
| Men | n | 24 (38%) | 21 (33%) | 28 (29%) | |
| Height (cm) | 184.2 (8.7) | 187.0 (8.6) | 187.1 (7.9) | 0.39 | |
| Weight (kg) | 76.7 (10.9) | 87.2 (12.0) | 90.1 (10.5) | <0.01 | |
| BMI | 22.6 (2.8) | 24.8 (2.0) | 25.7 (2.6) | <0.01 | |
| Waist girth (cm) | 79.1 (7.1) | 84.7 (5.8) | 89.5 (7.8) | <0.01 | |
| Hip girth (cm) | 98.2 (5.4) | 103.1 (6.7) | 105.3 (6.5) | <0.01 | |
| WHR | 0.80 (0.04) | 0.82 (0.03) | 0.85 (0.04) | <0.01 | |
| Women | n | 19 (47%) | 16 (40%) | 5 (13%) | |
| Height (cm) | 173.3 (4.7) | 173.2 (7.1) | 174.5 (3.1) | 0.90 | |
| Weight (kg) | 66.9 (7.8) | 68.8 (7.7) | 76.0 (5.6) | 0.07 | |
| BMI | 22.3 (2.2) | 23.0 (2.7) | 25.0 (2.0) | 0.09 | |
| Waist girth (cm) | 74.0 (5.1) | 74.8 (7.4) | 80.5 (6.9) | 0.13 | |
| Hip girth (cm) | 99.9 (6.7) | 97.5 (9.6) | 106.4 (4.8) | 0.10 | |
| WHR | 0.74 (0.04) | 0.76 (0.04) | 0.76 (0.04) | 0.48 |
BMI, body mass index; WHR, waist‐to‐hip ratio. Number in brackets is SD unless % shown.
Weight, BMI, waist girth, hip girth and WHR were associated with abnormal imaging among men (p<0.01) (table 1). Among women, there was only a trend towards an association between BMI, weight, hip girth in the three groups (table 1); therefore post‐hoc tests were only performed among men.
Post‐hoc tests indicated that waist girth was the only factor that had an ordinal relationship with the three groups (p<0.05) (table 1). Men with abnormal unilateral or bilateral imaging were more likely to be heavier and have a larger BMI than men with normal imaging (p<0.05) (table 1). Men with abnormal imaging bilaterally were more likely to have a larger hip girth and a larger WHR than their counterparts who had either abnormal imaging on one side or normal imaging on both (p<0.05) (table 1).
No factors were retained in the multivariable model among women. Among men, only waist girth retained significance in the multivariable model and explained 40% of the variance between the groups (table 2). Waist girth improved accuracy in classifying men into the groups by 18% compared with chance alone. Waist girth was strongly correlated with several factors (table 3) and this may explain why these factors were not significant in the discriminant model.
Table 2 Results of discriminant function analysis among men.
| Discriminant model | |
|---|---|
| Factor(s)* | Waist girth (1.00) |
| Wilk's lambda | 0.71† |
| Eigenvalue | 0.41 |
| Classification accuracy‡ | 56.2% (38.4%) |
*Significant factors (standardised discriminant function coefficients).
†p<0.01.
‡Percentage of cases correctly classified (percentage correctly classified by chance).
Table 3 Correlation between factors in the discriminant function analysis.
| Weight | BMI | Waist girth | Hip girth | WHR | |
|---|---|---|---|---|---|
| Weight | ___ | 0.79* | 0.79* | 0.85* | 0.38* |
| BMI | ___ | 0.80* | 0.74* | 0.52* | |
| Waist girth | ___ | 0.86* | 0.75* | ||
| Hip girth | ___ | 0.31* | |||
| WHR | ___ |
*Correlation is significant at the 0.01 level
BMI, body mass index; WHR, waist‐to‐hip ratio.
A ROC curve was plotted to identify the waist‐girth score that best discriminated between players with normal imaging and players with abnormal imaging either unilaterally or bilaterally (fig 1). Men with abnormal unilateral or bilateral imaging were grouped together because the purpose was to investigate the waist‐girth score that could identify abnormal imaging on one or both sides of the body. The area under the ROC curve between players with normal and abnormal imaging either unilaterally or bilaterally was 0.82. Based on the coordinates of the ROC curve, 83 cm was the waist‐girth score that most accurately identified tendons with abnormal unilateral or bilateral imaging.
Figure 1 Receiver operator characteristic curve for waist girth among men.
There was a post‐test probability of approximately 74% that players with a waist‐girth score greater than 83 cm had abnormal imaging on one or both sides (table 4). By contrast, there was a post‐test probability of 15% that players with a waist‐girth score below 83 cm had abnormal imaging on one or both sides (table 4).
Table 4 Relative risk, pre‐test and post‐test probability, absolute risk increase, sensitivity and specificity.
| Pre‐test probability (prevalence) | Post‐test probability (<83 cm waist girth) | Post‐test probability (>83 cm waist girth) | Absolute risk increase | Relative risk (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| 62% | 15% | 74% | 59% | 2.4 (1.5 to 3.8) | 76% (62% to 85%) | 83% (64% to 93%) |
Discussion
This study investigated the association between various anthropometric factors and abnormal imaging in the patellar tendons of men and women. Waist girth was associated with abnormal imaging in the multivariable model, but only among men. This finding indicates that waist girth had the dominant statistical association with abnormal imaging and a greater likelihood of being involved in the development of tendon pathology, which is revealed by abnormal imaging.1 Men with a waist girth greater than 83 cm were two and a half times more likely to have abnormal patellar tendon imaging on one or both sides.
Waist girth was also the only factor that demonstrated a significant ordinal relationship with abnormal unilateral and bilateral imaging—that is, players with bilateral abnormal imaging were likely to have a larger waist girth than players with unilateral abnormal imaging, who tended to have a larger waist girth than players with normal imaging. This dose‐dependent relationship between waist girth and abnormal imaging unilaterally and bilaterally strengthens the evidence that waist girth is associated with the development of tendon pathology and abnormal imaging.
Increased waist girth may have a mechanical influence on tendon pathology. Waist girth was significantly correlated with weight (table 3), which is likely to reflect patellar tendon load. Load has been shown to stimulate an adaptive process within the tendon as it attempts to remodel and strengthen.19 Tendon remodelling may fail when load is excessive and/or repetitive, resulting in tendon pathology.20 As weight was not retained in the multivariate model, however, patellar tendon load may not entirely explain the association between waist girth and abnormal patellar tendon imaging.
Waist girth is a good measure of abdominal adipose tissue,21,22 and this tissue releases free fatty acids into the circulation during adipocyte lipolysis, as well as pro‐inflammatory cytokines.22 Free fatty acids and cytokines have been linked to disorders such as heart disease and diabetes.22 These biochemical substances may also adversely affect tendon function and metabolism23 and predispose to pathology and abnormal imaging. Therefore, waist girth may have a biochemical as well as a mechanical influence on the development of patellar tendon pathology.
The association between waist girth and abnormal patellar tendon imaging may also be partly explained by sex hormones. Sex hormones, including oestrogen, influence the distribution of adipose tissue around the body.24 Differential oestrogen levels contribute to greater adipose tissue stores around the waist in men (android body type) and the pelvis in women (gynoid body type).24 Oestrogen receptors have been identified in human tendons, although their function is not clear.25 It is possible that lower oestrogen levels may explain the link between a larger waist girth and abnormal patellar tendon imaging among men. However, WHR is likely to be a better indicator of body type and hormone levels than is waist girth,24,27 but this factor was not significant in the multivariable model. This suggests that abdominal adipose tissue is likely to have a greater influence on patellar tendon health than oestrogen among men.
Abdominal adipose tissue may partly explain the univariable association between heavier weight, greater BMI, larger hip girth, greater WHR and abnormal patellar tendon imaging as these factors were significantly correlated with waist girth (table 3). The current findings also suggest that waist girth may partly explain the association between weight and patellar tendon injury in previous studies among male volleyball players that did not consider the influence of waist girth in a multivariable model.10,28
What is already known on this topic
Anthropometric factors including taller height, heavier weight and a larger waist‐to‐hip ratio have been associated with abnormal patellar‐tendon imaging
These anthropometric factors are likely to be correlated and the strongest predictor of abnormal patellar‐tendon imaging is not known
What this study adds
Waist girth seems to have a stronger association with abnormal patellar‐tendon injury than other anthropometric factors among men
As waist girth is an accurate anthropometric surrogate for abdominal adipose tissue, free fatty acids and cytokines released by abdominal adipose tissue may have a role in tendon pathology
The strongest anthropometric predictor of abnormal patellar‐tendon imaging among women is unclear at present.
The association between waist girth and abnormal patellar tendon imaging was not found among women. This may be explained by the relatively small sample of women in the study. There was, however, a trend towards an association between abnormal patellar tendon imaging and weight, BMI and hip girth among women. A previous study reported that WHR, but not waist girth, was associated with abnormal patellar tendon injury among female basketball players.11 These findings suggest that waist girth may have less influence on tendon pathology and abnormal imaging among women compared with men. This may be because women are likely to deposit adipose tissue around their pelvis rather than their abdomen.24 Adipose tissue around the pelvis is less metabolically active and releases lower levels of free fatty acids and cytokines than abdominal adipose tissue.22
Based on the findings of this study, clinicians should consider waist girth when assessing risk of patellar tendon pathology among male volleyball players. Modification of other known risk factors, such as activity level10 may assist in preventing tendon pathology and abnormal imaging among men with a waist girth greater than 83 cm. This is important as abnormal imaging may be associated with pain in two thirds of male volleyball players.2 In addition, further research into the potential biochemical role of waist girth in tendon injury may reveal other treatment options.
A limitation of this cross‐sectional study is that the anthropometric factors identified as being associated with abnormal imaging can vary in their magnitude over time and, therefore, may have developed secondarily to injury. Prospective longitudinal studies are required to confirm that the significant factors are risk factors for patellar tendon injury. In addition, it is possible that the findings of this study may only apply to adult volleyball players, as the factors associated with abnormal imaging may vary among participants of different sports or age groups. The sample of women included may have also been too small to identify any significant associations between women.
Conclusion
Waist girth has a strong association with abnormal patellar tendon imaging and may be involved in the development of tendon pathology via mechanical or biochemical mechanisms. Players with a waist girth greater than 83 cm may be at increased risk of injury on one or both sides of the body.
Abbreviations
BMI - body mass index
ROC - receiver operator characteristic
WHR - waist‐to‐hip ratio
Footnotes
Ethics approval was granted by the Human Ethics Committee at La Trobe University, Victoria, Australia. Participants gave informed consent before the start of the study.
References
- 1.Mourad K, King J, Guggiana P. Computed tomography and ultrasound imaging of jumper's knee—patellar tendinitis. Clin Radiol 198839162–165. [DOI] [PubMed] [Google Scholar]
- 2.Lian O, Holen K J, Engebretson L.et al Relationship between symptoms of jumper's knee and the ultrasound characteristics of the patellar tendon among high level male volleyball players. Scand J Med Sci Sports 19966291–296. [DOI] [PubMed] [Google Scholar]
- 3.Laforgia R, Capocasale N, Saracino N.et al A clinical and ultrasonographic study of jumper's knee and the achilles tendon in volleyball players. J Sports Traumatol Related Research 199214127–138. [Google Scholar]
- 4.Ferretti A. Epidemiology of jumper's knee. Sports Med 19863289–295. [DOI] [PubMed] [Google Scholar]
- 5.Popp J E, Yu J S, Kaeding C C. Recalcitrant patellar tendinitis: magnetic resonance imaging, histologic evaluation and surgical treatment. Am J Sports Med 199725218–222. [DOI] [PubMed] [Google Scholar]
- 6.Visnes H, Hoksrud A, Cook J.et al No effect of eccentric training on jumper's knee in volleyball players during the competitive season. A randomised controlled trial. Clin J Sport Med 200515225–232. [DOI] [PubMed] [Google Scholar]
- 7.Young M, Cook J, Purdam C.et al Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med 200539102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook J, Khan K. What is the most appropriate treatment for patellar tendinopathy? Br J Sports Med 200135291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook J L, Khan K M, Kiss Z S.et al Asymptomatic hypoechoic regions on patellar tendon ultrasound: a 4‐year clinical and ultrasound followup of 46 tendons. Scand J Med Sci Sports 2001111–7. [DOI] [PubMed] [Google Scholar]
- 10.Lian O, Engebretsen L, Ovrebo R V.et al Characteristics of the leg extensors in male volleyball players with jumper's knee. Am J Sports Med 199624380–385. [DOI] [PubMed] [Google Scholar]
- 11.Gaida J, Cook J, Bass S.et al Are unilateral and bilateral patellar tendinopathy distinguished by differences in anthropometry, body composition, or muscle strength in elite female basketball players? Br J Sports Med 200438581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puddu G, Cipolla M, Selvanetti A.et al Patellar tendinopathies. J Sports Traumatol Related Research 19992141–48. [Google Scholar]
- 13.Norton K, Olds T.Anthropometrica. Sydney: University of New South Wales Press, 1996
- 14.Chen M M, Lear S A, Gao M.et al Intraobserver and interobserver reliability of waist circumference and the waist‐to‐hip ratio. Obes Res 20019651–652. [DOI] [PubMed] [Google Scholar]
- 15.Khan K M, Cook J L, Kiss Z S.et al Patellar tendon ultrasonography and jumper's knee in elite female basketball players: a longitudinal study. Clin J Sports Med 19977199–206. [DOI] [PubMed] [Google Scholar]
- 16.Keppel G, Sheldon Z.Data analysis for research designs. New York: WH Freeman and Co, 1989
- 17.Hanley J A, McNeil B J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 198214329–36. [DOI] [PubMed] [Google Scholar]
- 18.Sackett D, Richardson W, Rosenberg W.et alEvidence‐based medicine: how to practice and teach EBM. 1st edn. Edinburgh: Churchill Livingstone, 1998
- 19.Kjaer M. Role of Extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 200484649–698. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter W. Cell matrix response in tendon injury. Clin Sports Med 199211533–578. [PubMed] [Google Scholar]
- 21.Dalton M, Cameron A J, Zimmet P Z.et al Waist circumference, waist‐hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Int Med 2003254555–563. [DOI] [PubMed] [Google Scholar]
- 22.Misra A, Vikram N K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tisue deposits. Nutrition 200319457–466. [DOI] [PubMed] [Google Scholar]
- 23.Wong S, Janssen I, Ross R. Abdominal adipose tissue distribution and metabolic risk. Sports Med 200333709–726. [DOI] [PubMed] [Google Scholar]
- 24.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 199620291–302. [PubMed] [Google Scholar]
- 25.Hart D A, Archambault J M, Kydd A.et al Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin Orthop 199835144–56. [PubMed] [Google Scholar]
- 26.Liu S H, Al‐Shaikh R A, Panossian V.et al Estrogen affects the cellular metabolism of the anterior cruciate ligament: A potential explanation for female athletic injury. Am J Sports Med 199725704–709. [DOI] [PubMed] [Google Scholar]
- 27.Fink B, Neave N, Manning J T. Second to fourth digit ratio, body mass index, waist‐to‐hip ratio, and waist‐to‐chest ratio: their relationship in heterosexual men and women. Ann Human Biol 200330728–738. [DOI] [PubMed] [Google Scholar]
- 28.Lian O, Refsnes P, Engebretsen L.et al Performance characteristics of volleyball players with patellar tedinopathy. Am J Sports Med 200331408–413. [DOI] [PubMed] [Google Scholar]