Abstract
Background
With the advent of molecular techniques, self‐collected specimens without a clinician's examination are often adequate to detect common genital infections.
Objective
To evaluate the additional information that speculum and bimanual examinations provides clinicians in the routine evaluation of genital infections among attendees of a sexually transmitted disease (STD) clinic.
Methods
Cross‐sectional study from a database of all visit records to two STD clinics in Baltimore between 1996 and 2002. Women were stratified on the basis of reason for visit. Proportional and likelihood ratio estimates of the speculum examination in detecting clinically relevant cervicovaginal lesions (leading to a diagnosis of other infections or outside referral for further management) and bimanual examination in detecting abnormalities (leading to a diagnosis of pelvic inflammatory disease or referral) are presented.
Results
15 918 of 21 703 records were included: 12 073 were symptomatic (SYM; discharge, rash, abdominal pain, dysuria, genital irritation or odour), 1676 were asymptomatic contacts of an infected partner (CON) and 2169 were asymptomatic and presented for checkup (ASYM). The median age was 26 years; 94% were black. 11.8% of SYM, 4.6% of CON and 3.9% of ASYM patients had clinically meaningful lesions detected on speculum examination. The bimanual examination detected clinically relevant abnormalities in 6.5% of SYM, 0.8% of CON and 0.6% of ASYM patients.
Conclusion
Symptomatic women are most likely to benefit from speculum and bimanual examinations. However, their yield in evaluating asymptomatic women is low. Prospective studies are needed to determine whether eliminating speculum and bimanual examinations in a subset of women would offer an operational advantage without compromising patient safety.
Sexually transmitted diseases (STDs) and reproductive health clinics are under increasing resource constraints. The scope of services has increased, with the provision of HIV testing and counselling and contraceptives.1 This has led to increased attendance at these clinics, and, in some areas, logistic limitations and shrinking resources may prohibit a comprehensive evaluation of all patients.2,3,4,5 Clinicians need efficient and effective triage algorithms.
Women presenting to STD clinics represent a particularly susceptible group at high risk for the most serious sequelae of sexually transmitted infections (STIs).6,7 Clinical practice in some areas, including the US, dictates that the evaluation of all women presenting to STD clinics for care include speculum and bimanual examinations. The speculum examination allows visualisation of the lower genital tract and is necessary to obtain endocervical swabs to test for the most prevalent STIs, gonorrhoea and chlamydia. The bimanual examination allows evaluation of the upper reproductive tract, which cannot be visualised, and its main goal is to aid in the diagnosis of pelvic inflammatory disease (PID).
The advent of molecular techniques that detect common cervical and vaginal infections,8,9 the validity and reliability of self‐collected vaginal and urine specimens to detect these infections10,11,12,13,14 and their acceptability to patients15 may render the routine use of a speculum examination on all patients unnecessary, thus streamlining triage and improving diagnostic efficiency. We hypothesise that with contemporary diagnostics, most cervical and vaginal infections can be detected without a clinical examination. We explored how speculum and bimanual examinations further inform clinical diagnosis and management of women presenting to STD clinics for care in a busy urban clinic environment.
Methods
Study setting
This is a records‐based cross‐sectional cohort study from a large clinical electronic database of all female visits to two urban STI clinics in Baltimore, Maryland, USA, between 1996 and 2002. The cohort consisted of female patients aged 12–79 years. This analysis was granted approval by the institutional review boards of the Johns Hopkins Medical Institutions, Baltimore, Maryland, USA.
Data collection
The standardised female clinical assessment at the Baltimore STI clinics includes a structured interview on current symptoms, STI history, behavioural risk factors, a physical examination, clinician impressions, treatment, referrals (gynaecology, medicine, emergency room, etc.) and laboratory testing. Women are asked about the reason for their visit (HIV testing, symptoms, contact with an infected partner with an STI, or routine check‐up), current symptoms (discharge, dysuria, irritation/odour, genital lesion, genital itching, rash and abdominal pain), duration of symptoms, antibiotic use, pregnancy history, current contraceptive use and sexual risk behaviours. A directed physical examination is performed, which includes evaluation of the eyes, oropharynx, skin, pubis, abdomen, rectum, vulva/vagina (documenting erythema, discharge, ulcers, vesicles, rash, warts, cysts and other lesions), speculum examination of the cervix (documenting ectopy, discharge, ulcers, vesicles, contact bleeding and other lesions) and a bimanual examination (documenting motion tenderness, adnexal tenderness, adnexal fullness, masses and abnormalities of the uterine fundus). All findings on the history, physical examination and outside referrals are documented on the encounter form and captured in the electronic clinical database. Gonorrhoea is diagnosed by culture, chlamydia by amplification testing (Amplicor, Roche Diagnostic Systems, Branchburg, New Jersey, USA), bacterial vaginosis using Amsel's clinical criteria,16 trichomoniasis using a wet mount, vulvovaginal candidiasis based on the finding of yeast on potassium hydroxide, and vulvovaginal oedema or erythema. If consistent lesions are noted on examination, viral culture for herpes simplex virus and/or dark‐field microscopy for syphilis are performed. Infections are treated with directly dispensed drugs following current Centers for Disease Control and Prevention STD treatment guidelines.
Definitions and data analyses
Women were stratified into three separate groups based on the reason for their visit: group 1 included all women with symptoms (SYM), group 2 included asymptomatic women who presented as known contacts of a partner with an STI (CON), and group 3 included asymptomatic women presenting for a general check‐up or for HIV testing (ASYM). Women with missing symptom information or physical findings were excluded. Only the first clinic visit was used in women with multiple visits. We assumed that self‐collected vaginal swabs would provide adequate specimens for the diagnosis of gonorrhoea, chlamydia and trichomoniasis using amplification tests,12,17,18,19 and bacterial vaginosis and vulvovaginal candididiasis using a Gram's stain and wet mount. For the final analysis, any vulvar, vaginal or cervical lesion detected on the speculum examination that resulted in a clinical diagnosis of an STI or referral (emergency room, internal medicine or gynaecology) was considered clinically meaningful. For the bimanual examination, documentation of cervical motion tenderness, adnexal tenderness, adnexal fullness, masses or abnormalities of the uterine fundus were assumed to represent a positive clinical finding, and PID was diagnosed on the basis of uterine/adnexal tenderness or cervical motion tenderness. (http://www.cdc.gov/STD/treatment/TOC2002TG.htm).
Independent continuous mean values were compared using Student's t test, and proportions were compared using the χ2 test. Positive and negative likelihood ratios (LRs; LR+ = sensitivity/(1−specificity) and LR− = (1−sensitivity)/specificity) were calculated to determine whether the presence of individual variables altered the index of suspicion based on the pretest probability (post‐test odds = pretest odds×LR).20 LR values >10 and <0.1 usually represent a clinically meaningful difference between pretest and post‐test probability. p Values <0.05 were assumed to represent significance. Data analyses were performed using STATA V.9.0.
Results
Of 21 703 patient visit records, 15 918 met the entry criteria. A total of 12 073 women were SYM (discharge, abdominal pain, dysuria, genital irritation or odour), 1676 were CON, and 2169 were ASYM; 5785 records were excluded from the study because of missing information (62%) or multiple visits (38%). The majority of women were African‐American and the mean age was 28 years. Table 1 summarises the additional demographic and clinical information for all three groups and women excluded from the study.
Table 1 Demographic and behavioural characteristics.
| SYM (n = 12 073) | CON (n = 1676) | ASYM (n = 2169) | Excluded (n = 5785) | |
|---|---|---|---|---|
| Mean (SD) age (years) | 27.1 (9.6) | 29.9 (10.6) | 29.1 (11.1) | 30.4 (11.4) |
| Race (%) | ||||
| White | 3.6 | 3.3 | 6.6 | 7.5 |
| Black | 95.2 | 95.8 | 91.8 | 90.5 |
| Other | 1.2 | 0.9 | 1.6 | 2 |
| Reported symptoms (%) | ||||
| Discharge | 63.9 | — | — | — |
| Dysuria | 14.4 | — | — | — |
| Irritation/odour | 35.7 | — | — | — |
| Lesion | 6.3 | — | — | — |
| Itching | 31.5 | — | — | — |
| Abdominal pain | 33.5 | — | — | — |
| Antibiotics taken in previous 2 weeks (%) | 11.2 | 10.3 | 7 | 11.3 |
| Prior STI (%) | 52.9 | 43.2 | 42.8 | 27.2 |
| Sex partners in past 30 days | ||||
| 1 | 72.5 | 77.9 | 66.3 | 47.2 |
| 2–3 | 14.4 | 11.6 | 11.2 | 7.8 |
| >3 | 13.1 | 10.5 | 22.5 | 44.9 |
| Substance use (%) | ||||
| IVDU | 4.6 | 3.1 | 9.7 | 8.7 |
| Cannabis | 43.1 | 34.7 | 36.7 | 34.5 |
| Alcohol | 49.3 | 45.2 | 47.5 | 37.4 |
| Exposure sites (%) | ||||
| Genital | 96.2 | 96.8 | 87.4 | 57.1 |
| Oral | 30.2 | 26.8 | 24.3 | 18.4 |
| Rectal | 5.8 | 4.5 | 4.2 | 3.2 |
| Contraception (%) | ||||
| Pill | 5.8 | 7.4 | 5.4 | 3.6 |
| DMPA | 8.7 | 12.1 | 8.8 | 5.2 |
| Condom | 47.9 | 38.7 | 41.7 | 27.2 |
| Pregnant (%) | 2.3 | 3.3 | 2 | 2.2 |
| Physical examination (%) | ||||
| Cervicovaginal discharge | 45.4 | 30.3 | 21.5 | 5.3 |
| Cervicovaginal lesion | 24.7 | 19.9 | 12.8 | 8.9 |
| Abdominal tenderness | 7.6 | 1.1 | 0.7 | 0.6 |
| Cervical motion tenderness | 3.9 | 0.5 | 0.3 | 1.1 |
| Adnexal tenderness | 3.8 | 0.7 | 0.2 | 0.4 |
| Diagnosis at clinic visit (%) | ||||
| Gonorrhoea | 6.8 | 9.2 | 3.4 | 3.4 |
| Chlamydia | 12.8 | 36.5 | 6 | 7.9 |
| Trichomoniasis | 17.5 | 15.8 | 9.1 | 5.9 |
| Vulvovaginal candidiasis | 16.6 | 5.2 | 5.5 | 3.3 |
| Bacterial vaginosis | 37.3 | 23.9 | 22 | 11.2 |
| Pelvic inflammatory disease | 5.4 | 0.6 | 0.3 | 0.5 |
| Herpes (ulcer) | 4.2 | 0.6 | 1.7 | — |
| Primary syphilis | 3.8 | 1.6 | 0.1 | — |
| Secondary syphilis | 2.1 | 0.1 | 0.5 | — |
–, Not applicable; ASYM, asymptomatic patients, not known to be contacts of partners with an STI presenting to clinic for a check‐up; CON, asymptomatic women who presented as known contacts of a partner with an STI; DMPA, depot medroxyprogesterone acetate; IVDU, intravenous drug use; STI, sexually transmitted infection; SYM, symptomatic.
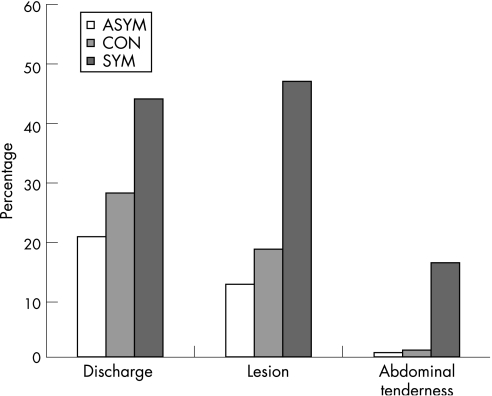
Among the SYM women, there was a 50.5%, 52.4%, and 18% concordance between self‐reports of vaginal discharge, genital lesion, and abdominal pain and physical examination findings confirming an abnormal discharge, any cervicovaginal lesion or abdominal tenderness, respectively. In the asymptomatic groups (CON and ASYM), abdominal tenderness was uncommon, whereas findings of a cervicovaginal discharge and any cervicovaginal lesion on examination were more common (fig 1).
Figure 1 Comparison of self‐reported complaints (or lack thereof) with objective physical findings on clinician examination. The figure represents the percentage of women in each group who were found to have any discharge, cervicovaginal lesion or abdominal pain on physical examination. The asymptomatic groups (asymptomatic women presenting for a general check‐up or for HIV testing (ASYM) and asymptomatic women who presented as known contacts of a partner with a sexually transmitted infection (CON)) comprised women with an unexpected clinical finding (discordance between reported symptoms and physical examination findings). For the symptomatic group (SYM), the values represent concordance between self‐reported symptoms and physical findings.
Although 25% of SYM, 20% of CON and 13% of ASYM patients had cervicovaginal lesions (ulcers, vesicles, rash or “other” lesions) noted on speculum examination, these lesions resulted in a clinical diagnosis or referral for further management in 11.8% of SYM (n = 1425), 4.6% of CON (n = 77) and 3.9% of ASYM (n = 85) groups (table 2). The clinicians did not consider the rest of the reported lesions to be clinically relevant. Table 2 summarises the performance measures, including LRs, for the speculum examination to identify clinically relevant cervicovaginal lesions.
Table 2 Diagnostic and performance measures of the speculum examination in the three patient groups.
| SYM (n = 12 073) | CON (n = 1676) | ASYM (n = 2169) | |
|---|---|---|---|
| Speculum examination, relevant lesion, % (n) | 11.8 (1425) | 4.6 (77) | 3.9 (85) |
| Syphilis | 5.9 (713) | 1.7 (29) | 0.6 (14) |
| Herpes | 4.2 (513) | 0.6 (11) | 1.7 (37) |
| Referral | 1.6 (199) | 2.2 (37) | 1.6 (34) |
| Performance* | |||
| Sensitivity (%, 95% CI) | 10.8 (9.6 to 12.1) | 30.2 (25.2 to 35.5) | 2.2 (0.8 to 4.7) |
| Specificity (%, 95% CI) | 95.3 (94.9 to 95.7) | 75 (72.6 to 77.3) | 99.3 (98.8 to 99.6) |
| PPV (95% CI) | 40 (36.3 to 43.8) | 22.5 (18.6 to 26.7) | 30 (11.9 to 54.3) |
| NPV (95% CI) | 78.7 (77.9 to 79.5) | 81.7 (79.4 to 83.8) | 87.6 (86.2 to 89) |
| LR+ (95% CI) | 2.3 (2 to 2.7) | 1.21 (1 to 1.5) | 3 (1.16 to 7.71) |
| LR– (95% CI) | 0.9 (0.9 to 1) | 0.9 (0.9 to 1) | 1 (0.9 to 1) |
| Bimanual examination, any sign, % (n) | 8 (966) | 2.2 (34) | 1.2 (26) |
| PID | 5.4 (657) | 0.6 (10) | 0.3 (7) |
| Referral | 1.1 (129) | 0.2 (4) | 0.3 (6) |
ASYM, asymptomatic patients not known to be contacts of partners with a sexually transmitted infection (STI) presenting to clinic for a check‐up; CON, asymptomatic patients who are known contacts of partners with an STI; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PID, pelvic inflammatory disease; PPV, positive predictive value; SYM, symptomatic group.
*Likelihood that a cervicovaginal lesion found on speculum examination would lead to a clinical diagnosis of herpes or syphilis, or a referral for further clinical evaluation.
In all, 8% of SYM (n = 966), 2.2% (n = 34) of CON and 1.2% (n = 26) of ASYM women were noted to have some abnormality (cervical motion tenderness, adnexal tenderness, uterine tenderness, adnexal mass or uterine enlargement/fullness) on bimanual examination. By group, 5.4% (n = 657), 0.6% (n = 10) and 0.3% (n = 7), respectively, were diagnosed with PID or were referred for further management. In the SYM group, 78% of PID cases were diagnosed in patients who presented with abdominal pain as their chief complaint.
Discussion
The LR incorporates both the sensitivity and specificity of the test and provides a direct estimate of how much a test result will change the odds of having a disease. We found that the odds of being diagnosed with a clinically meaningful cervicovaginal lesion did not increase significantly after a speculum examination. This finding was independent of self‐reported symptoms. Thus, the speculum examination, which takes the most time, may not add to the evaluation of a large subset of women in the era of molecular testing. Speculum examination is performed to obtain specimens for diagnostic testing, and to evaluate cervicovaginal abnormalities. As self‐collected vaginal swabs are adequate to diagnose the majority of the common STIs, specimen collection as a rationale may be a moot issue. Of the clinically meaningful lesions detected on speculum examination, 36% (n = 513), 14% (n = 11) and 17% (n = 14) of the SYM, CON and ASYM patients, respectively, would have been diagnosed with herpes, and 50% (n = 713) in the SYM, 38% (n = 29) in the CON and 43% (n = 37) in the ASYM groups with primary syphilis. Both these infections can be detected by serological testing. For those with primary syphilis, the serologies obtained on the day of evaluation would have been positive in 79%. This would suggest that, at the very least, symptomatic clinic attendees should still be triaged to undergo a speculum examination because of the probability of missing a primary syphilitic lesion. On the other hand, the positive yield of a speculum examination among asymptomatic individuals is fairly low, especially if serological testing for herpes and syphilis is performed.
Key messages
Current molecular diagnostic methods performed on specimens obtained by patient self‐swabs without a clinician's examination are often adequate to diagnose lower genital tract infections in women.
This study evaluates the additional information that speculum and bimanual examinations provides in the evaluation of lower genital tract infections.
We found that the odds of being diagnosed with a clinically meaningful cervicovaginal lesion did not increase significantly after a speculum examination. This finding was true, independent of self‐reported symptoms. Thus, the speculum examination, which takes most time, may not add to the evaluation of a large subset of women in the era of molecular testing.
In this era of increasing fiscal constraints and improved molecular diagnostics, eliminating unnecessary speculum or bimanual examinations in a subset of women who are asymptomatic and who do not need a routine cervical smear test may provide an operational advantage without compromising patient care.
A relatively small number of the ASYM group were diagnosed with PID as compared with 5.4% of the SYM group. The definition of PID in this study was clinical, and the updated 2002 Centers for Disease Control and Prevention criteria for diagnosing PID have been simplified (thus increasing their sensitivity but decreasing their specificity). Simms et al21 found that LRs of clinical criteria to diagnose laparoscopically proved PID were low, suggesting that the currently used clinical criteria were not specific for the diagnosis. These clinical criteria, however, are routinely used in clinical practice. Omitting the bimanual examination would have missed few cases of PID in asymptomatic women.
In summary, if self‐collected vaginal specimens and serologies for syphilis and herpes simplex virus were obtained from all patients and no speculum and bimanual examinations were performed, clinically relevant diagnoses (ie, 21% of primary syphilis diagnoses missed by serology, a missed PID diagnosis and lesions detected by speculum or bimanual examination leading to referral) would have been missed in 9.3% of SYM, 3.3% CON and 2.3% of ASYM patients.
Our study has several limitations. Firstly, this was a retrospective study, and both selection and information biases may be present. The majority of visits excluded from the study were of women who had multiple visits to the STI clinics. A number of women had missing data, leading to their exclusion. Many of these women did not undergo a physical examination as they were attending the clinic for other services provided, such as follow‐up for HIV care or contraception. In addition, as it was a retrospective analysis, the study may have misclassified outcomes of interest. We attempted to identify clinically meaningful outcomes, but a prospective evaluation of these patients may have allowed us to identify important diagnoses not captured in the standard electronic clinical assessment form. Similarly, we may have misclassified a cervicovaginal lesion noted on examination as clinically meaningful as a result of a documented referral made at the end of the clinical encounter, but that referral may have been made for an unrelated issue not captured by the clinical encounter form. Finally, this study may not apply to other clinical settings; our clinic population comprises high‐risk patients in a city with a high prevalence of most STIs.
Women presenting to STD clinics with symptoms are most likely to benefit from speculum and bimanual examinations. The yield of these tests in ASYM women, even in a city with high STI morbidity, is relatively low. In an audit of 421 ASYM women attending the Melbourne Sexual Health Service, Lee et al22 identified 5.4% cervicovaginal findings, of which only 1.3% were clinically relevant compared with 13% and 3.9% in our ASYM group of women. On the basis of their audit, the authors estimate that about 10 min per patient would be saved by eliminating the speculum examination, allowing allocation of resources to high‐risk and SYM patients. The Melbourne clinic has eliminated routine examination of ASYM women examined in the preceding two years. Eliminating unnecessary speculum or bimanual examinations in a subset of women who are ASYM and who do not need a routine smear test may provide an operational advantage without compromising patient care. In this era of increasing fiscal constraints and improved molecular diagnostics, these data may be used to frame and inform discussions on this issue, and help guide prospective studies aimed at defining the risks and benefits of streamlining clinical care.
Abbreviations
ASYM - asymptomatic patients, not known to be contacts of partners with a STI presenting to clinic for a check‐up
CON - asymptomatic women who presented as known contacts of a partner with a STI
PID - pelvic inflammatory disease
STD - sexually transmitted disease
STI - sexually transmitted infection
SYM - women with symptoms
Footnotes
Funding: None.
Competing interests: None.
References
- 1.Rogstad K E, Ahmed‐Jushuf I H, Robinson A J.et al MSSVD Adolescent Sexual Health Group. Standards for comprehensive sexual health services for young people under 25 years. Int J STD AIDS 200213420–424. [DOI] [PubMed] [Google Scholar]
- 2.Fenton K A, Mercer C H, Johnson A M.et al Reported sexually transmitted disease clinic attendance and sexually transmitted infections in Britain: prevalence, risk factors, and proportionate population burden. J Infect Dis 2005191(Suppl 1)S127–S138. [DOI] [PubMed] [Google Scholar]
- 3.Laverty S, Pugh R N, Joseph A T. The crisis in sexual health and developing genitourinary medicine services: lessons from a primary care trust. Int J STD AIDS 20061737–43. [DOI] [PubMed] [Google Scholar]
- 4.Van Leent E J M, Heijman R L J, de Vries H J C.et al Low time consuming STI screening for low risk STI clinic visitors. 16th Biennial Meeting of the International Society for Sexually Transmitted Diseases Research (ISSTDR), July 2005, Amsterdam
- 5.Bradbeer C, Mears A. STI services in the United Kingdom: how shall we cope? Sex Transm Infect 200379435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westrom L, Joesoef R, Reynolds G.et al Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 199219185–192. [PubMed] [Google Scholar]
- 7.Rhoton‐Vlasak A. Infections and infertility. Prim Care Update Ob Gyns 20007200–206. [DOI] [PubMed] [Google Scholar]
- 8.Buimer M, Van Doornum G J, Ching S.et al Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by ligase chain reaction‐based assays with clinical specimens from various sites: implications for diagnostic testing and screening. J Clin Microbiol 1996342395–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stary A, Najim B, Lee H H. Vulval swabs as alternative specimens for ligase chain reaction detection of genital chlamydial infection in women. J Clin Microbiol 199735836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garland S M, Tabrizi S N. Diagnosis of sexually transmitted infections (STI) using self‐collected non‐invasive specimens. Sex Health 20041121–126. [DOI] [PubMed] [Google Scholar]
- 11.Garrow S C, Smith D W, Harnett G B. The diagnosis of chlamydia, gonorrhoea, and trichomonas infections by self obtained low vaginal swabs, in remote northern Australian clinical practice. Sex Transm Infect 200278278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesenfeld H C, Lowry D L, Heine R P.et al Self‐collection of vaginal swabs for the detection of chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis 200128321–325. [DOI] [PubMed] [Google Scholar]
- 13.Nelson D B, Bellamy S, Gray T S.et al Self‐collected versus provider‐collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. J Clin Epidemiol 200356862–866. [DOI] [PubMed] [Google Scholar]
- 14.Strauss R A, Eucker B, Savitz D A.et al Diagnosis of bacterial vaginosis from self‐obtained vaginal swabs. Infect Dis Obstet Gynecol 20051331–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman S B, Nelson M B, Gaydos C A.et al Female prisoners' preferences of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis 200330306–319. [DOI] [PubMed] [Google Scholar]
- 16.Amsel R, Totten P A, Spiegel C A.et al Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 19837414–22. [DOI] [PubMed] [Google Scholar]
- 17.Schachter J, McCormack W M, Chernesky M A.et al Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol 2003413784–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K S, Tabrizi S N, Fethers K A.et al Comparison of conventional testing to polymerase chain reaction in detection of Trichomonas vaginalis in indigenous women living in remote areas. Int J STD AIDS 200516811–815. [DOI] [PubMed] [Google Scholar]
- 19.Wendel K A, Erbelding E J, Gaydos C A.et al Trichomonas vaginalis polymerase chain reaction compared with standard diagnostic and therapeutic protocols for detection and treatment of vaginal trichomoniasis. Clin Infect Dis 200235576–580. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke R, Guyatt G H, Sackett D L. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence‐Based Medicine Working Group. JAMA 1994271703–707. [DOI] [PubMed] [Google Scholar]
- 21.Simms I, Warburton F, Westrom L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect 200379491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D M, Chen M Y, Bradshaw C S.et al Is routine vaginal examination necessary for asymptomatic women attending sexual health services? Int J STD AIDS 200617631–632. [DOI] [PubMed] [Google Scholar]