Abstract
Four antigen preparations from Rhizopus arrhizus were made and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and column chromatography. Electrophoretic analyses of these antigens indicated that there are 18 to 28 component bands with a molecular mass range of approximately 10,500 to 83,000 daltons. Seven of these bands appear to be components common to three antigen preparations. Several of the bands identified by SDS-PAGE were composed of glycoproteins or carbohydrates as determined by their affinity for concanavalin A. Western blots, using sera from five patients with mucormycosis, consistently identified five different determinants in the R. arrhizus antigens separated by SDS-PAGE. This suggests that several of the Rhizopus antigens are present during mucormycosis. Four of the antigenic determinants recognized by patient sera reacted with the concanavalin A-peroxidase stain, indicating that they are composed of glycoproteins or carbohydrate. Enzyme-linked immunosorbent assays of sera from five patients with mucormycosis and with rabbit antisera resulted in antibody titers ranging from 1:64 to 1:32,000 for the R. arrhizus antigens.
Full text
PDF




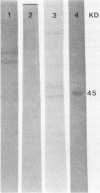
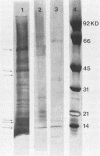
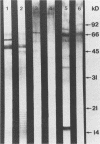
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Eades S. M. Experimental phycomycosis in mice; examination of the role of acquired immunity in resistance to Absidia ramosa. J Hyg (Lond) 1976 Oct;77(2):221–233. doi: 10.1017/s0022172400024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Wessels J. G., van den Ende H. The hyphal wall of Mucor mucedo. 2. Hexosamine-containing polymers. Eur J Biochem. 1977 Nov 1;80(2):621–626. doi: 10.1111/j.1432-1033.1977.tb11919.x. [DOI] [PubMed] [Google Scholar]
- Datema R., van den Ende H., Wessels J. G. The hyphal wall of Mucor mucedo. 1. Polyanionic polymers. Eur J Biochem. 1977 Nov 1;80(2):611–619. doi: 10.1111/j.1432-1033.1977.tb11918.x. [DOI] [PubMed] [Google Scholar]
- Hearn V. M., Mackenzie D. W. The preparation and partial purification of fractions from mycelial fungi with antigenic activity. Mol Immunol. 1980 Sep;17(9):1097–1103. doi: 10.1016/0161-5890(80)90106-6. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Kaufman L. Development and evaluation of an immunodiffusion test for diagnosis of systemic zygomycosis (mucormycosis): preliminary report. J Clin Microbiol. 1978 Jan;7(1):97–103. doi: 10.1128/jcm.7.1.97-101.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Standard P. G., Kaufman L. Specific immunological test for the rapid identification of members of the genus Histoplasma. J Clin Microbiol. 1976 Feb;3(2):191–199. doi: 10.1128/jcm.3.2.191-199.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf A. R., Halde C., Vedros N. A. Immunodiffusion and complement fixation assays with sera from mucormycotic-infected mice. Mycopathologia. 1983 Nov 25;83(3):157–160. doi: 10.1007/BF00437022. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Halde C., Vedros N. A. Passive immunization in murine mucormycosis. Mycopathologia. 1983 Nov 25;83(3):149–155. doi: 10.1007/BF00437021. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R. Host-parasite relationship in opportunistic mycoses. Crit Rev Microbiol. 1986;13(2):133–172. doi: 10.3109/10408418609108737. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Peter L., Polak A. Mucormycotic infection in mice following prolonged incubation of spores in vivo and the role of spore agglutinating antibodies on spore germination. Sabouraudia. 1984;22(2):101–108. doi: 10.1080/00362178485380171. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Ruderman N., Diamond R. D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984 Jul;74(1):150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Sarinana F. O. The staining of sciatic nerve glycoproteins on polyacrylamide gels with concanavalin A-peroxidase. Anal Biochem. 1975 Nov;69(1):320–322. doi: 10.1016/0003-2697(75)90597-7. [DOI] [PubMed] [Google Scholar]
- Wu F. S., Wang M. Y. Extraction of proteins for sodium dodecyl sulfate-polyacrylamide gel electrophoresis from protease-rich plant tissues. Anal Biochem. 1984 May 15;139(1):100–103. doi: 10.1016/0003-2697(84)90394-4. [DOI] [PubMed] [Google Scholar]