Summary
Inhibition of Na+/H+ exchange (NHE) during ischemia reduces cardiac injury due to reduced reverse mode Na+/Ca2+ exchange. We hypothesized that activating NHE-1 at buffer pH 8 during ischemia increases mitochondrial oxidation, Ca2+ overload and reactive O2 species (ROS) levels, and worsens functional recovery in isolated hearts and that NHE inhibition reverses these effects. Guinea pig hearts were perfused with buffer at pH 7.4 (control) or pH 8 +/− NHE inhibitor eniporide for 10 min before and for 10 min after 35 min ischemia and then for 110 min with pH 7.4 buffer alone. Mitochondrial NADH and FAD, [Ca2+], and superoxide were measured by spectrophotofluorometry. NADH and FAD were more oxidized and cardiac function was worse throughout reperfusion after pH 8 vs. pH 7.4, Ca2+ overload was greater at 10 min reperfusion, and superoxide generation was higher at 30 min reperfusion. The pH 7.4 and eniporide groups exhibited similar mitochondrial function and cardiac performance was most improved after pH 7.4+eniporide. Cardiac function on reperfusion after pH 8+eniporide was better than after pH 8. % infarction was largest after pH 8 and smallest after pH 7.4+eniporide. Activation of NHE with pH 8 buffer and the subsequent decline in redox state with greater ROS and Ca2+ loading underlie the poor functional recovery after ischemia and reperfusion.
Keywords: energy metabolism, free radicals, ischemia, mitochondria, reperfusion, Na+/H+ exchange
Cardiac ischemia reperfusion (I/R) injury describes the injury a heart sustains when deprived of coronary perfusion followed by a sudden reperfusion. Major factors underlying I/R injury are cytosolic and mitochondrial (mt) Ca2+ loading and excess generation of reactive O2 species (ROS).1 The increase in mtCa2+ loading is a result of an I/R –induced increase in cytosolic Ca2+ loading and occurs largely via the mitochondrial Ca2+ uniporter (CaU).2 Na+/H+ exchange (NHE) activity is believed to be minimal under normal pH conditions but increases with an increase in buffer pH.3 NHE may become activated during ischemia in response to intracellular acidosis during anaerobic metabolism, but is especially activated during early reperfusion when the transmembrane pH gradient is largest. Inhibition of cytosolic Na+ accumulation induced by activation of NHE, and reduction of excess cytosolic Ca2+ influx via reverse mode Na+/Ca2+ exchange (NCE), are the probable mechanisms of acute cardioprotection afforded by NHE inhibitors.4–6 The relative role of sarcolemmal vs. mitochondrial NHE (mtNHE) in these events is unknown, but mtCa2+ loading could also result from mtNHE and mtNCE.7,8 Our objective was to test if buffer pH –induced activation of NHE is responsible for the subsequent increase in mtCa2+ overload and how this might lead to mitochondrial as well as cardiac dysfunction.
In previous reports we showed that blocking the NHE-1 isoform with eniporide (ENI) improved function and reduced infarct size on reperfusion after 6 h of no-flow 3°C storage in acidic cardioplegic solution,9 and that inhibition of NHE was as effective as cardioplegia alone in reducing cytosolic [Ca2+] after 4 h of cold ischemia.10 We,11 and others,12–14 have also shown that blocking NHE reduced ischemia -induced Na+ and Ca2+ overload and improved warm post-ischemic contractile recovery. The most direct involvement of NHE-1 in I/R injury comes from a study showing that mice carrying a null mutation in the Nhe1 gene were protected against I/R injury.15
In previous studies10,11 we measured the effects of blocking NHE on cytosolic Ca2+ in intact hearts. In the present study our aim was to assess the effects of augmented NHE by alkalosis on mitochondrial Ca2+ and energetics in the intact heart during I/R injury and the reversibility of these effects with inhibition of NHE. We hypothesized that brief perfusion of hearts at pH 8.0 before and after ischemia would cause an additional increase in the trans-sarcolemmal proton gradient, so that cell acidosis during late ischemia and early reperfusion would augment activation of NHE. In turn NHE would cause an exaggerated increase in mt[Ca2+] and lead to a more oxidized redox state (less NADH, more FAD) and an increase in ROS generation during and after ischemia, thereby contributing to poor recovery from I/R injury.
We predicted that inhibition of NHE with ENI during I/R-induced cytosolic Ca2+ and mtCa2+ loading would not only improve myocardial function and reduce cell death, but also restore the mitochondrial redox state, reduce mtCa2+ loading and lower ROS production, which together contribute to reducing cardiac cell injury. To test this, we measured myocardial function and tissue damage, and used fluorescence techniques to assess on-line changes in redox state (NADH and FAD), mt[Ca2+], and superoxide (O2−•) generation in the isolated beating heart.
METHODS
Langendorff Heart Preparation
The experiments conformed to the Guide for the Care and Use of Laboratory Animals (US NIH Publication No. 85–23, Revised 1996) and were approved by the Medical College of Wisconsin Biomedical Resources Studies Committee. Guinea pigs (n=84) were anesthetized with ketamine (50 mg/kg, IP) and decapitated. After thoracotomy, hearts were removed and perfused at 55 mmHg via the aortic root as described previously11,16–18 with a HEPES buffer solution (gassed with 5% CO2, 95% O2) containing (in mM) 140 Na+, 4.5 K+, 2.5 Ca2+, 1.2 Mg2+, 134 Cl−, 11.5 glucose, 2 pyruvate, 16 mannitol, 0.1 probenecid, 0.05 EDTA, 5U/L insulin, 5 HEPES [4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid N-(2-hydorxyethyl) piperazine-N’-(2-ethanesulfonic acid)]) at pH 7.4 and 37°C. Buffer pH 8 was obtained by titrating CO2 into HEPES buffer as described19 and with 1 M NaOH. Final buffer [Na+] was 140±2 mM at pH 7.4 and 148±3 mM at pH 8. HEPES buffer was used to maintain extracellular [Ca2+] constant during changes in buffer pH because a large increase in buffer pH in bicarbonate/phosphate buffers causes a large fall in free [Ca2+].19 Buffer [Ca2+] was 2.26±0.02 mM at pH 7.4 and 2.23±0.02 mM at pH 8.
Isovolumetric left ventricular pressure (LVP) and its first derivatives (dLVP/dtmax, contractility; dLVP/dtmin, relaxation), heart rate (HR), and coronary flow were measured as described.11,16–18 Coronary arterial (aortic inflow) and coronary venous (right ventricular outflow) Na+, K+, Ca2+, pCO2, pO2 and pH were measured off-line with an intermittently self-calibrating analyzer system; venous pO2 was also measured continuously with an elecrode placed in the coronary effluent tubing. Cardiac O2 delivery was defined as coronary flow•heart weight−1•(paO2)•24 µL O2/mL (37°C); cardiac O2 consumption (MVO2) as coronary flow•heart weight−1•(paO2-pvO2)•24 µL O2/mL (37°C) at 760 mmHg; cardiac efficiency as developed LVP•HR/MVO2; and %O2 extraction as 100•(paO2 - pvO2)/paO2 (where paO2 and pvO2 are arterial and venous pO2, respectively).
Measurements of Cardiac Mitochondrial Redox State, O2−• and [Ca2+]
FAD fluorescence is derived only from mitochondria; the majority of the NADH signal also arises from mitochondria20–22 and mitochondria comprise about 1/3 the volume of cardiac myocytes.23,24 The majority of superoxide (O2−•) likely originates from cardiac mitochondria because its generation in the isolated heart is very sensitive to mitochondrial inhibitors and insensitive to inhibitors of xanthine oxidase.25 Myocardial [Ca2+] signals arise from non-cytosolic sources after quenching by MnCl2;31 the major non-cytosolic source is the mitochondrial compartment because of its large volume relative to cell volume.23,24
NADH and FAD, mt[Ca2+], or O2−• was measured near continuously via a trifurcated fiberoptic probe (3.8 mm2/bundle) placed directly on the free LV wall using one of four excitation (λex) and emission (λem) fluorescence wavelengths11,16–18,26 assessed by spectrophotofluorometery (SLM Instruments Inc, Urbana IL; and Photon Technology International, London ON) in different subsets of hearts. Fluorescence light intensity is transmural but attenuated at the endocardial surface to 20–30% of that at the epicardial surface.27 In a subset of hearts, as described,16,18,26 10 µM dihydroethidium (DHE) was loaded for 20 min; at 540 nm λex and 590 nm λem the fluorescence is primarily a marker of O2−• radicals.28–30 An intermediate product of DHE is 2-hydroxyethidium, which is labile and fluoresces at a slightly shorter wavelength.29,30 We speculate that this labile intermediate forms rapidly, is reversible, and is not necessarily dependent on DNA chelation to generate the fluorescence signal. In other hearts NADH autofluorescence (350 nm λex and λem 450/390 nm) and FAD autofluorescence (480 nm λex and λem 540 nm) were measured near simultaneously.17,18,26 Alternatively, hearts were loaded with 6 µM indo 1 AM for 30 min; after washout, the cytosolic signal (350 nm λex and λem 390/450 nm) was quenched with 100 µM MnCl2, which permitted measurement of mt[Ca2+].31 mt[Ca2+] was corrected for NADH autofluorescence during I/R for each group. Calibration of indo 1 for [Ca2+] was described previously.32 Changing perfusate pH from 7.4 to 8 had no significant effect on Indo 1 fluorescence signal. Each signal was digitized and recorded at 200 Hz and computed later for mt[Ca2+]. Loading of DHE and indo 1 transiently decreases contractility; washout restores contractility.
Protocol
There was a time control group and two pH ischemia groups treated or untreated with 10 µM eniporide (ENI). This concentration was chosen because ten Hove et al.13 reported that 3 µM eniporide should block NHE by at least 95%. In each heart either mt[Ca2+], O2−• or NADH plus FAD were assessed under the same protocol. After baseline measurement, hearts of the four ischemia groups were perfused for 10 min either with pH 7.4 (ischemia control), pH 8 alone, pH 7.4+ENI, or pH 8+ENI. ENI alone did not alter fluorescence characteristics or spectra of any dye. This was followed by 35 min of no flow global ischemia induced by clamping the aortic inflow tubing. After ischemia, hearts were treated in the same manner as before ischemia for 10 min before reverting to perfusion at pH 7.4 for the remainder of reperfusion (110 min). At the end of each experiment hearts were removed and atria discarded; ventricles (1.3±0.2 g) were cut into 3–4 mm transverse sections and immersed in 0.1% 2,3,5-triphenyltetrazolium chloride (TTC) for measurement of infarct size.9–11,17,18,26
Experiments were also conducted in two additional groups, pH 6.5 and pH 6.5+ENI. Specific results from these studies are only displayed in Table 1. We observed that pH 6.5 alone or with ENI was as efficacious as pH 7.4+ENI in protecting mitochondria and improving functional recovery.
Table 1.
Effects of pH 6.5 with or without 10 µM eniporide (ENI) on cardiac function and mitochondrial bioenergetics during 30 and 60 min reperfusion.
| pH 6.5 | pH 6.5+ENI | |||||
|---|---|---|---|---|---|---|
| Variable measured | Baseline | Reperfusion 30 min | Reperfusion 60 min | Baseline | Reperfusion 30 min | Reperfusion 60 min |
| developed LVP (mmHg) | 83±1 | 47±4 | 51±4 | 87±2 | 50±4 | 46±5 |
| diastolic LVP (mmHg) | 0±0 | 26±5 | 18±5 | 0±0 | 12±3 | 10±2 |
| dLVP/dt max (mmHg/s) | 1674±79 | 869±133 | 1014±108 | 1589±93 | 940±154 | 962±130 |
| dLVP/dt min (mmHg/s) | −1270±38 | −500±118 | −726±64 | −1284±51 | −623±101 | −618±94 |
| coronary flow (ml/g/min) | 7.8±0.1 | 5.5±0.3 | 5.3±0.3 | 7.9±0.2 | 6.5±0.2 | 5.2±0.3 |
| NADH (afu) | 51±1 | 44±2 | 45±2 | 51±1 | 47±1 | 47±1 |
| FAD (afu) | 40±0 | 42±0 | 43±1 | 40±0 | 41±1 | 42±1 |
| superoxide free radical (afu) | 4.18±0.02 | 4.21±0.17 | 4.01±0.09 | 4.14±0.08 | 4.01±0.10 | 3.91±0.11 |
| mitochondrial [Ca2+] (nM) | 191±5 | 294±46 | 225±32 | 190±5 | 378±44 | 346±17† |
P <0.05: † pH 6.5+ENI vs. pH 6.5
Statistical Analysis
All data are expressed as mean ±SEM. Statistical differences were measured by two-way analysis of variance for repeated measures of a given variable across the four groups at specific time points (baseline, at 15 and 30 min ischemia, and at 10, 30 and 60 min during reperfusion). One-way analysis of variance was used to determine changes over time for a given variable at the same time points. If F tests were significant (P<0.05), appropriate ad hoc tests (Student-Newman-Keuls or Duncan) were used to compare means (P <0.05; two-tailed). As in our previous studies,11,18,26 functional recovery and changes in fluorescent signals at 60 min reperfusion were not significantly different from those at 120 min reperfusion (not displayed). Values for NADH, FAD and O2−• are expressed in arbitrary fluorescence units (afu) and m[Ca2+] in nM. Infarct size was determined in a blinded manner after 120 min reperfusion.
RESULTS
Mitochondrial Redox State, mt[Ca2+] and ROS Production
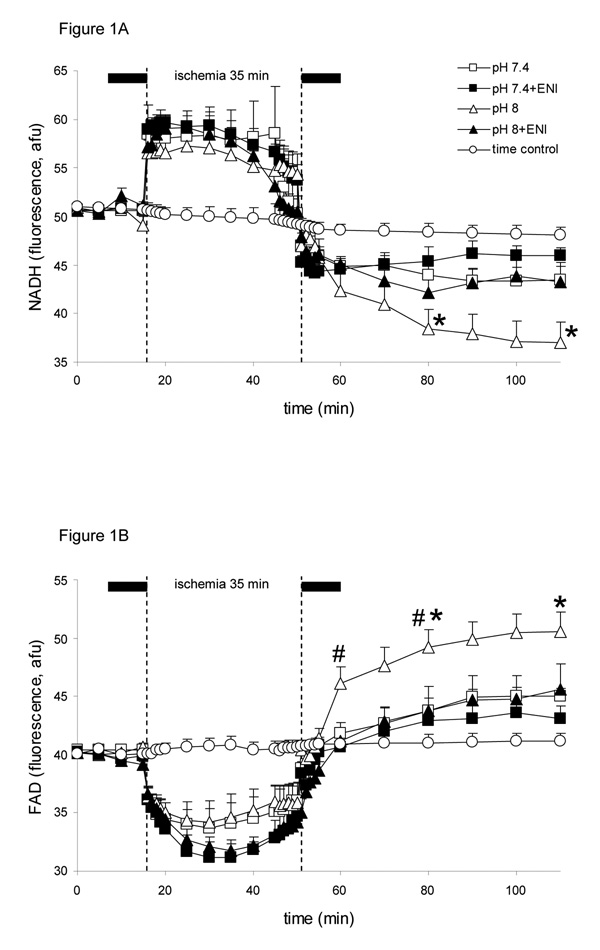
All variables in the time control (i.e., no ischemia) experiments remained unchanged during the 3 h period of perfusion. Baseline values for NADH (Fig. 1A) and FAD (Fig. 1B) (redox state) were not different among groups and did not change before ischemia due to pH or ENI. At the onset of ischemia NADH abruptly increased by approximately 17%, while FAD more slowly decreased by approximately 19%. Note that on reperfusion, NADH levels decreased and FAD levels increased, so that by 60 min reperfusion, NADH and FAD levels, respectively, were farther from basal values in the pH 8 group and more normalized in the pH 7.4+ENI group and pH 7.4 and pH 8+ENI groups. The biphasic changes in NADH and FAD during ischemia and reperfusion were not different between the pH 7.4+ENI and pH 7.4 groups. Acidic buffer (pH 6.5) had similar myocardial protective effects on NADH and FAD as did pH 7.4+ENI; pH 6.5+ENI did not improve mitochondrial redox state any better than pH 6.5 alone (Table 1).
Figure 1.
Changes in NADH (A) and FAD (B) (autofluorescence units, afu), during perfusion with HEPES buffer at pH 7.4 (control; n=7), pH 8 (n=7), pH 7.4+eniporide (ENI, 10 µM) (n=6), or pH 8+ENI (10 µM) (n=6) 10 min before and 10 min after 35 min no flow, global ischemia. A non-ischemia, pH 7.4 time control group (n=4) is also displayed for all variables. For P <0.05: * pH 8 vs. 7.4; # pH 8+ENI vs. pH 8; † pH 7.4+ENI vs. pH 7.4. Attenuated Na+/H+ exchange by the lower pH and or ENI led to a less oxidized redox state.
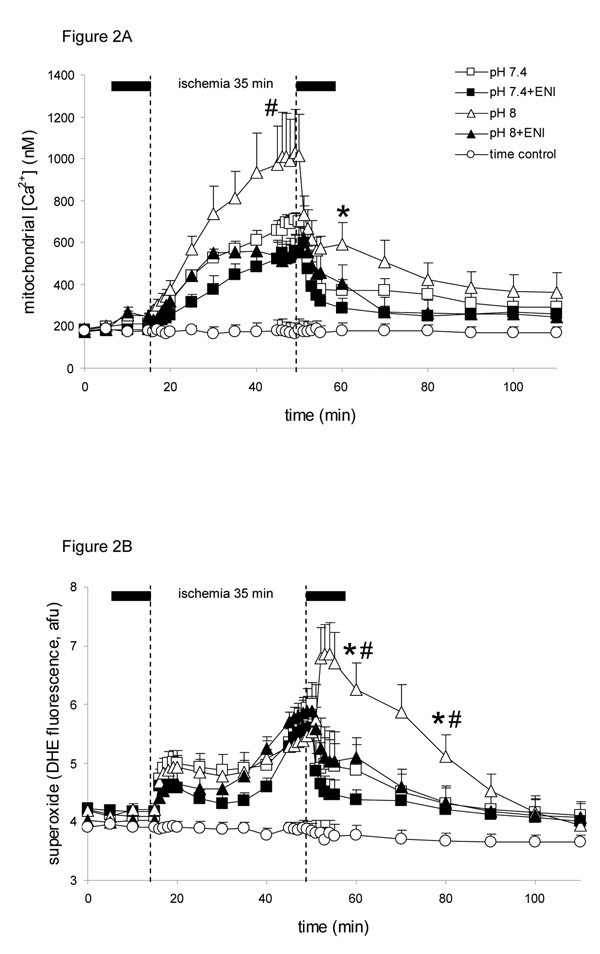
Baseline mt[Ca2+] was not different among groups (Fig. 2A). Note that toward the end of ischemia, mt[Ca2+] increased in all groups, but much more so in the pH 8 group than in the pH 7.4 and ENI treated groups. Compared to the pH 8 group, addition of ENI at pH 8 and pH 7.4 attenuated the rise in mt[Ca2+] during late ischemia. At 10 min reperfusion mt[Ca2+] was significantly elevated in the pH 8 group compared to other groups. mt[Ca2+] was reduced similarly in pH 7.4+ENI and pH 6.5 (Table 1) groups and mt[Ca2+] was higher in the pH 6.5+ENI group than in the pH 6.5 group at 60 min reperfusion (Table 1).
Figure 2.
Changes in mt[Ca2+] in nM (A) and superoxide (O2−•) in afu (B), during perfusion with HEPES buffer at pH 7.4 (control; n=8 each variable), pH 8 (n=7 each variable), pH 7.4+ENI (10 µM) (n=7 each variable), or pH 8+ENI (10 µM) (n=7 each variable) 10 min before and 10 min after 35 min no flow, global ischemia. For P <0.05: * pH 8 vs. 7.4; # pH 8+ENI vs. pH 8; † pH 7.4+ENI vs. pH 7.4. Attenuated Na+/H+ exchange by the lower pH and or ENI led to a smaller increase in mt[Ca2+] during ischemia and smaller increases in both mt[Ca2+] and O2−• during early reperfusion.
Baseline O2−• levels (Fig. 2B) were not different among groups. In late ischemia O2−• levels increased significantly in all groups to values not significantly different among groups. However, note that during 10 and 30 min reperfusion, O2−• surged higher in the pH 8 alone group, whereas it fell in all other groups, with pH 7.4+ENI showing the least increase in O2−• levels at 10 min reperfusion. O2−• levels remained significantly elevated during the first 30 min of reperfusion, but addition of ENI at pH 8 significantly reduced the reperfusion -induced increase in O2−• production to a level similar to that in the two pH 7.4 groups. The pH 6.5 and 6.5+ENI groups (Table 1) displayed similarly less O2−• production during reperfusion at a level equivalent to that of the pH 7.4+ENI group.
Cardiac Function and Infarct Size
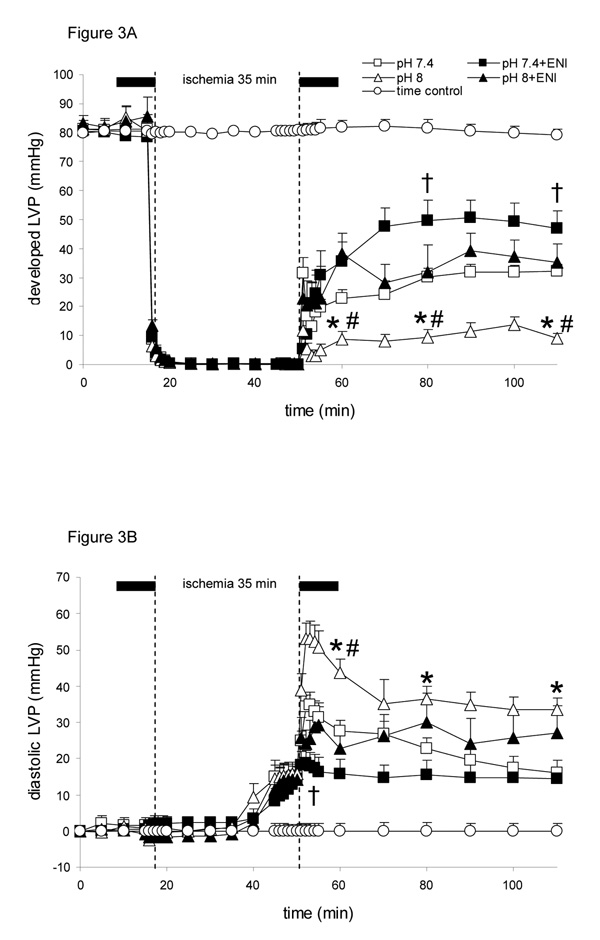
Baseline values were not different among groups for developed LVP (devLVP) (Fig. 3A); diastolic LVP (diaLVP) (Fig. 3B) was set initially at 0 mmHg. Throughout reperfusion devLVP recovered least in the pH 8 (11 ± 2 % of baseline after 60 min reperfusion) group compared to all other groups; recovery was better in the pH 7.4+ENI (58 ± 7 %) group and intermediate in the pH 8+ENI (42 ± 8 %) and pH 7.4 (40 ± 4 %) groups. Note that during late ischemia, diaLVP rose above baseline in all groups and on reperfusion continued to increase but remained higher in the pH 8 group throughout reperfusion. The pH 7.4+ENI group had the least increase in diaLVP during early reperfusion and the pH 7.4 and pH 8+ENI groups were intermediate. The pH 6.5+/− ENI groups exhibited similar increases in devLVP and decreases in diaLVP during reperfusion (Table 1); these variables were similar to those of the pH 7.4+ENI group.
Figure 3.
Changes in developed left ventricular pressure (systolic-diastolic LVP or devLVP in mmHg; A), and diastolic left ventricular pressure (diaLVP in mmHg; B) during perfusion with HEPES buffer at pH 7.4 (control; n=12), pH 8 (n=12), pH 7.4+ENI (10 µM) (n=12), or pH 8+ENI (10 µM) (n=12) 10 min before and 10 min after 35 min no flow, global ischemia. For P <0.05: * pH 8 vs. 7.4; # pH 8+ENI vs. pH 8; † pH 7.4+ENI vs. pH 7.4. Attenuated Na+/H+ exchange by the lower pH and or ENI led to better cardiac muscle function throughout reperfusion.
Functional and metabolic variables did not change over time in the time control (non ischemia) group (data not shown). Table 2 shows that dLVP/dtmax, dLVP/dtmin and cardiac efficiency recovered poorly at 10 and 60 min of reperfusion in the pH 8 group compared to the pH 7.4 group; adding ENI to either pH group improved recovery of dLVP/dtmax and dLVP/dtmin throughout reperfusion; these variables were similar among the pH 7.4+ENI and pH 6.5 groups (Table 1). Cardiac efficiency was slower to recover in all groups (10 min reperfusion) but remained severely depressed at 60 min reperfusion only in the pH 8 group. The pH 8+ENI group exhibited improved recovery of cardiac efficiency compared to the pH 8 group. Heart rate, O2 consumption, and %O2 extraction were not significantly different among the pH 7.4 and pH 8 groups on reperfusion.
Table 2.
Effects of perfusate pH and Na+/H+ exchange inhibition with eniporide (ENI) on cardiac variables during reperfusion after 35 min global cardiac ischemia.
| Variable measured | Baseline | Reperfusion 10 min | Reperfusion 60 min |
|---|---|---|---|
| dLVP/dt max (mmHg/s) | |||
| pH 7.4 | 1379±197 | 313±72 | 531±75 |
| pH 7.4+ENI | 1544±130 | 588±159† | 968±98† |
| pH 8 | 1369±128 | 88±35* | 211±47* |
| pH8 +ENI | 1531±69 | 470±234# | 620±152# |
| dLVP/dt min (mmHg/s) | |||
| pH 7.4 | −1055±148 | −206±40 | −406±58 |
| pH 7.4+ENI | −1275±103 | −440±117† | −628±79† |
| pH 8 | −1145±114 | −64±21* | −155±41* |
| pH 8+ENI | −1314±93 | −287±142# | −415±88# |
| Heart Rate (beats/min) | |||
| pH 7.4 | 247±17 | 242±18 | 255±14 |
| pH 7.4+ENI | 261±6 | 269±10 | 275±12 |
| pH 8 | 260±6 | 269±63 | 302±39 |
| pH 8+ENI | 263±6 | 245±36 | 272±12 |
| Oxygen Delivery (units) | |||
| pH 7.4 | 152.9±6.4 | 120.1±7.9 | 85.4±6.5 |
| pH 7.4+ENI | 151.7±13.1 | 130.8±6.9 | 106.1±2.2† |
| pH 8 | 166±12.8 | 95.5±9.8 | 86.4±14.7 |
| pH 8+ENI | 179±19.8 | 117.6±6.6 | 128.5±9.7# |
| Oxygen Consumption (units) | |||
| pH 7.4 | 86.8±6.7 | 71.7±4.9 | 53.1±5.4 |
| pH 7.4+ENI | 101.3+6.3 | 67.2±2.4 | 68.4±2.1† |
| pH 8 | 89.2±4.8 | 67.9±6.6 | 57.1±4.2 |
| pH 8+ENI | 107.3±7.6 | 81.2±10.7 | 66.6±1.8 |
| Oxygen Extraction (%) | |||
| pH 7.4 | 66.5±3.1 | 59.9±2.3 | 62.7±4.8 |
| pH 7.4+ENI | 67.4±3.2 | 51.7±3.8 | 64.6±3.2 |
| pH 8 | 64.9±3.1 | 71.7±2.8* | 61.3±3.1 |
| pH 8+ENI | 60.5±3.1 | 68.8±3.3 | 62.6±4.8 |
| Cardiac Efficiency (units) | |||
| pH 7.4 | 13.2±1.6 | 1.3±0.4 | 4.9±0.3 |
| pH 7.4+ENI | 12.5±2.2 | 2.3±0.1† | 6.8±1.4 |
| pH 8 | 11.8±1.5 | 1.0±0.7* | 2.1±0.5* |
| pH 8+ENI | 12.7±7 | 1.8±0.8 | 4.7+0.7# |
See Methods for calculation and units for oxygen delivery, consumption, and extraction and for cardiac efficiency.
For P <0.05: * pH 8 vs. 7.4
pH 8+ENI vs. pH 8
pH 7.4+ENI vs. pH 7.4.
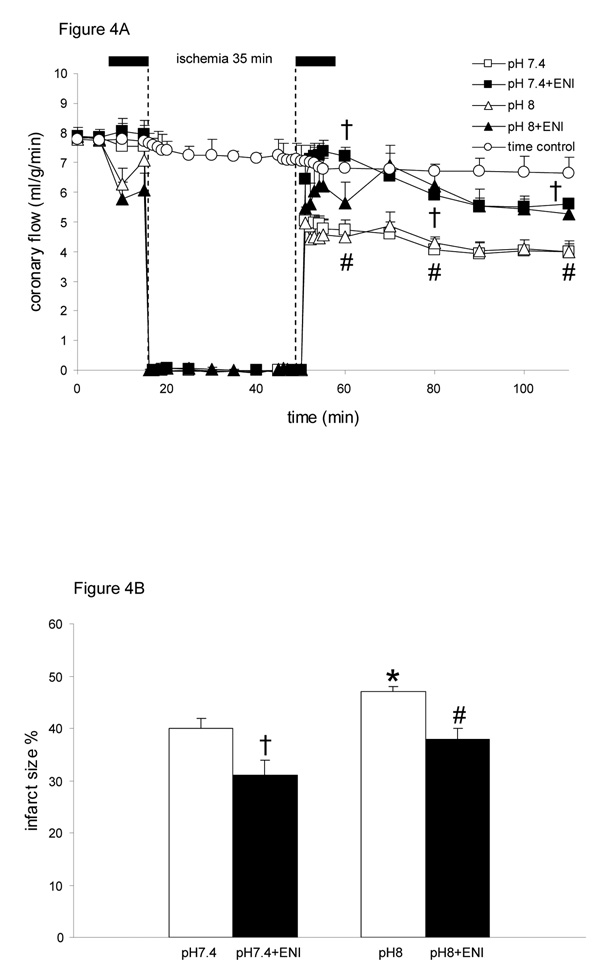
Baseline values for coronary flow (Fig. 4A) were not different among groups. At 60 min reperfusion, flow was higher in pH 7.4+ENI, pH 8+ENI and both pH 6.5 (Table 1) groups, and lower in 7.4 and pH 8 groups. Similarly O2 delivery was higher in the ENI groups than the non-ENI groups on reperfusion (Table 2). Infarct size (Fig. 4B) was smaller in the pH 8+ENI group than in the pH 8 group, not different in the pH 8+ENI and pH 7.4 groups, and lowest in the pH 7.4+ENI (31±3%). Infarct size was not significantly different between pH 6.5 (34±2%), pH 6.5+ENI (36±3%).
Figure 4.
Changes in coronary flow (A) during perfusion with HEPES buffer at pH 7.4 (control; n=12), pH 8 (n=12), pH 7.4+ENI (10 µM) (n=12), or pH 8+ENI (10 µM) (n=12) 10 min before and 10 min after 35 min no flow, global ischemia. For P <0.05: * pH 8 vs. 7.4; # pH 8+ENI vs. pH 8; † pH 7.4+ENI vs. pH 7.4. Infarct size (B) as a percentage of total ventricular weight measured after 120 min reperfusion. For P <0.05: * pH 8 vs. 7.4; # pH 8+ENI vs. pH 8; † pH 7.4+ENI vs. pH 7.4. Attenuated Na+/H+ exchange by ENI at both pH’s led to a higher coronary flow on reperfusion and less infarction.
DISCUSSION
Our objective was to activate and block NHE while examining changes in mitochondrial bioenergetics during I/R when hearts were perfused just before and after ischemia at pH 6.5, 7.4 or 8. The major findings are: 1) Brief perfusion of hearts at pH 8 before and after ischemia caused the most mitochondrial dysfunction. This was evidenced by the largest mt[Ca2+] overload during and after ischemia, the most oxidized mitochondrial redox state, and the highest level of O2−• production during reperfusion. 2) NHE (and NCE) are activated early during ischemia as suggested by the rapid rise in mt[Ca2+], particularly in the pH 8 group. 3) Cardiac functional recovery was least and infarct size was largest in the pH 8 group. 4) Inhibition of NHE by ENI largely reversed the deleterious mitochondrial and cardiac functional effects of alkalosis (pH 8 group) on reperfusion.
The improvements in contractility and relaxation in the eniporide groups were likely due to the lesser damaging effects of Ca2+ overload, ROS production, and the more reduced redox state. It is likely that the differences in mtCa2+ overload between the eniporide treated and untreated groups can be explained in part by the contribution of sarcolemmal NCE, secondary to NHE, to increase cytosolic Ca2+ loading. NCE and NHE activity in the mitochondrial membrane may also play a role. The mtCa2+ overload that remained in the eniporide groups could arise from other cytosolic sources such as enhanced Ca2+ release from the sarcoplasmic reticulum50 due to oxidative stress with greater passage of Ca2+ into the matrix via the CaU.2
The eniporide-induced improvement in coronary flow on reperfusion likely stems from less vascular edema and/or improved endothelial and vascular responsiveness. The higher coronary flow and better contractility after eniporide treatment may underlie the higher O2 consumption. These observations clearly demonstrate that augmented activation of NHE with alkaline pH during I/R results in even worse cardiac functional recovery and point out the effectiveness of NHE inhibitors to effectively reverse this dysfunction. Improved mitochondrial bioenergetics with eniporide treatment may also underlie the improved cardiac function as discussed below.
Mitochondrial Ca2+ Loading and Na+/H+ Exchange
NHE is relatively quiescent under non-ischemic conditions at an extracellular pH of 7.4.33 However, NHE becomes activated during ischemia when intracellular acidosis ensues, and especially during early reperfusion when a larger proton gradient develops across the cell membrane. NHE is both pH and Na+ dependent.34–36 The larger the transsarcolemmal H+ gradient, the more active is the NHE.37 It was shown that reperfusion of myocardial tissue at a high pH (7.9) significantly increased cell Na+ and Ca2+ content but only when NHE was not inhibited; this indicates the requirement for NHE activity to indirectly activate NCE.38 Increased NHE activity leads to increased cytosolic [Na+]11–14 and subsequently cytosolic Ca2+ overload as a result of activation of the reverse mode of NCE. The increase in cytosolic [Ca2+]10,11,39 additionally leads to mtCa2+ loading2,18 largely through the mtCa2+ uniporter (CaU) despite the apparent large buffering capacity for calcium in mitochondria.
Our study clearly shows that mtCa2+ overload is augmented at a more alkaline pH during I/R injury but that it can be markedly reduced when NHE is inhibited with ENI or at pH 6.5. The additional increase in mt[Ca2+] in the pH 8 group is attributed to the added increase in the pH gradient across the cell membrane, which results in greater activation of NHE and reverse mode NCE. Thus, our study demonstrates that NHE during I/R injury has a consequence not only to augment cytosolic Ca2+ but also mtCa2+. Moreover, blocking NHE during and after ischemia reduced this cascade of events that culminated in mtCa2+ overload and impaired mitochondrial function on reperfusion.
A previous study reported that in mitochondria isolated after I/R, NHE inhibition before and after ischemia exhibited improved state 3 respiration and oxidative phosphorylation and decreased mt[Ca2+] compared to the controls.40 In another study inhibition of NHE with cariporide in rat cardiomyocytes reduced markers of oxidant –induced (H2O2) cell death by attenuating cytosolic Na+ and Ca2+ loading and mtCa2+ loading, and by preventing depolarization of ΔΨm.33 The present study conducted in intact, beating hearts supports the results derived previously in isolated mitochondria40 and isolated myocytes33 that NHE activation and inhibition also alter mtCa2+ and that mtCa2+ loading contribute to the impaired function that occurs with I/R injury.
A small increase in mt[Ca2+] during increased workload is believed to stimulate the mitochondrial TCA cycle to furnish NADH via Ca2+-dependent mitochondrial dehydrogenases to match energy demand with supply. However, a high mt[Ca2+], as observed during I/R, can impair ATP synthesis and lead to a loss of ionic homeostasis, opening of the mitochondrial permeability transitional pore (mPTP), matrix swelling, and outer membrane rupture.33 Irreversible mPTP opening causes collapse of the ΔΨm and release of cytochrome c to induce apoptosis.41–44 The collapse of ΔΨm and the subsequent release of cytochrome c can lead to more ROS production, resulting in the vicious cycle of further amplification of cellular ROS production, mtCa2+overload, and increasing cell injury.1,44
Mitochondrial Ca2+ and ROS with Na+/H+ Exchange
Although mtCa2+ loading and formation of ROS are major causative factors in reperfusion stunning and permanent damage after ischemia, the cause-effect relationship between mtCa2+ loading and excess ROS during I/R injury remains unsettled.45,46 Numerous studies from our laboratory16,18 and others28,47 show that ROS are produced not only during reperfusion but also during ischemia. Our experiments may shed some light on whether the initial excess in mtCa2+ leads to ROS production or increased ROS leads to excess mtCa2+. In each group we observed similarly reduced redox states and moderate increases in O2−• during the early ischemic period and an increasingly oxidized redox state and higher levels of O2−• during the late ischemic period. However, mt[Ca2+] rose faster and much higher in the pH 8 group compared to other groups. This suggests that an increase in mt[Ca2+] during ischemia does not directly cause any additional change in redox state or increase in O2−• level during ischemia. However, during early reperfusion mt[Ca2+] and O2−• were highest and redox state was lowest in the pH 8 group. Proportional but smaller changes occurred in the other groups, so on reperfusion these variables may all be interrelated. Increased mt[Ca2+] has been reported to alter the integrity of cytochromes a/a3 in complex IV48 and to increase nitration of mitochondrial proteins by ONOO−.49 Impaired electron flow can result in increased electron leak and excess O2−• production. Conversely, ROS production may modulate mt[Ca2+] by its action on cytosolic Ca2+ regulation. H2O2 was shown to modify the thiol residues of the ryanodine receptor and to stimulate Ca2+ release from sarcoplasmic reticulum.50 H2O2 at low concentrations may also directly activate NHE and lead to increased diastolic [Ca2+] in cultured neonatal myocytes.51,52 ROS was also found to increase activity of NCE in the reverse mode to increase cytosolic Ca2+ influx.45,53
Mitochondrial Redox State and Na+/H+ Exchange
Redox state (ratio of NADH/NAD and FADH2/FAD) is a qualitative measure of the reducing equivalents available to drive respiration. We,17,18 and others,20–22 showed that as the supply of O2 diminishes during early ischemia, electron flux through the ETC falters, NADH accumulates, and oxidative phosphorylation rapidly declines.54–56 In the present study, the NADH and FAD signals likely represent the average redox state of a volume of cells underlying the fiberoptic probe. The marked and irreversible decline in NADH and increase in FAD during reperfusion in the pH 8 group could represent greater dead cell volume57 or increased volume of irreversibly oxidized and energy-depleted mitochondria.17 The latter seem to be the case in this study because the continued decline in NADH with the rise in FAD during reperfusion does not likely represent a reduction in the number of viable cells. It is interesting that the increasingly more oxidized state in the pH 8 group during reperfusion correlated with a gradual decline in the O2−• level. This suggests ROS cannot be produced as the damaged mitochondria become more oxidized. In contrast, in the pH 8+ENI group and the two pH 7.4 groups, both NADH and FAD returned nearly to their baseline values on reperfusion, and this was associated with less O2−• and mtCa2+overload. Thus, the more reduced redox state during reperfusion implies greater availability of reducing equivalents and electrons for oxidative phosphorylation along with less electron leak and normalization of mtCa2+.
Although many studies imply that NHE blockers confer cardiac protection by reducing cytosolic Ca2+ loading during I/R, the present study expresses the importance of NHE in inducing additional damage to mitochondria via mtCa2+ loading. Protecting mitochondria from deleterious increases in mt[Ca2+] and ROS is also key to reducing I/R –induced cell injury. Cardiomyocytes exposed to oxidative stress show Ca2+-dependent morphological changes in mitochondria such as swelling and loss of cristae, which is followed by collapse of the ΔΨm, and finally cytosolic fragmentation.58
Mitochondrial Na+/H+ Exchange
NHE inhibitors may exert their protective effects by a direct action on mtNHE59 as well as on sarcolemmal NHE, although there is controversy about the existence of mtNHE-1.39,60 Cariporide, an NHE-1 inhibitor, was shown to block mtNHE and to delay matrix acidification and ATP depletion during simulated ischemia in cardiac myocytes.8 mtCa2+ uptake by the CaU is largely dependent on the magnitude of ΔΨm; an increase in mt[H+], which depolarizes ΔΨm, will in turn reduce mtCa2+ uptake.7,61 In the presence of respiratory inhibitors (oligomycin, KCN), inhibition of mtNHE was shown to enhance mitochondrial acidification in permeabilized rat myocytes.7 Therefore, in our study an added increase in mt[H+] by mtNHE inhibition with ENI during ischemia may be in part responsible for decreasing mtCa2+ loading via CaU or mtNCE during reperfusion. It is also possible that the increased transmembrane H+ gradient at pH 8, which lowers cytosolic [H+], also lowers mitochondrial [H+] by mtNHE. This effect in turn could increase mt[Ca2+] via the CaU and mtNCE. In recent preliminary studies,62,63 we showed that eniporide altered matrix cation balance in isolated mitochondria, which supports the presence of NHE-1 in the inner mitochondrial membrane.
Inhibition of NHE may reduce mtCa2+ loading during ischemia by reducing cytoplasmic [Ca2+] but also by reducing Ca2+ flux through the ΔΨm –dependent CaU, particularly when ΔΨm is more depolarized by a higher mt[H+]. Acidification of the mitochondrial matrix reduces proton influx through complex V (ATP synthase), i.e. uncouples mitochondria, so NHE inhibitors (or matrix acidosis) may also protect indirectly by more efficiently restoring oxidative phosphorylation on reperfusion. Thus on the basis of current knowledge, inhibition of either sarcolemmal NHE or mtNHE would appear to be beneficial in reducing mtCa2+ loading. Future studies using CaU blockers and mitochondrial selective NHE inhibitors may help to delineate the relative importance of these exchangers on reducing cytosolic vs. mtCa2+ loading through the cell and mitochondrial membranes.
CONCLUSIONS AND LIMITATIONS
We have shown that enhanced activation of NHE with pH 8 during ischemia leads to an additional increase in mtCa2+ loading. This contributes to a greater deterioration of mitochondrial bioenergetics and ROS production on reperfusion and poor functional return and greater tissue damage. Blocking NHE with ENI at an alkaline pH markedly improved functional return on reperfusion by minimizing the increase in mt[Ca2+], by better preserving the mitochondrial redox state, and by reducing ROS production. Both sarcolemmal and mitochondrial NHE may be involved in promoting mtCa2+ loading with I/R injury.
As in the present study, a large number of experimental studies have shown beneficial effects by inhibiting NHE during deliberate I/R injury. Clinical trials of NHE inhibitors, however, have so far failed to show significant benefits for patients suffering I/R injury. A potential problem is the NHE inhibitors exhibit their most protective effects when the drug is given just before ischemia or immediately on reperfusion.11,64,65 But administration of a NHE inhibitor before cardioplegic arrest66 also failed to show significant protection in pigs subjected to cardiopulmonary bypass. Nevertheless, the quite beneficial cellular effects of avoiding extracellular and mitochondrial alkalosis during cardiac ischemia and early reperfusion in this model are clearly reflected by the preservation not only of myocardial function but also of mitochondrial bioenergetics.
ACKNOWLEDGMENTS
The authors thank Steve Contney and Anita Tredeau for administrative assistance.
This research was supported in part by grants from the National Institutes of Health (HL07324 to Amadou KS Camara); American Heart Association (0355608Z to David F Stowe); Veterans Affairs Research Service (to David F Stowe)
Footnotes
Portions of this work have appeared in abstract form: Camara et al. FASEB J 20:468.15, 2006; Spence et al. FASEB J. 21, 899.16; 2007.
Disclosures
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 2.Miyamae M, Camacho SA, Weiner MW, et al. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am J Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan-Jones RD, Wu ML, Bountra C. Sodium-hydrogen exchange and its role in controlling contractility during acidosis in cardiac muscle. Mol Cell Biochem. 1989;89:157–162. doi: 10.1007/BF00220769. [DOI] [PubMed] [Google Scholar]
- 4.Karmazyn M. The role of the myocardial sodium-hydrogen exchanger in mediating ischemic and reperfusion injury. From amiloride to cariporide. Ann N Y Acad Sci. 1999;874:326–334. doi: 10.1111/j.1749-6632.1999.tb09248.x. [DOI] [PubMed] [Google Scholar]
- 5.Pike MM, Kitakaze M, Marban E. 23Na-NMR measurements of intracellular sodium in intact perfused ferret hearts during ischemia and reperfusion. Am J Physiol. 1990;259:H1767–H1773. doi: 10.1152/ajpheart.1990.259.6.H1767. [DOI] [PubMed] [Google Scholar]
- 6.Tani M. Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol. 1990;52:543–559. doi: 10.1146/annurev.ph.52.030190.002551. [DOI] [PubMed] [Google Scholar]
- 7.Gursahani HI, Schaefer S. Acidification reduces mitochondrial calcium uptake in rat cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2004;287:H2659–H2665. doi: 10.1152/ajpheart.00344.2004. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Meana M, Garcia-Dorado D, Pina P, et al. Cariporide preserves mitochondrial proton gradient and delays ATP depletion in cardiomyocytes during ischemic conditions. Am J Physiol Heart Circ Physiol. 2003;285:H999–H1006. doi: 10.1152/ajpheart.00035.2003. [DOI] [PubMed] [Google Scholar]
- 9.Stowe DF, Heisner JS, An J, et al. Inhibition of Na+/H+ isoform-1 exchange protects hearts perfused after 6-hour cardioplegic cold storage. J Heart Lung Transplant. 2002;21:374–382. doi: 10.1016/s1053-2498(01)00383-7. [DOI] [PubMed] [Google Scholar]
- 10.Camara AK, An J, Chen Q, et al. Na+/H+ exchange inhibition with cardioplegia reduces cytosolic [Ca2+] and myocardial damage after cold ischemia. J Cardiovasc Pharmacol. 2003;41:686–698. doi: 10.1097/00005344-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 11.An J, Varadarajan SG, Camara A, et al. Blocking Na+/H+ exchange reduces [Na+]i and [Ca2+]i load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 12.Hove MT, Jansen MA, Nederhoff MG, et al. Combined blockade of the Na+ channel and the Na+/H+ exchanger virtually prevents ischemic Na+ overload in rat hearts. Mol Cell Biochem. 2007;297:101–110. doi: 10.1007/s11010-006-9334-0. [DOI] [PubMed] [Google Scholar]
- 13.ten Hove M, van Emous JG, van Echteld CJ. Na+ overload during ischemia and reperfusion in rat hearts: comparison of the Na+/H+ exchange blockers EIPA, cariporide and eniporide. Mol Cell Biochem. 2003;250:47–54. doi: 10.1023/a:1024985931797. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann M, Decking UK. Blocking Na+-H+ exchange by cariporide reduces Na+-overload in ischemia and is cardioprotective. J Mol Cell Cardiol. 1999;31:1985–1995. doi: 10.1006/jmcc.1999.1029. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Meyer JW, Ashraf M, et al. Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774.DC. [DOI] [PubMed] [Google Scholar]
- 16.Kevin LG, Camara AK, Riess ML, et al. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 17.Riess ML, Camara AK, Chen Q, et al. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 18.Aldakkak M, Stowe DF, Chen Q, et al. Inhibited mitochondrial respiration by amobarbital during cardiac ischemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2007 doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Stowe DF, Bosnjak ZJ, Kampine JP. Cardiac cell action potential duration is dependent upon induced changes in free Ca2+ activity during pH changes in vitro. J Electrocardiol. 1986;19:143–154. doi: 10.1016/s0022-0736(86)80022-x. [DOI] [PubMed] [Google Scholar]
- 20.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996;71:1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chance B, Williamson JR, Jamieson D, et al. Properties and kinetics of reduced pyridine nucleotide fluorescence of the isolated and in vivo rat heart. Biochem Z. 1965;341:357–377. [Google Scholar]
- 22.Nuutinen EM. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984;79:49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- 23.Barth E, Stammler G, Speiser B, et al. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 24.Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004;286:H1742–H1749. doi: 10.1152/ajpheart.00478.2003. [DOI] [PubMed] [Google Scholar]
- 25.Camara AK, Riess ML, Kevin LG, et al. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–H1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 26.Camara AK, Aldakkak M, Heisner JS, et al. ROS scavenging before 27aC ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state. Am J Physiol Cell Physiol. 2007;292:C2021–C2031. doi: 10.1152/ajpcell.00231.2006. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes SS, Ropella KM, Camara AK, et al. How inotropic drugs alter dynamic and static indices of cyclic myoplasmic [Ca2+] to contractility relationships in intact hearts. J Cardiovasc Pharmacol. 2003;42:539–553. doi: 10.1097/00005344-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Vanden Hoek TL, Li C, Shao Z, et al. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Joseph J, Fales HM, et al. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Kalivendi S, Zhang H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 31.Miyata H, Silverman HS, Sollott SJ, et al. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 32.Stowe DF, Fujita S, An J, et al. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol. 1999;277:H2321–H2332. doi: 10.1152/ajpheart.1999.277.6.H2321. [DOI] [PubMed] [Google Scholar]
- 33.Teshima Y, Akao M, Jones SP, et al. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 34.Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- 35.Piwnica-Worms D, Lieberman M. Microfluorometric monitoring of pHi in cultured heart cells: Na+-H+ exchange. Am J Physiol. 1983;244:C422–C428. doi: 10.1152/ajpcell.1983.244.5.C422. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan-Jones RD. Regulation of intracellular pH in cardiac muscle. Ciba Found Symp. 1988;139:23–46. doi: 10.1002/9780470513699.ch3. [DOI] [PubMed] [Google Scholar]
- 37.Pierce GN, Philipson KD. Na+-H+ exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1985;818:109–116. doi: 10.1016/0005-2736(85)90553-x. [DOI] [PubMed] [Google Scholar]
- 38.Meng HP, Lonsberry BB, Pierce GN. Influence of perfusate pH on the postischemic recovery of cardiac contractile function: involvement of sodium-hydrogen exchange. J Pharmacol Exp Ther. 1991;258:772–777. [PubMed] [Google Scholar]
- 39.Javadov S, Choi A, Rajapurohitam V, et al. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2007 doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2002;39:569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 42.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 43.Regula KM, Ens K, Kirshenbaum LA. Mitochondria-assisted cell suicide: a license to kill. J Mol Cell Cardiol. 2003;35:559–567. doi: 10.1016/s0022-2828(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JN, Korge P, Honda HM, et al. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 45.Bagchix D, Wetscher GJ, Bagchi M, et al. Interrelationship between cellular calcium homeostasis and free radical generation in myocardial reperfusion injury. Chem Biol Interact. 1997;104:65–85. doi: 10.1016/s0009-2797(97)03766-6. [DOI] [PubMed] [Google Scholar]
- 46.Flitter WD. Free radicals and myocardial reperfusion injury. Br Med Bull. 1993;49:545–555. doi: 10.1093/oxfordjournals.bmb.a072629. [DOI] [PubMed] [Google Scholar]
- 47.Becker LB, vanden Hoek TL, Shao ZH, et al. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 48.Wikstrom M, Saari H. A spectral shift in cytochrome a induced by calcium ions. Biochim Biophys Acta. 1975;408:170–179. doi: 10.1016/0005-2728(75)90009-2. [DOI] [PubMed] [Google Scholar]
- 49.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol. 2004;286:H39–H46. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- 50.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 51.Rothstein EC, Byron KL, Reed RE, et al. H2O2-induced Ca2+ overload in NRVM involves ERK1/2 MAP kinases: role for an NHE-1-dependent pathway. Am J Physiol Heart Circ Physiol. 2002;283:H598–H605. doi: 10.1152/ajpheart.00198.2002. [DOI] [PubMed] [Google Scholar]
- 52.Sabri A, Byron KL, Samarel AM, et al. Hydrogen peroxide activates mitogen-activated protein kinases and Na+-H+ exchange in neonatal rat cardiac myocytes. Circ Res. 1998;82:1053–1062. doi: 10.1161/01.res.82.10.1053. [DOI] [PubMed] [Google Scholar]
- 53.Goldhaber JI, Qayyum MS. Oxygen free radicals and excitation-contraction coupling. Antioxid Redox Signal. 2000;2:55–64. doi: 10.1089/ars.2000.2.1-55. [DOI] [PubMed] [Google Scholar]
- 54.Cairns CB, Ferroggiaro AA, Walther JM, et al. Postischemic administration of succinate reverses the impairment of oxidative phosphorylation after cardiac ischemia and reperfusion injury. Circulation. 1997;96(II):260–265. [PubMed] [Google Scholar]
- 55.Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol. 1996;28 Suppl 1:S1–S10. doi: 10.1097/00005344-199600003-00002. [DOI] [PubMed] [Google Scholar]
- 56.Fryer RM, Eells JT, Hsu AK, et al. Ischemic preconditioning in rats: role of mitochondrial KATP channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- 57.Di Lisa F, Menabo R, Canton M, et al. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 58.Akao M, O'Rourke B, Teshima Y, et al. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- 59.Numata M, Petrecca K, Lake N, et al. Identification of a mitochondrial Na+/H+ exchanger. J Biol Chem. 1998;273:6951–6959. doi: 10.1074/jbc.273.12.6951. [DOI] [PubMed] [Google Scholar]
- 60.Javadov S, Purdham DM, Zeidan A, et al. NHE-1 inhibition improves cardiac mitochondrial function through regulation of mitochondrial biogenesis during postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;291:H1722–H1730. doi: 10.1152/ajpheart.00159.2006. [DOI] [PubMed] [Google Scholar]
- 61.Fry CH, McGuigan JA. The influence of pH on Ca2+ exchange in ferret heart mitochondria. Biochem Biophys Res Commun. 1990;166:1352–1357. doi: 10.1016/0006-291x(90)91015-k. [DOI] [PubMed] [Google Scholar]
- 62.Dash R, Aldakkak M, Huang M, Rhodes S, Camara AKS, Stowe DF, Beard DA. Modeling the roles of Ca2+ uniporter, Na+-Ca2+ exchanger and Na+- H+ exchanger in regulating Ca2+, Na+ and pH flux in cardiac mitochondria using in vitro spectrofluorometry. FASEB J. 2007;21:946.3. [abstract] [Google Scholar]
- 63.Rhodes S, Sharma S, Aldakkak M, Camara AKS, Dash RK, Beard DA, Stowe DF. Regulation of Ca2+ flux in isolated mitochondria; Novel spectrofluorometric measurements. Bio Med Eng Soc J. 2007:2.14. [abstract] [Google Scholar]
- 64.Hendrikx M, Mubagwa K, Verdonck F, et al. New Na+-H+ exchange inhibitor HOE 694 improves postischemic function and high-energy phosphate resynthesis and reduces Ca2+ overload in isolated perfused rabbit heart. Circulation. 1994;89:2787–2798. doi: 10.1161/01.cir.89.6.2787. [DOI] [PubMed] [Google Scholar]
- 65.Klein HH, Pich S, Bohle RM, et al. Myocardial protection by Na+-H+ exchange inhibition in ischemic, reperfused porcine hearts. Circulation. 1995;92:912–917. doi: 10.1161/01.cir.92.4.912. [DOI] [PubMed] [Google Scholar]
- 66.Klass O, Fischer UM, Perez E, et al. Effect of the Na+/H+ exchange inhibitor eniporide on cardiac performance and myocardial high energy phosphates in pigs subjected to cardioplegic arrest. Ann Thorac Surg. 2004;77:658–663. doi: 10.1016/S0003-4975(03)01604-7. [DOI] [PubMed] [Google Scholar]