Abstract
Leptin receptor dysfunction results in overeating and obesity. Leptin regulates hypothalamic signaling that underlies the motivation to hyperphagia, but the interaction between leptin and cannabinoid signaling is poorly understood. We evaluated the role of cannabinoid 1 receptors (CB1R) in overeating and the effects of food deprivation on CB1R in the brain. One-month-old Zucker rats were divided into unrestricted and restricted (fed 70% of unrestricted rats) diet groups and maintained until adulthood (4 months). Levels of relative binding sites of CB1R (CB1R binding levels) were assessed using [3H] SR141716A in vitro autoradiography. These levels were higher (except cerebellum and hypothalamus) at 4 months than at 1 month of age. One month CB1R binding levels for most brain regions did not differ between Ob and Lean (Le) rats (except in frontal and cingulate cortices in Le and in the hypothalamus in Ob). Four month Ob rats had higher CB1R binding levels than Le in most brain regions and food restriction was associated with higher CB1R levels in all brain regions in Ob, but not in Le rats. CB1R binding levels increased between adolescence and young adulthood which we believe was influenced by leptin and food availability. The high levels of CB1R in Ob rats suggest that leptin's inhibition of food-intake is in part mediated by downregulation of CB1R and that leptin interferes with CB1R upregulation under food-deprivation conditions. These results are consistent with prior findings showing increased levels of endogenous cannabinoids in the Ob rats corroborating the regulation of cannabinoid signaling by leptin.
Keywords: endocannabinoids; obesity; reward, drug abuse; cannabinoid; β-imager; Δ9-THC
INTRODUCTION
Cannabis has been known and used for its analgesic and appetitive properties. The active compound delta(9)tetrahydrocannabinol (Δ9-THC), the endogenous cannabinoid anandamide (AEA) (N-Arachidonoylethanolamide), and the cannabinoid receptor 1 (CB1R) agonist, WIN55,212-2, increased food intake in rodents (Gomez et al., 2002; Jarbe and DiPatrizio, 2005; Williams and Kirkham, 2002) by binding with high affinity to the CB1R (Bayewitch et al., 1996; Felder et al., 1993). Furthermore, studies have shown that activation of CB1R results in fatty-acid synthesis (Osei-Hyiaman et al., 2005) and lipogenesis, both strong markers of obesity (Cota et al., 2003; Osei-Hyiaman et al., 2005). There is also evidence that endogenous cannabinoids may regulate food preferences from a study that showed a correlation between CB1R density and the intake of palatable (sweet and high-fat food) (Harrold et al., 2002). Since CB1R activation has been associated with obesity-related behavioral manifestations such as overeating and lipogenesis, many efforts have focused on discovering an “antimarijuana” like drug in hopes of finding a treatment for obesity (Gadde and Allison, 2006).
Indeed, CB1R inactivation has been associated with a decrease in food intake and weight gain. Studies have shown that the CB1R antagonist, Rimonabant (SR141716A), reduced body weight and food intake in obese (Ob) Zucker rats (Vickers et al., 2003) and obese mice (Ravinet Trillou et al., 2003). This CB1R antagonist also suppressed food intake induced by administration of the endocannabinoid anandamide into the ventromedial hypothalamus (Jamshidi and Taylor, 2001). Another CB1R antagonist, AM 251, has also been shown to reduce food intake and weight gain in Ob rats (Chambers et al., 2004). Furthermore, Ob mice treated with CB1R antagonists gained less weight and showed lower levels of the obesity-related messengers (insulin, glucose, and leptin) compared with age-matched untreated animals (Poirier et al., 2005). Finally, mice lacking CB1R are resistant to the effects of diet induced obesity and when fed a high fat-diet show lower food intake, and a diminished response to the rewarding properties of food compared with controls (Sanchis-Segura et al., 2004). Thus, it is not surprising that CB1R antagonists are a main target in the development of medications to treat obesity.
CB1R and endogenous cannabinoids are distributed throughout the brain. However, the role of endocannabinoids in the hypothalamus has been most consistently linked with regulation of food intake. Indeed leptin appears to decrease food intake in part by reducing the levels of endogenous cannabinoids (anandamide and 2-arachidonoyl glycerol) in the hypothalamus (Di Marzo et al., 2001). Here we assess the role of leptin on CB1R expression in the brain. For this purpose we compared the regional brain distribution of CB1R in obese Zucker rats, which are leptin deficient (Chua et al., 1996), with that of their lean counterparts at different developmental stages and diet conditions. We hypothesized that leptin inhibits CB1R expression in the brain and thus Ob rats would have higher levels of CB1R than their lean counterparts; an effect that would be accentuated in food deprivation conditions.
MATERIALS AND METHODS
Animals
One-month-old male Zucker Obese {fa/fa} (Ob; n = 20) and Lean {Fa/FA} (Le; n = 20) rats were used. Rats were divided into four groups, 10 rats in each group. Specifically: (i) Ob rats with unrestricted (U) food access, (ii) Ob rats with restricted (R) food access, (iii) Le U rats, and (iv) Le R rats. At 1 month of age half the rats were placed on restricted food access (70% of ad libitum fed animals) and the rest on free access to food (ad libitum). Rats were obtained from Harlan (Indianapolis, IN) and all experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
Behavior assessment—Food intake and weight
Rats were fed a standard (Purina) laboratory rat chow. Food intake was monitored daily at 1500 h and all rats were weighed every other day. Restricted diet rats were fed daily at 1500 h and the amount of food given was continuously adjusted to 70% of food intake of ad libitum pair-fed rats.
CB1R autoradiography
Each animal was deeply anesthetized with a mixture of Xylazine (10 mg/kg) and Ketamine (100 mg/kg). The brain was rapidly removed and frozen in an isopentane and dry-ice bath and stored in a −80°C freezer. The brain was transferred to the cryostat and sections of 14 μm thick were cut at −22°C and then stored at −80°C until binding. Slides were then preincubated at room temperature for 10 min in 50 mM Tris-HCl buffer (pH 7.4) and were then incubated in binding buffer (50 mM Tris-HCl, 0.4 nM [3H] SR 141716A) (Amersham Biosciences, Piscataway, New Jersey) at room temperature for 90 min. To determine nonspecific binding some slides in parallel were similarly incubated in binding buffer in the presence of 100 μM HU-210 (Tocris Bioscience, Ellisville, Missouri). Next, the slides were washed 3 × 30 min in ice-cold 50 mM Tris-HCl and then rapidly rinsed in ice-cold dH2O. Slides were then dried over night at room temperature in a desiccator and placed in a glass slide cassette for image acquisition scanning and analysis using a β-Imager 2000 (Biospace Mesures, FR). Scans were acquisitioned in the β-Imager 2000 for 6 h at high resolution. Using β vision+ software (Biospace Measures, FR, USA), regions of interest (ROIs) were drawn on the left and right: striatum (ST), nucleus accumbens (NAc), cerebellum (CB), cingulate (CC), frontal (FC), parietal (PC), insulate (IC) cortices, and hypothalamus (HYP) of each brain slice. These data were averaged and expressed initially in counts per minute per millimeter square (cpm/mm2) and were subsequently converted into microcuries per gram (μCi/g) of tissue by using a brain homogenate standard of known radioactivity value and mass (provided by Y. Piyis at Brookhaven National Laboratory) taking into account apparatus recovery (90%) and autoabsorption (40%) due to section thickness (Langlois et al., 2001; Poisnel et al., 2006). The μCi/g estimation is a relative measure of radioactivity/mass of tissue since the tissue section and homogenate differ in their respective tissue densities.
RESULTS
Body weight
Body weight was examined in each group over a 4-month period. A three-way ANOVA revealed significant main effects in body weight with respect to strain (F = 461.683; df = 1, 79; P < 0.001), diet (F = 320.027; df = 1, 79; P < 0.001), and age (F = 5288.733; df = 1, 79; P < 0.001). At 1 month, there were no significant differences in weight between any of the groups (Ob U: 110.3 ± 9.61 g; Le U: 119.3 ± 10.81 g; Ob R: 107.4 ± 11.47 g; Le R: 114.7 ± 8.38 g). At 4 months, Ob U rats showed significantly higher weight gain than Le R (t = 23.873; P < 0.05), Le U (t = 26.545; P < 0.05), and Ob R (t = 28.154; P < 0.05). Similarly, at 4 months, Le U and Ob R showed significantly higher weight gain compared with Le R, (t = 7.441; P < 0.05) and (t = 5.832; P < 0.05) respectively (Ob U: 564.23 ± 15.4 g; Le U: 408.84 ± 7.81 g; Ob R: 364.14 ± 7.04 g; Le R: 270.55 ± 4.22 g). There were no weight differences between the Ob R and the Le U rats.
Food intake
Throughout the study food intake was monitored in the two unrestricted rat groups. A two-way ANOVA showed significant main effects with respect to strain (F = 39.044; df = 1, 39; P < 0.001) and age (F = 272.048; df = 1, 39; P < 0.001). At 1 month, there were no differences in food intake between Ob and Le rats (Ob U: 19.7 ± 0.56 g; Le U: 19.46 ± 1.16 g). At 4 months Ob rats had higher food intake levels compared with Le (t = 6.477; P < 0.05) (Ob U: 33.85 ± 0.82 g; Le U: 23.83 ± 0.46 g). Specifically, Ob rats consumed on average an additional 10 g of food than Le U rats (30% more). Finally, food intake levels were significantly higher at 4 compared with 1 month for both Ob (t = 13.722; P < 0.05) and Le rats (t = 9.604; P < 0.05).
CB1R autoradiography
The findings from this study were as follows: (1) at 1 month of age most brain regions did not differ between Ob and Le animals (except lower relative CB1R binding levels in frontal and cingulate cortices in Le and in the hypothalamus in Ob); (2) CB1R were significantly higher at 4 months than at 1 month of age in all animals (in all brain regions except the CB, and HYP); (3) Ob had significantly higher relative CB1R binding levels than Le animals (4 months of age) (except in HYP); and (4) diet restriction was associated with higher relative CB1R binding levels in all brain regions in Ob but not in Le animals (4 months of age) (except in the HYP). These findings are described later.
Brain sections were analyzed for [3H] SR 141716ACB1R binding at 1 and 4 months (Fig. 1). A one-way ANOVA showed no significant (P > 0.05) differences between the left and right for all eight brain regions of interest (ROI) examined: (ST, NAc, CB, CC, FC, PC, IC, and HYP). The left and right values from each ROI were combined and treated as a single ROI value for the purpose of maximizing statistical power. A two-way ANOVA on CB1R levels revealed a significant group (F = 55.72; df = 5.7289; P < 0.001) brain region (F = 32.18; df = 7.7289; P < 0.001) and group X brain region interaction effect (F = 3.62; df = 35.7289; P < 0.05); along with several significant pairwise multiple comparisons using the Holm-Sidak method (see later).
Fig. 1.
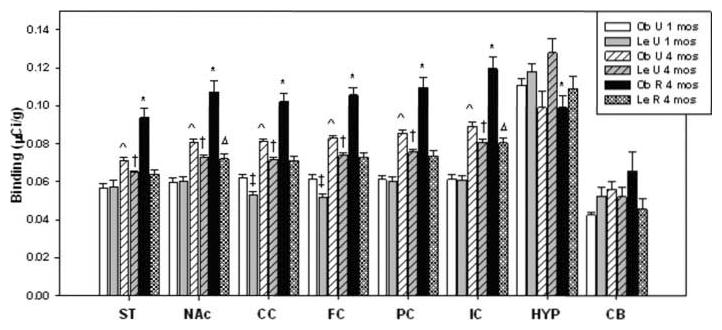
Mean (+SEM) [3H]SR141716A CB1 receptor binding in obese and lean Zucker rats. P < 0.05 *Ob R rats show the greatest CB1R binding compared across all groups. However, lower than Le U in HYP. P < 0.05 ^4-month-old Ob U rats show significantly greater CB1R binding compared to 1-month-old Ob U and Le U rats. P < 0.05 †Le U and Le R rats showed significantly greater CB1R binding at 4 months compared to 1 month of age. P < 0.05 ‡1-month-old Le U rats show lower CB1R binding compared to similarly aged Ob U rats. P < 0.05 ΔLe R rats at 4 months show greater CB1R binding than 1-month-old Ob U rats.
Age effects
At 4 months Ob R rats had significantly higher relative CB1R binding levels than 1-month-old OB U in IC (t = 11.41, P < 0.001), NAc (t = 9.88, P < 0.001), PC (t = 9.33, P < 0.001), FC (t = 8.49, P < 0.001), CC (t = 7.85, P < 0.001), ST (t = 7.42, P < 0.001), but lower than 1-month-old Le U rats (t = 2.48, P < 0.05) in the HYP (Fig. 1). Similarly, 4-month-old Ob U rats also showed significantly higher relative CB1R binding levels than 1-month-old Ob U rats in the ST (t = 2.46, P < 0.05), NAc (t = 4.23, P < 0.001), CC (t = 3.33, P < 0.001), FC (t = 3.61, P < 0.001), PC (t = 4.23, P < 0.001), and in the IC (t = 5.09, P < 0.001) (Fig. 1). Differences were also significant in 4-month-old Le R rats compared with 1-month-old Le U rats in the ST (t = 2.21, P < 0.05, NAc (t = 3.70, P < 0.001), CC (t = 5.22, P < 0.001), the FC (t = 5.75, P < 0.001), the PC (t = 3.92, P < 0.001), and the IC (t = 5.34, P < 0.001), which Le R rats showed higher CB1R binding than Le U rats (Fig. 1). Le R 4-month-old rats also showed higher binding in the IC (t = 3.34, P < 0.001) and NAc (t = 1.98, P < 0.05) than 1-month-old Ob U rats (Fig. 1). At 4 months, Le U rats showed higher respective CB1R binding than 1 month Le U rats in the ST (t = 2.44, P < 0.05), NAc (t = 3.72, P < 0.001), CC (t = 5.00, P < 0.001), FC (5.70, P < 0.001), PC (t = 4.26, P < 0.001), and IC (t = 5.26, P < 0.001) (Fig. 1). Overall these results show an increase in CB1R binding with respect to age, except in the CB and HYP.
Diet and strain effects
At 1 month, Ob U rats displayed significantly higher respective CB1R binding levels than Le U rats in both the CC (t = 4.93, P < 0.001) and FC (t = 5.30, P < 0.001), but lower although not significant in the CB (t = 0.24; P > 0.05) and HYP (t = 1.47, P > 0.05) (Fig. 1). In contrast, at 4 months, Ob rats displayed significantly higher CB1R binding in most brain regions than Le rats (except in the CB and HYP). The greatest CB1R binding levels were observed in the Ob R rats. Specifically Ob R had higher CB1R levels in ST than Ob U (t = 4.66, P < 0.001), Le U (t = 6.24, P < 0.001), and Le R (t = 6.48, P < 0.001); in NAc than Ob U (t = 5.52, P < 0.001), Le U (t = 7.26, P < 0.001), and Le R (t = 7.64, P < 0.001); in CC than Ob U (t = 4.21, P < 0.001), Le U (t = 6.21, P < 0.001), and Le R (t = 6.58, P < 0.001); in FC than Ob U (t = 4.51, P < 0.001), Le U (t = 6.45, P < 0.001), and Le R (t = 6.89, P < 0.001); in PC than Ob U (t = 4.74, P < 0.001), Le U (t = 6.93, P < 0.001), and Le R (t = 7.57, P < 0.001); in IC than Ob U (t = 5.89, P < 0.001), Le U (t = 7.83, P < 0.001), Le R (t = 8.15, P < 0.001), but lower in HYP than Le U (t = 2.61, P < 0.05) (Fig. 1).
Diet restriction significantly affected brain regional CB1R binding levels in the Ob but did not affect the respective levels in the Le rats (4 months of age), except in the HYP. Specifically CB1R binding levels were significantly higher in Ob R than Ob U in all regions; but not significantly higher (t = 0.65, P > 0.05) in the CB and lower in the HYP (t = 0.01, P > 0.05). In contrast at 4 months, respective CB1R binding levels did not differ in any of the brain regions between the Le R and the Le U rats, except in the HYP.
DISCUSSION
Here we show significant differences in CB1R binding expression in the brain between Ob and Le rats; specifically in frontal, limbic, and striatal regions, CB1R binding levels were higher in Ob than Le rats. Since Ob rats are deficient in leptin receptor function this suggests that leptin inhibits CB1R expression in regions that process rewarding and motivational signals associated with food intake (Kelley et al., 2005). When leptin levels are low and the animals are on a restricted diet, CB1R expression increased in Ob but not in the Le rats. This suggests that leptin receptor activity may play a role in inhibiting CB1R in the brain. Leptin's regulation may be developmentally triggered since the differences between strains, both for CB1R expression as well as food intake and weight, were observed at 4 months but not at 1 month of age. In addition, we also show that CB1R levels increased with age; CB1R were higher at 4 months than at 1 month of age in both strains.
Strain effects on CB1R: Leptin signaling
Zucker obese rats have been widely used as a model of obesity and characterized by a mutation in the leptin receptor (Chua et al., 1996) leading to obesity, diabetes, and hyperphagia (Fetissov et al., 2000). In our study, the only observed differences in CB1R between Ob and Le strains of Zucker rats at 1 month of age occurred in the CC, FC (Ob > Le rats), and HYP (Le > Ob). This suggests that the CB1R in these specific brain structures was affected by leptin-receptor deficiency or behavioral and physiological manifestations associated with this deficiency. Leptin activation inhibits endocannabinoid release (Jo et al., 2005) in the HYP, which is neuroanatomically connected both with the FC and CC (Morecraft et al., 1992; Pajolla et al., 2001). Work done in ob/ob mice (leptin deficient) recently has shown a higher concentration of 2-AG (an endocannabinoid) in the HYP relative to wild-type mice (Di Marzo et al., 2001). Therefore, increased CB1R binding levels in Ob rats at this early age in the CC and FC may reflect neuroadaptive changes associated with increased endocannabinoid release, which may be involved in increased food intake regulated by leptin (Di Marzo et al., 2001).
Compared with 1-month-old adolescent rats, adult 4-month-old rats' CB1R binding levels showed significant differences between Ob and Le rats in all brain regions examined apart from the CB. Indeed, it is possible that leptin does not regulate CB1R in the CB. Defective leptin signaling is associated with elevated hypothalamic, but not cerebellar levels of endocannabinoids in Zucker rats (Di Marzo et al., 2001). On the other hand, the increased levels of CB1R binding in brain regions involved in the rewarding effects of food (NAc, IC, CC, CPU, FC, and PC) suggests that CB1R in these regions may contribute to hyperphagia observed in 4-month-old Ob rats (Durham and Truett, 2006; Kurtz et al., 1989). Whereas there is clear evidence that leptin modulates cannabinoid signaling in HYP, our findings suggest that the modulatory role of leptin on cannabinoid signaling extends to other brain regions.
Developmental effects on CB1R
All rats showed significantly higher CB1R binding at 4 months compared with 1 month of age in all regions except for the CB of Le rats and the HYP of Ob rats. This suggests that in the rodent there is an increase in CB1R binding levels in most of the brain regions in the transition from childhood to adolescence. The increases in CB1R with age are consistent with prior rodent studies showing gradual increases in CB1R mRNA in the FC, hippocampus, basal ganglia, and CB between the fetal period and adulthood (Berrendero et al., 1999) and with human postmortem studies showing increases in CB1R mRNA and CB1R binding levels between prenatal and postnatal brain development (Mato et al., 2003; Wang et al., 2003). The increases in CB1R we observed with age may contribute to the developmentally related increases in food intake and weight gain.
The developmental changes in CB1R, which differed between the strains, paralleled the changes in weight gain and food intake. Ob and Le rats did not differ at 1-month but at 4-months of age; Ob rats weighed 39% more and consumed 42% more than their lean counterparts. This is consistent with the developmental regulation of leptin receptors in rat pituitary and HYP (Morash et al., 2003).
Diet effects on CB1R
Food restriction significantly reduced body weight at 4 months of age in both Ob and Le rats; Ob R rats weighed 55% less than Ob U rats and Le R rats weighed 51% less than Le U rats. However food restriction increased CB1R in Ob rats (deficient leptin receptors) but not in the Le rats (normal leptin receptors). This suggests that leptin receptors may exert an inhibitory role on CB1R upregulation under conditions of food deprivation. Since leptin levels drop with food deprivation, this suggests that constitutive activity of leptin receptors may be involved in this inhibition. However, to our knowledge constitutive activity of leptin receptors has previously been documented only for peripheral tissues (adipose tissue, placenta, and stomach) (Morash et al., 1999). Thus, further work is required to assess if constitutive activity for leptin receptors occurs in the brain.
Modulation of CB1R by diet is consistent with the recognized role of CB1R in feeding behavior (Gomez et al., 2002; Williams and Kirkham, 2002) and blocking CB1R reduces food intake in both Le and Ob Zucker rats, with a greater effect on Ob rats (Vickers et al., 2003). Moreover, CB1R knockout mice are resistant to diet-induced obesity, show lower food intake, and show a diminished response to rewarding stimuli compared with controls (Sanchis-Segura et al., 2004). The CB1R antagonist, Rimonabant (SR141716A), reduced body weight and decreased food intake [of obese Zucker rats (Bensaid et al., 2003; Vickers et al., 2003) and mice (Ravinet Trillou et al., 2003)]; reduced ethanol intake [in C57BL/6 mice (Arnone et al., 1997; Thanos et al., 2005a); Sardinian alcohol-prefering (sP) rats (Colombo, 1997; Colombo et al., 2004); alcohol preferring (P) rats (Thanos et al., 2005b)]; and decreased place preference for alcohol (Houchi et al., 2005; Thanos et al., 2005a).
In summary, the high levels of CB1R levels in limbic cortical and subcortical brain region of obese Zucker rats suggests that leptin's regulation of food-intake is in part mediated by CB1R in areas of the brain involved with the rewarding and motivational aspects of food intake. The increases in CB1R in Ob but not in Le animals with diet restriction when leptin levels are low, suggests that constitutive leptin receptor activity interferes with CB1R upregulation.
Acknowledgments
Contract grant sponsor: NIAAA Intramural Research Program; Contract grant numbers: AA 11034, AA07574, AA07611; Contract grant sponsor: US Department of Energy; Contract grant number: DE-AC02-98CH10886.
REFERENCES
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Bayewitch M, Rhee MH, Avidor-Reiss T, Breuer A, Mechoulam R, Vogel Z. (−)-Delta9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J Biol Chem. 1996;271:9902–9905. doi: 10.1074/jbc.271.17.9902. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist. AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004;82:863–869. doi: 10.1016/j.physbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chua SJ, Chung W, Wu-Peng X. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Colombo G. ESBRA-Nordmann 1996 Award Lecture: Ethanol drinking behaviour in Sardinian alcohol-preferring rats. Alcohol Alcohol. 1997;32:443–453. doi: 10.1093/oxfordjournals.alcalc.a008279. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Carai MA, Gessa GL. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol's motivational properties in alcohol-preferring rats. Eur J Pharmacol. 2004;498:119–123. doi: 10.1016/j.ejphar.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Durham HA, Truett GE. Development of insulin resistance and hyperphagia in zucker fatty rats. Am J Physiol. 2006;290:R652–R658. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Shafiroff M, Miyata G, Torelli GF. Dopamine (DA) in the VMN of the hypothalamus is important for diurnal distribution of eating in obese male zucker rats. Nutrition. 2000;16:65–66. doi: 10.1016/s0899-9007(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Allison DB. Cannabinoid-1 receptor antagonist, rimonabant, for management of obesity and related risks. Circulation. 2006;114:974–984. doi: 10.1161/CIRCULATIONAHA.105.596130. [DOI] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodríguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: A role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal DA D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, DiPatrizio NV. Delta9-THC induced hyperphagia and tolerance assessment: Interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol. 2005;16:373–380. doi: 10.1097/00008877-200509000-00009. [DOI] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua SC, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Morris RC, Pershadsingh HA. The zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- Langlois X, te Riele P, Wintmolders C, Leysen JE, Jurzak M. Use of the beta-imager for rapid ex vivo autoradiography exemplified with central nervous system penetrating neurokinin 3 antagonists. J Pharmacol Exp Ther. 2001;299:712–717. [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morash BA, Imran A, Wilkinson D, Ur E, Wilkinson M. Leptin receptors are developmentally regulated in rat pituitary and hypothalamus. Mol Cell Endocrinol. 2003;210:1–8. doi: 10.1016/j.mce.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajolla GP, Crippa GE, Correa SA, Moreira KB, Tavares RF, Correa FM. The lateral hypothalamus is involved in the pathway mediating the hypotensive response to cingulate cortex-cholinergic stimulation. Cell Mol Neurobiol. 2001;21:341–356. doi: 10.1023/a:1012650021137. [DOI] [PubMed] [Google Scholar]
- Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B, O'Connor SE, Janiak P, Herbert JM. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- Poisnel G, Quentin T, Barre L, Coquerel A, Debruyne D. Competitive displacement binding assay on rat brain sections and using a beta-imager: Application to mu-opioid ligands. J Neurosci Methods. 2006;154:60–67. doi: 10.1016/j.jneumeth.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrié P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Cline BH, Marsicano G, Lutz B, Spanagel R. Reduced sensitivity to reward in CB1 knockout mice. Psychopharmacology. 2004;176:223–232. doi: 10.1007/s00213-004-1877-8. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005a;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Volkow ND. Ethanol self-administration is markedly reduced in ethanol preferring (P) rats treated with the CB1 antagonist SR 141716. Alcohol Clin Exp Res Suppl. 2005b;29:12A. [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]


