Abstract
Reactive oxygen species (ROS) and oxidative stress are thought to play a central role in the etiology of cell dysfunction and tissue damage in sepsis. However, there is limited and controversial evidence from in vivo studies that ROS mediate cell signaling processes that elicit acute inflammatory responses during sepsis. Since NADPH oxidase is one of the main cellular sources of ROS, we investigated the role of this enzyme in lipopolysaccharide (LPS)-induced acute inflammation in vivo, utilizing mice deficient in the gp91phox or p47phox subunits of NADPH oxidase. Age and body-weight matched C57BL/6J wild-type (WT) and gp91phox−/− and p47phox−/− mice were injected i.p. with 50 µg LPS or saline vehicle, and sacrificed at different time points up to 24 hours. We found that LPS-induced acute inflammatory responses in serum and tissues were not significantly diminished in gp91phox−/− and p47phox−/− mice compared to WT mice. Rather, genetic deficiency of NADPH oxidase was associated with enhanced gene expression of inflammatory mediators and increased neutrophil recruitment to lung and heart. Furthermore, no protection from LPS-induced septic death was observed in either knockout strain. Our findings suggest that NADPH oxidase-mediated ROS production and cellular redox signaling do not promote but instead limit LPS-induced acute inflammatory responses in vivo.
Keywords: NADPH oxidase, inflammation, reactive oxygen species, redox-sensitive cell signaling, TLR4
INTRODUCTION
A central feature of the pathophysiology of acute inflammation and septic shock triggered by the bacterial endotoxin, lipopolysaccharide (LPS), is the generation of reactive oxygen species (ROS) and inflammatory mediators, such as cellular adhesion molecules, cytokines, and chemokines, by multiple phagocytic and non-phagocytic cells (1–3). These inflammatory mediators not only trigger an acute inflammatory response but also further enhance oxidative stress. It is now widely accepted that oxidative stress is central to the etiology of cell and organ dysfunction and tissue damage in sepsis (4–6). ROS play an important role in inflammatory responses by altering vascular cell signaling and function, leading to recruitment of polymorphonuclear neutrophils (PMN) and monocytes to the vascular wall and subsequent infiltration of these cells into tissues, where they trigger an inflammatory response (7). The regulation of inflammatory cytokine, chemokine, and adhesion molecule expression has been linked to oxidative stress through specific, reduction-oxidation (redox) sensitive signaling pathways and transcription factors, such as nuclear factor κB (NFκB) and activator protein-1 (AP-1) (8–11). In particular, the NFκB pathway impacts host defense against infectious agents by upregulating inflammatory genes that cause acute neutrophilic inflammation and the systemic inflammatory response syndrome (12).
NADPH oxidase is a highly regulated membrane-bound enzyme complex found in a variety of phagocytic and non-phagocytic cells (13,14). The enzyme catalyzes the production of superoxide by the one-electron reduction of oxygen, using NADPH as the primary electron donor. In phagocytic cells the core enzyme consists of five subunits: p40phox, p47phox, p67phox, p22phox, and gp91phox. In the basal state, p40phox, p47phox, and p67phox exist in the cytosol as a complex, whereas p22phox and gp91phox form a heterodimeric flavohemoprotein called cytochrome b558, which is located in the membranes of secretory vesicles and specific granules of leukocytes. Also, two low molecular weight GTP-binding proteins, Rap 1A and rac1/2, are involved in the activation of NADPH oxidase. Upon stimulation, the cytosolic subunit p47phox is phosphorylated, and the entire cytosolic complex migrates to the membrane, where it associates with cytochrome b558 to form active NADPH oxidase. Activation of NADPH oxidase can take place either at the plasma membrane, leading to release of superoxide to the extracellular environment, or at intracellular membranes, leading to ROS formation in intracellular compartments.
Phagocytic NADPH oxidase was originally described in neutrophils and macrophages, where it serves a critical function in host-defense against invading microorganisms. It is well documented that superoxide production is almost completely abrogated in myeloid-derived cells that lack gp91phox. The non-phagocytic NADPH oxidase, which is found in smooth muscle cells, synoviocytes, chondrocytes, endothelial cells, and epithelial cells, appears to be qualitatively similar to the phagocytic enzyme in its ability to induce redox signaling, although ROS generation is markedly lower in nonphagocytic than phagocytic cells (14). It has been shown that mice deficient in gp91phox or p47phox have a reduced capacity to generate superoxide via the NADPH oxidase pathway (15–17). However, the relative concentrations and activities of NADPH oxidase in each of the non-phagocytic cells have not been examined.
In recent years, there has been increasing evidence that ROS act as mediators in cell signaling in a broad array of physiological and pathophysiological responses, from cell proliferation to gene expression and apoptosis (7,14,18,19). Many in vitro studies have suggested that ROS act as second messengers that mediate NFκB activation (20–22). Thus, it is possible that superoxide generated by NADPH oxidase plays an important role in regulating host inflammatory responses through modulation of redoxsensitive signaling pathways in phagocytic and non-phagocytic cells. However, there is limited, and controversial, evidence from in vivo studies to support this notion (16,17,23–26).
The purpose of the present study was to examine the hypothesis that elimination or reduction of ROS production by NADPH oxidase results in reduced bacterial LPS-induced inflammatory responses in vivo. To this end, we utilized two experimental mouse models: a knockout of the gp91phox subunit of NADPH oxidase, in which production of ROS by phagocytic cells is greatly reduced (gp91phox−/−), and a knockout of the p47phox subunit, in which ROS production by both phagocytic and non-phagocytic cells is impaired (p47phox−/−)
MATERIALS AND METHODS
Animals and experimental procedures
Age- and body weight-matched female C57BL/6J wild-type (WT), gp91phox−/−, and p47phox−/− mice at 10–12 weeks of age weighing 20–22 g were purchased from Jackson Laboratories (Bar Harbor, Maine). The mice were housed in specific pathogen-free conditions and a temperature and humidity controlled environment (12-hour light/dark cycle) with unlimited access to tap water and food. Mice were acclimated for 7–10 days before initiation of experiments. All animal procedures were reviewed and approved by the Oregon State University Institutional Animal Care and Use Committee.
Mice (WT, gp91phox−/−, or p47phox−/−) were randomly assigned to receive i.p. injection of the vehicle HBSS (control) or 50 µg LPS (serotype 055:B5 from Escherichia coli, Sigma Aldrich) and sacrificed after 1, 3, 8, or 24 hours. In some studies, different doses of LPS (12.5, 25, 50, 100, 200 µg) were used and animals sacrificed after 3 hours. Each group consisted of 4 to 8 animals. After sacrifice, blood and tissues were collected for further analysis.
For survival experiments, mice (WT, gp91phox−/− or p47phox−/−) were injected i.p. with 1.5 mg LPS, which has been shown to cause 75–80% mortality (27), and monitored for endotoxemia and death trice daily for up to five days.
Serum concentrations of inflammatory mediators
Serum concentrations of soluble sVCAM-1 (sVCAM-1), soluble ICAM-1 (sICAM-1), MCP-1, and TNFα were measured by quantitative colorimetric sandwich ELISA kits from R&D Systems (Minneapolis, MN), following the manufacturer’s instructions. The reported sensitivity of the assay is 5 pg/ml for sVCAM-1, 29 pg/ml for sICAM-1, 2 pg/ml for MCP-1, and 5 pg/ml for TNFα.
Tissue mRNA levels of inflammatory mediators
Total RNA was isolated from various tissues using TRIzol Reagent (Invitrogen). cDNA synthesis was performed using the high capacity cDNA archive kit from Applied Biosystems (Foster City, CA). mRNA levels of VCAM-1, ICAM-1, MCP-1, TNF, IL-1β, IL-6, Toll-like receptor 4 (TLR4), myeloperoxidase (MPO), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were quantitated by real-time PCR. All primers and probes were purchased as kits (Assays on Demand, Applied Biosystems). The assays are supplied as a 20× mixture of PCR primers and TaqMan minor groove binder 6-FAM dye labeled probes with a nonfluorescent quencher at the 3' end of the probe. STAR TaqMan quantitative PCR (40 cycles at 95°C for 15 seconds and 60°C for 1 min) was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) in 96-well plates with the ABI Prism 7500 Sequence Detection System (Applied Biosystems). To obtain relative quantitation, two standard curves were constructed in each plate with one target gene and an internal control gene (GAPDH). Standard curves were generated by plotting the threshold cycle number values against the log of the amount of input cDNA and used to quantitate the expression of the various target genes and GAPDH gene in the same sample. After normalization to internal GAPDH in each sample, the results were expressed as percentage of GAPDH.
Activation of nuclear transcription factors
Nuclear extracts were prepared from various tissues using nuclear extraction kits (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. For analysis of activation of nuclear transcription factors, ELISA-based assays (Active Motif) were used to determine the DNA binding activity of NFκB (p65) and AP-1 (c-fos) according to the manufacturer’s instructions. The specificity of binding was confirmed by competition with either wild type or mutant oligonucleotides. The wild type oligonucleotides prevent NFκB and AP-1 binding to the probe immobilized on the plates. Conversely, the mutated oligonucleotides should have no effect on NFκB and AP-1 binding activity.
Statistical analysis
The data were calculated as means ± SEM and analyzed by an unpaired Student's t test. The survival of groups of animals was analyzed by log-rank test using Statview software. Statistical significance was set at a value of P<0.05.
RESULTS
Deficiency of gp91phox or p47phox does not diminish the LPS-induced increase in serum levels of inflammatory mediators
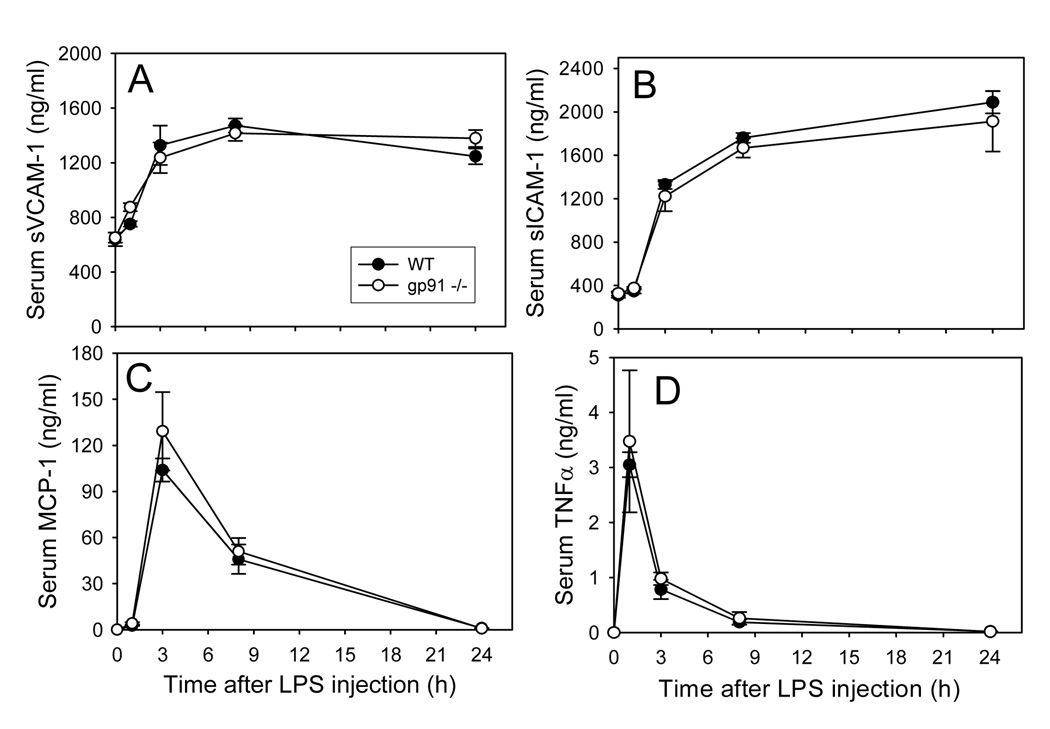
We first determined the effect of LPS on systemic inflammation in WT mice, as assessed by increased serum levels of inflammatory mediators. As shown in Fig. 1 and Fig. 2, LPS treatment induced a time- and dose-dependent increase in serum concentrations of sVCAM-1, sICAM-1, MCP-1, and TNFα. TNFα peaked at 1 hour, and the other inflammatory molecules were significantly increased at 3 hours (Fig. 1). sVCAM-1 and sICAM-1 remained elevated up to 24 hours, while MCP-1 and TNFα gradually returned to baseline after 24 hours. Interestingly, the LPS-induced increases in serum levels of inflammatory mediators were indistinguishable in gp91phox−/− and WT mice (Fig. 1).
Fig. 1. Time-course of LPS-induced increases in serum levels of sVCAM-1 (A), sICAM-1 (B), MCP-1 (C), and TNF (D) in wild-type and gp91phox−/− mice.
Wild-type (solid circles) and gp91phox−/− (open circles) mice were injected i.p. with HBSS (0 time point control) or 50µ g LPS as described in Methods. At the indicated time points after LPS injection, the animals were sacrificed and blood was collected. Serum sVCAM-1, sICAM-1, MCP-1, and TNFα were measured by ELISA. Data shown are mean values ± SEM of 4–8 animals.
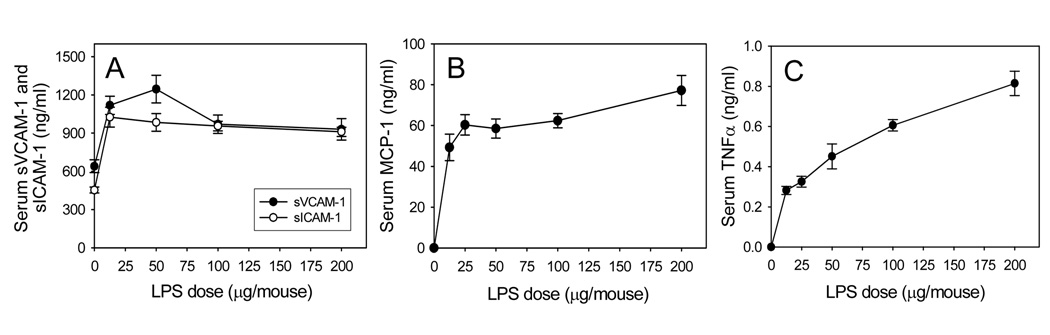
Fig. 2. Dose-response of LPS-induced increases in serum levels of sVCAM-1 and sICAM-1 (A), MCP-1 (B), and TNFα (C) in wild-type mice.
Wild-type mice were injected i.p. with HBSS (control) or different doses of LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and blood was collected. Serum sVCAM-1, sICAM-1, MCP-1, and TNFα were measured by ELISA. Data shown are mean values ± SEM of 4–8 animals.
Based on the data in Fig. 1 and Fig. 2, the 3-hour time point and 50-µg LPS dose were selected for further assessment of the role of NADPH oxidase in LPS-induced systemic inflammation. As shown in Table 1, the serum levels of sVCAM-1, sICAM-1, MCP-1, and TNFα were not significantly different in gp91phox−/− and p47phox−/− mice compared to WT mice, suggesting that NADPH oxidase is not essential for LPS-induced systemic inflammatory responses.
Table 1. Genetic deficiency of gp91phox or p47phox does not diminish the LPS-induced increase in serum levels of inflammatory mediators.
Wild-type, gp91phox−/−, and p47phox−/− mice were injected i.p. with HBSS (control) or 50 µg LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and blood was collected. Serum sVCAM-1, sICAM-1, MCP-1, and TNFα were measured by ELISA. Data shown are from LPS-treated groups and presented as mean values ± SEM of 4–8 animals.
| sVCAM-1 | sICAM-1 | MCP-1 | TNFα | |
|---|---|---|---|---|
| (ng/ml) | (ng/ml) | (ng/ml) | (pg/ml) | |
| WT | 1327 ± 144 | 1331 ± 42 | 94 ± 21 | 416 ± 87 |
| gp91−/− | 1237 ± 112 | 1221 ± 137 | 114 ± 16 | 700 ± 133 |
| WT | 1231 ± 183 | 1171 ± 161 | 82 ± 46 | 488 ± 188 |
| p47−/− | 1747 ± 183 | 1395 ± 103 | 119 ± 15 | 965 ± 288 |
Deficiency of gp91phox or p47phox does not diminish but rather enhances LPS-induced inflammatory gene expression
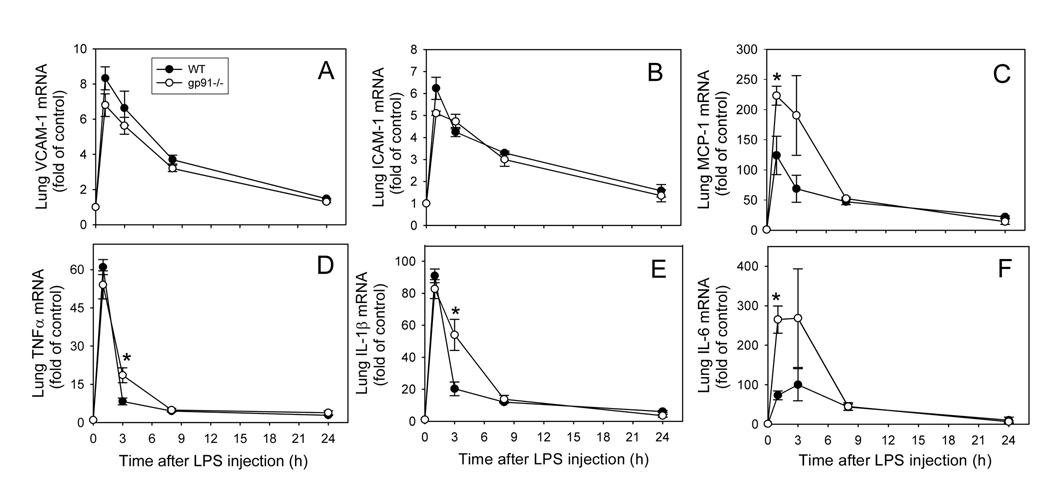
Gene expression of VCAM-1, ICAM-1, MCP-1, TNFα, IL-1β, IL-6, and TLR4 was assessed in lung, heart, liver, and kidney, using real-time quantitative PCR. As shown in Fig. 3 and Fig. 4, lung mRNA levels of inflammatory mediators increased in a time- and dose-dependent manner following LPS administration to WT mice. Expression of inflammatory genes was increased substantially 1 hour after LPS injection (Fig. 3). VCAM-1, ICAM-1, MCP-1 and IL-6 mRNA levels remained elevated for at least 2 more hours and then gradually declined to baseline after 24 hours. TNFα and IL-1β mRNA levels (Fig. 3D and E, respectively) peaked at 1 hour and then rapidly declined to about 25% at 3 hours and returned to baseline after 24 hours. However, none of the LPS-induced increases in inflammatory gene expression were diminished at any of the time points examined in gp91phox−/− mice compared to WT mice (Fig. 3). In fact, LPS-induced expression of MCP-1 and IL-6 at the 1-hour time point and TNFα and IL-1β at 3 hours were significantly increased in gp91phox−/− mice compared to WT mice (P<0.05, n=4–8). These data indicate that genetic deficiency of gp91phox increases rather than decreases LPS-induced cytokine and MCP-1 expression in lung.
Fig. 3. Time-course of LPS-induced increases in lung VCAM-1 (A), ICAM-1 (B), MCP-1 (C), TNFα (D), IL-1β (E), and IL-6 (F) mRNA levels in wild-type and gp91phox−/− mice.
Wild-type (solid circles) and gp91phox−/− (open circles) mice were injected i.p. with HBSS (0 time point control) or 50µ g LPS as described in Methods. At the indicated time points after LPS injection, the animals were sacrificed and total RNA was isolated from lung. Real-time quantitative PCR analysis was performed for VCAM-1, ICAM-1, MCP-1, TNFα, IL-1β, IL-6, and GAPDH mRNA. Data were expressed as fold of HBSS-treated control after normalization to the internal control gene, GAPDH, and are presented as mean values ± SEM of 4–8 animals. *P<0.05 compared to WT mice.
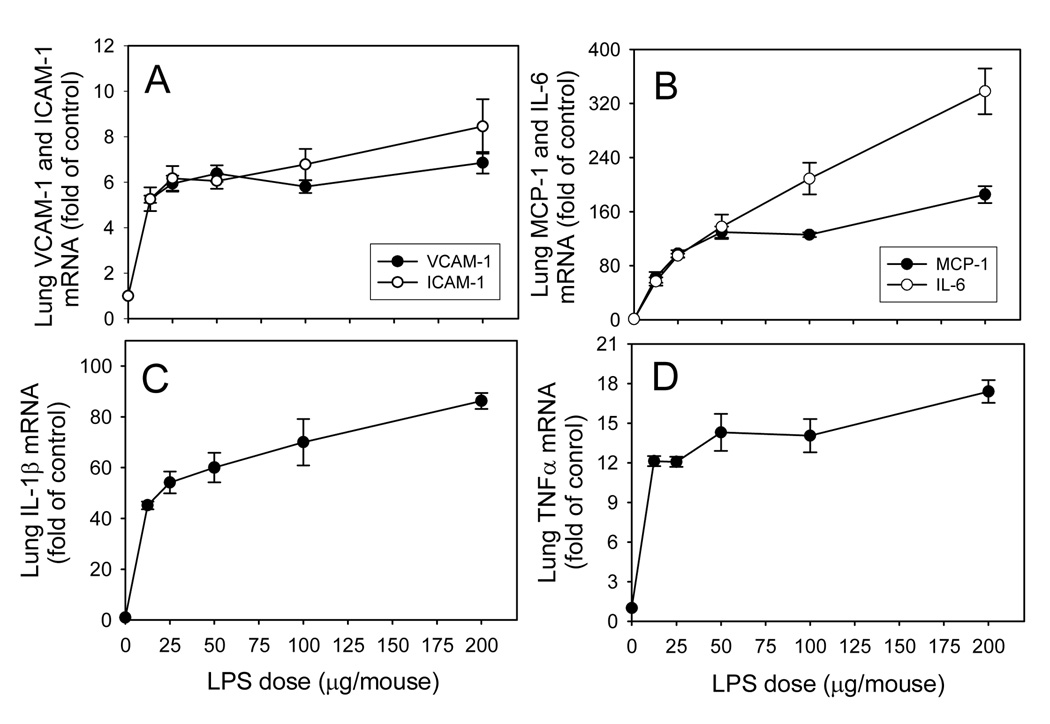
Fig. 4. Dose-response of LPS-induced increases in lung VCAM-1 and ICAM-1 (A), MCP-1 and IL-6 (B), IL-1β (C), and TNFα (D) mRNA levels in wild-type mice.
Wild-type mice were injected i.p. with HBSS (control) or different doses of LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and total RNA was isolated from lung. Real-time quantitative PCR analysis was performed for VCAM-1, ICAM-1, MCP-1, TNFα, IL-1β, IL-6, and GAPDH mRNA. Data were expressed as fold of HBSS-treated control after normalization to the internal control gene, GAPDH, and are presented as mean values ± SEM of 4–8 animals.
Exposing WT animals to 50 µg LPS for 3 hours also strongly increased VCAM-1, ICAM-1, MCP-1, TNFα, IL-1β, and IL-6 mRNA levels in heart, liver, and kidney (Table 2). However, none of these LPS-induced increases in inflammatory gene expression were diminished in gp91phox−/− or p47phox−/− mice compared to WT mice (Table 2). To the contrary, LPS-induced expression of ICAM-1, MCP-1, TNFα and IL-1β in heart, TNFα and IL-1β in lung, and IL-1β in liver was significantly higher in gp91phox−/− than WT mice, and hepatic IL-1β gene expression also was significantly higher in p47phox−/− mice (P<0.05, n=4–8). These data suggest that NADPH oxidase is not required for LPS-induced tissue inflammation and may in fact reduce LPS-induced transcriptional regulation of several inflammatory genes.
Table 2. Genetic deficiency of gp91phox or p47phox does not diminish but rather enhances LPS-induced inflammatory gene expression in various organs.
Wild-type, gp91phox−/−, and p47phox−/− mice were injected i.p. with HBSS (control) or 50 µg LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and total RNA was isolated from various organs. Real-time quantitative PCR analysis was performed for VCAM-1, ICAM-1, MCP-1, TNFα, IL-1β, IL-6, TLR-4, and GAPDH mRNA. After normalization to the internal control gene, GAPDH, the results for each target gene were expressed as percentage of GAPDH. Data shown are from LPS-treated groups and presented as mean values ± SEM of 4–8 animals. *P<0.05 compared to WT mice
| Heart | Lung | Liver | Kidney | ||
|---|---|---|---|---|---|
| WT | 114 ± 15 | 93 ± 22 | 81 ± 12 | 82 ± 5 | |
| gp91−/− | 152 ± 24 | 91 ± 20 | 87 ± 21 | 78 ± 21 | |
| VCAM-1 | |||||
| WT | 88 ± 9 | 87 ± 12 | 100 ± 2 | 114 ± 8 | |
| p47−/− | 102 ± 10 | 75 ± 9 | 128 ± 12 | 106 ± 6 | |
| WT | 124 ± 13 | 94 ± 18 | 102 ± 11 | 80 ± 8 | |
| gp91−/− | 189 ± 26* | 81 ± 20 | 98 ± 16 | 90 ± 31 | |
| ICAM-1 | |||||
| WT | 99 ± 10 | 111 ± 13 | 58 ± 2 | 94 ± 3 | |
| p47−/− | 84 ± 3 | 89 ± 14 | 73 ± 7 | 95 ± 6 | |
| WT | 150 ± 35 | 65 ± 31 | 64 ± 20 | 121 ± 21 | |
| gp91−/− | 362 ± 65* | 175 ± 64 | 82 ± 23 | 60 ± 25 | |
| MCP-1 | |||||
| WT | 157 ± 36 | 328 ± 100 | 197 ± 3 | 119 ± 11 | |
| p47−/− | 123 ± 24 | 327 ± 98 | 211 ± 22 | 104 ± 3 | |
| WT | 133 ± 13 | 66 ± 15 | 72 ± 16 | 99 ± 1 | |
| gp91−/− | 222 ± 27* | 174 ± 44* | 87 ± 24 | 80 ± 18 | |
| TNFα | |||||
| WT | 122 ± 27 | 65 ± 10 | 151 ± 10 | 88 ± 4 | |
| p47−/− | 187 ± 60 | 123 ± 26 | 191 ± 15 | 90 ± 10 | |
| WT | 51 ± 20 | 49 ± 15 | 65 ± 11 | 48 ± 2 | |
| gp91−/− | 156 ± 32* | 173 ± 20* | 117 ± 13* | 28 ± 6 | |
| IL-1β | |||||
| WT | 106 ± 12 | 95 ± 3 | 65 ± 3 | 62 ± 25 | |
| p47−/− | 86 ± 7 | 86 ± 6 | 117 ± 21* | 123 ± 17 | |
| WT | 39 ± 24 | 81 ± 47 | 56 ± 17 | 74 ± 26 | |
| gp91−/− | 165 ± 49 | 305 ± 147 | 123 ± 33 | 160 ± 112 | |
| IL-6 | |||||
| WT | 91 ± 6 | 113 ± 4 | 111 ± 31 | 102 ± 5 | |
| p47−/− | 84 ± 21 | 83 ± 23 | 216 ± 37 | 92 ± 11 | |
| WT | 95 ± 9 | 99 ± 5 | 90 ± 10 | 122 ± 20 | |
| gp91−/− | 104 ± 7 | 112 ± 8 | 101 ± 11 | 88 ± 18 | |
| TLR4 | |||||
| WT | 109 ± 4 | 99 ± 3 | 75 ± 1 | 104 ± 28 | |
| p47−/− | 97 ± 3 | 109 ± 6 | 107 ± 13* | 125 ± 5 | |
Finally, there was no significant difference in TLR4 gene expression in gp91phox−/− and p47phox−/− mice compared to WT mice, except in the liver of p47phox−/− mice, where TLR4 mRNA levels were significantly higher than in WT animals (P<0.05, n=4–8). These data suggest that the increase in LPS-induced expression of several inflammatory genes in gp91phox−/− mice is not due to increased TLR4 expression.
Deficiency of gp91phox or p47phox does not diminish LPS-induced NFκB or AP-1 activation
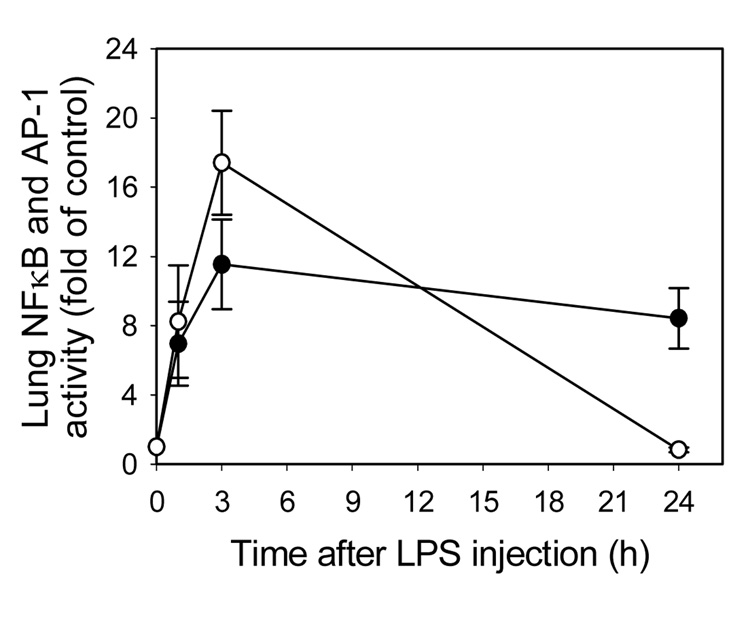
It is well documented that the redox-sensitive transcription factors, NFκB and AP-1, play prominent roles in LPS-induced transcriptional regulation of most inflammatory genes that contribute to the development of septic shock and multiple organ failure (13). Hence, we determined the role of NADPH oxidase in the activation of these transcription factors in various organs. Treatment with LPS increased NFκB and AP-1 DNA binding activity in a time-dependent manner in lung of WT mice (Fig. 5). Both NFκB and AP-1 binding activities appeared to peak at 3 hours. AP-1 activity returned to baseline after 24 hours, while NFκB activity remained elevated throughout the experiment. Treatment of WT mice with LPS for 3 hours markedly activated NFκB and AP-1 in lung, heart, liver, and kidney (Table 3), which was associated with increased protein levels and gene expression of inflammatory mediators in serum and tissues (Table 1 and Table 2), respectively. However, genetic deficiency of gp91phox and p47phox did not diminish LPS-induced activation of NFκB and AP-1 in all organs examined (Table 3), suggesting that NADPH oxidase is not essential for LPS-induced activation of these redox-sensitive transcription factors.
Fig. 5. Time-course of LPS-induced activation of lung NFκB and AP-1 in wild-type mice.
Wild-type mice were injected i.p. with HBSS (0 time point control) or 50µ g LPS as described in Methods. One, 3, and 24 hours after LPS injection, the animals were sacrificed and nuclear extracts were isolated from lung. NFκB (p65)/DNA (closed circles) and AP-1 (c-fos)/DNA (open circles) binding activities were quantified by ELISA. Data were expressed as fold of HBSS-treated control and are presented as mean values ± SEM of 4–8 animals.
Table 3. Genetic deficiency of gp91phox or p47phox does not diminish LPS-induced NFκB or AP-1 activation.
Wild-type, gp91phox−/−, and p47phox−/− mice were injected i.p. with HBSS (control) or 50 µg LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and nuclear extracts were isolated from heart, lung, liver, and kidney. NFκB (p65)/DNA and AP-1 (c-fos)/DNA binding activities were quantified by ELISA and expressed as OD450 nm. Data shown are from LPS-treated groups and presented as mean values ± SEM of 4–8 animals.
| Heart | Lung | Liver | Kidney | ||
|---|---|---|---|---|---|
| WT | 0.13 ± 0.04 | 0.33 ± 0.12 | 0.96 ± 0.13 | 0.63 ± 0.15 | |
| gp91−/− | 0.15 ± 0.05 | 0.67 ± 0.21 | 1.27 ± 0.31 | 1.08 ± 0.20 | |
| NFκB | |||||
| WT | 0.22 ± 0.06 | 0.55 ± 0.12 | 1.31 ± 0.20 | 0.45 ± 0.16 | |
| p47−/− | 0.24 ± 0.03 | 0.57 ± 0.09 | 1.78 ± 0.17 | 0.67 ± 0.09 | |
| WT | 0.25 ± 0.10 | 0.12 ± 0.05 | 0.42 ± 0.04 | 0.29 ± 0.07 | |
| gp91−/− | 0.17 ± 0.05 | 0.24 ± 0.04 | 0.66 ± 0.15 | 0.44 ± 0.13 | |
| AP-1 | |||||
| WT | 0.51 ± 0.04 | 0.51 ± 0.07 | 0.63 ± 0.02 | 0.68 ± 0.04 | |
| p47−/− | 0.65 ± 0.02 | 0.50 ± 0.02 | 0.70 ± 0.04 | 0.82 ± 0.07 | |
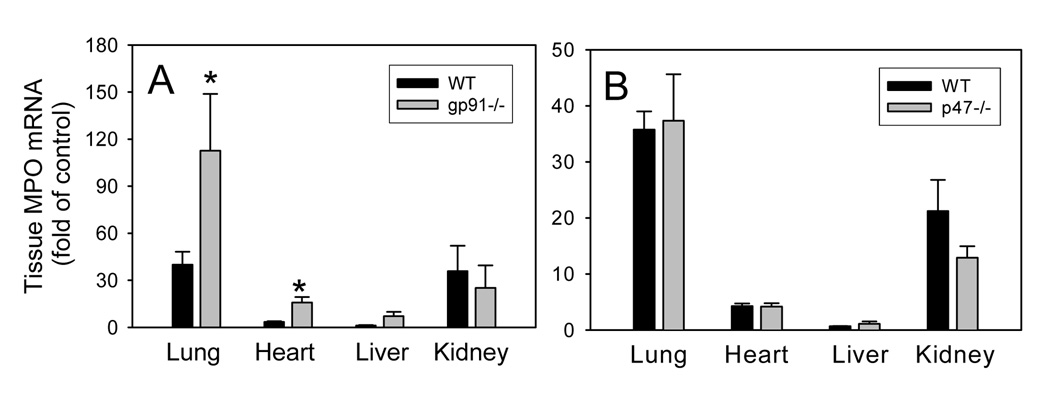
Deficiency of gp91phox increases LPS-induced neutrophil recruitment to lung and heart
As neutrophils and other phagocytic cells such as monocyte-macrophages play critical roles in acute tissue inflammatory responses, we assessed MPO gene expression, a biomarker of neutrophils, in various organs after LPS challenge. As shown in Fig. 6, treatment of animals with LPS strongly increased MPO mRNA levels in lung, heart, and kidney of gp91phox−/−, p47phox−/−, and WT mice. Interestingly, LPS-induced expression of MPO in lung and heart was significantly increased in gp91phox−/− mice compared to WT mice (P<0.05, n=4–8). These data further support the notion that genetic deficiency of gp91phox increases rather than decreases LPS-induced inflammatory responses in vivo.
Fig. 6. LPS-induced increases in myeloperoxidase mRNA levels in various organs of wild-type, gp91phox−/− (A), and p47phox−/− (B) mice.
Wild-type, gp91phox−/−, and p47phox−/− mice were injected i.p. with HBSS (control) or 50µ g LPS as described in Methods. Three hours after LPS injection, the animals were sacrificed and total RNA was isolated from various organs. Real-time quantitative PCR analysis was performed for MPO and GAPDH mRNA. After normalization to the internal control gene, GAPDH, the results for the MPO gene were expressed as fold of control. Data shown are mean values ± SEM of 4–8 animals. *P<0.05 compared to WT mice
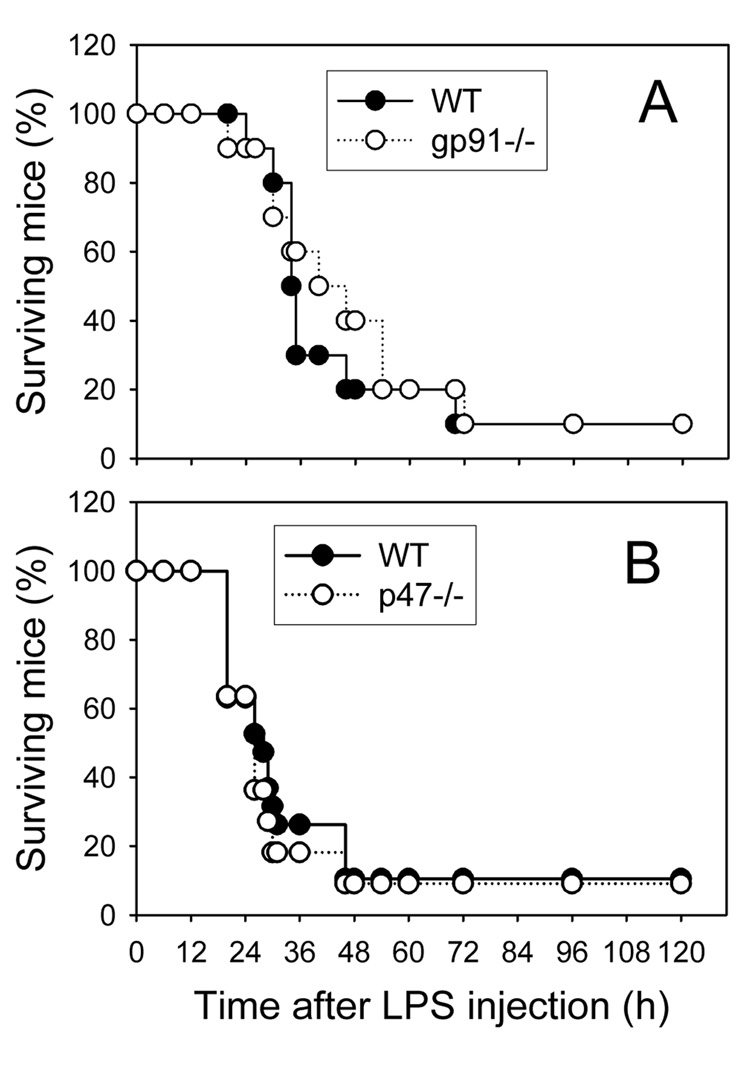
Deficiency of gp91phox or p47phox does not improve survival of endotoxemic mice
Finally, we investigated whether genetic deficiency of NADPH oxidase protects against LPS-induced endotoxemic death in mice. As shown in Fig. 7, i.p. injection of 1.5 mg LPS caused 90% of WT mice to expire within 48 to 72 hours. However, no reduction in mortality was observed for both gp91phox−/− and p47phox−/− mice, suggesting that NADPH oxidase is not playing a significant role in LPS-induced endotoxemic death.
Fig. 7. LPS-induced endotoxemic death in wild-type, gp91phox−/− (A), and p47phox−/− mice.
Wide-type (closed circles), gp91phox−/− (open circles, panel A) and p47phox−/− mice (open circles, panel B) were injected i.p. with 1.5 mg LPS as described in Methods. The animals were monitored for survival three times daily for up to five days. The numbers of animals used were: 10 and 19 wild-type mice, respectively, in Panels A and B; 10 gp91phox−/− mice; and 11 p47phox−/− mice.
DISCUSSION
Increased ROS production and oxidative stress during endotoxemia are thought to causally contribute to cell and tissue injury, systemic sepsis syndrome, and septic shock and death (4,25,28–30). ROS also modulate a number of cell signaling pathways resulting in transcription factor activation (17,18). Recent studies have suggested that NADPH oxidase-derived ROS (NOX-ROS) act as second messengers mediating LPS-induced inflammatory responses (16,17,29,31–33). Contrary to this notion, in the present study we found that LPS-induced inflammatory responses were not significantly diminished in gp91phox−/− and p47phox−/− mice compared to WT mice. In fact, genetic deficiency of NADPH oxidase was associated with increased gene expression of several inflammatory mediators and increased neutrophil recruitment to lung and heart. Furthermore, no protection from septic death was observed in either knockout mouse. These findings indicate that NOX-ROS and cellular redox signaling do not promote but instead limit LPS-induced inflammatory responses.
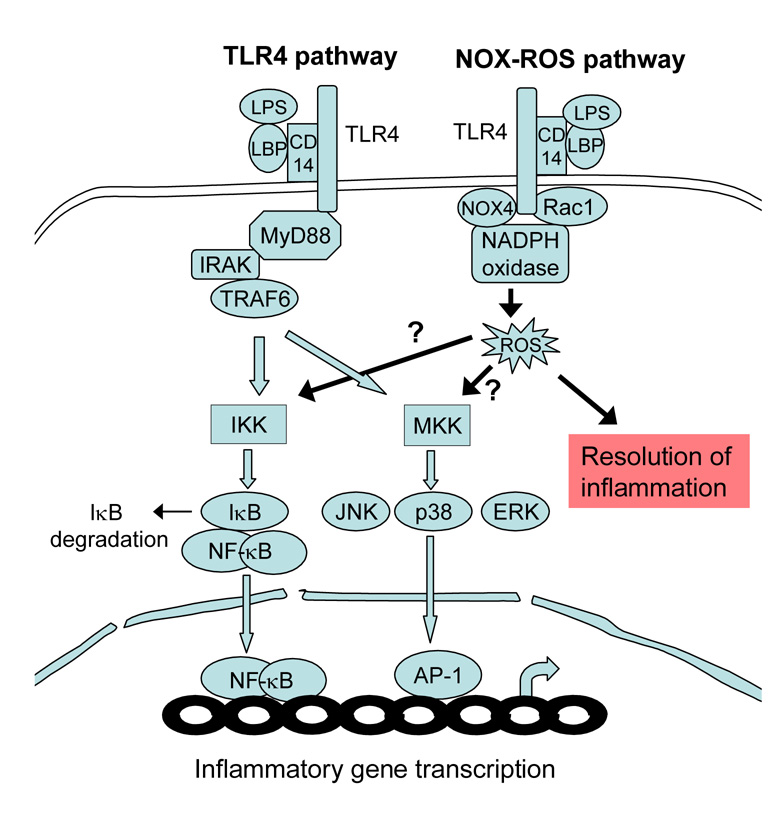
Our findings raise interesting questions about the mechanisms underlying ROS-mediated signal transduction pathways in sepsis. It is well documented that the “classic” TLR4 pathway plays a major role in LPS signaling during sepsis (34). TLR4 recognizes LPS from Gram-negative bacteria and mediates the innate immune response by activating IκB kinase (IKK) and mitogen-activated protein kinase kinases (MKK), which in turn activate NFκB and AP-1, respectively (12,34) (Scheme 1). Cell culture studies have shown that LPS also stimulates the NOX-ROS pathway via TLR4-mediated Rac 1 and NOX4 activation, with the resulting ROS acting as second messengers for the classic TLR4 pathway (18,19,22,35) (Scheme 1). However, several signaling cascades have been recently identified that activate NFκB without the involvement of ROS, and ROS-mediated NFκB activation may be restricted to certain cell types (36,37). Overall, the current evidence suggests that the NOX-ROS pathway is not sufficient to mediate LPS-induced inflammatory responses but may serve to amplify LPS signaling along the classic TLR 4 pathway (22,35, 38, 39) (Scheme 1).
Scheme 1. Diagram depicting the TLR4 and NOX-ROS pathways.
LPS binds to LPS-binding protein (LBP) and CD14, thus activating TLR4. TLR4 activates two major kinases: IκB kinase (IKK), which phosphorylates IκB, leading to its ubiquitylation and subsequent degradation and NFκB translocation to the nucleus; and mitogen-activated protein kinase kinases (MKK), which phosphorylate and activate c-Jun kinase (JNK), extracellular receptor-activated kinase (ERK), and p38 kinase, leading to AP-1 activation. Activated NFκB and AP-1 in the nucleus bind to DNA promoter regions to induce inflammatory gene transcription. In the NOX-ROS pathway, LPS activates TLR4, which in turn activates NADPH oxidase through Rac1 and Nox4. While it has been hypothesized that ROS generation by NADPH oxidase enhances IKK and MKK activity (arrows marked “?”), the results presented in this paper indicate that the NOX-ROS pathway contributes to the resolution of LPS-induced inflammation.
The evidence from in vivo studies for a role of the NOX-ROS pathway in inflammatory responses to LPS or bacterial infection, however, is scant and controversial (16,17,24–26,40–42). Koay et al. (17) reported that pulmonary NFκB activation by LPS (5 µg/g bw, i.p.) was similar in p47phox−/− and WT mice, but lower in p47phox−/− mice when a higher dose of LPS (20 µg/g bw) was used. In the present study, we used 2.5µ g LPS per g body weight, which is a low dose, and – in agreement with Koay et al. (17) – we did not observe a reduction in NFκB and AP-1 activation and inflammatory gene expression in gp91phox−/− and p47phox−/− mice compared to WT mice. These results suggest that either ROS are not required for NFκB activation following low-dose LPS, or gp91phox- and p47phox-deficient mice are able to compensate for the defect in NADPH oxidase by engaging alternate mechanisms of ROS production, such as mitochondria (43,44) or xanthine oxidase (45,46). Sato et al. (25) also found no differences between p47phox−/− and WT mice in LPS-induced lung injury, alveolar damage, and infiltration of neutrophils and monocytes, and Swain et al. (26) observed no amelioration of pulmonary damage in Pneumocystis-infected gp91phox−/− mice compared to WT mice. These results suggest that NADPH oxidase and oxidative mechanisms of neutrophil function do not play a major role in lung injury after exposure to bacteria or LPS.
In contrast, Gao et al. (16) reported that, in an E. coli -mediated septic mouse model, p47phox- and gp91phox-deficiency was associated with reduced lung microvascular injury, suggesting a role for ROS. Similarly, Sadikot et al. (24) found that NFκB activation and TNFα levels were significantly decreased in p47phox−/− compared to WT mice following P. aeruginosa infection. These discrepant results (16,17,24–26),may be attributed to different animal models, LPS doses, or bacterial stimuli used, as well as different time points at which measurements were performed.
In the present study, gene expression of MCP-1 and the inflammatory cytokines, IL-6, TNFα, and IL-1β, was significantly higher in lung and heart of LPS-treated gp91phox−/− mice than WT mice, and the same was true for hepatic IL-1β gene expression in gp91phox−/− and p47phox−/− mice. Further, genetic deficiency of NADPH oxidase appeared to promote neutrophil recruitment to lung and heart. These data suggest that NOX-ROS suppress–rather than enhance–the inflammatory response to LPS, and are in agreement with previous reports that lack or impairment of NADPH oxidase confers a state of hyperinflammation (17,47–50). For example, LPS-exposed p47phox−/− mice exhibited enhanced pulmonary inflammation due to increased neutrophil influx and macrophage-inflammatory protein-2 (MIP-2) levels compared to WT mice (17). Furthermore, increased lung infiltration of inflammatory cells, activation of alveolar monocyte-macrophages, and levels of MCP-1 and MIP-1α in bronchoalveolar lavage fluid were found in NADPH oxidase-deficient mice following cytoxan treatment, irradiation, or allogeneic bone marrow transplantation (47). Similarly, a recent study found a significant increase in lung inflammation and alveolar damage, TLR4 expression, and NFκB activation in cigarette-smoke exposed gp91phox−/− and p47phox−/− mice compared to WT mice (48). Deficiency of gp91phox was also reported to markedly increase pulmonary cytokine and chemokine expression and neutrophil recruitment in a mouse model of pneumococcal pneumonia (49). These results might also relate to clinical observations in subjects with chronic granulomatous disease (CGD), who have a genetic deficiency of NADPH oxidase: these patients are much more prone to infection and suffer from a variety of enhanced inflammatory conditions (50,51).
The putative anti-inflammatory activity of NADPH oxidase represents an interesting and surprising function, and the underlying molecular mechanisms need to be further studied. Marriott et al. (49) suggested increased neutrophil apoptosis due to increased NOX-ROS as a possible underlying mechanism, based on their findings of increased neutrophil numbers in lung of gp91phox−/− mice 24 and 48 hours after pneumococcal infection. However, our data do not favor this hypothesis because pulmonary MPO mRNA levels in gp91phox−/− mice were already significantly increased 3 hours after LPS injection, indicating that NOX-ROS may inhibit early phase proinflammatory signals that otherwise would induce acute neutrophil activation and infiltration into lung tissue.
Interestingly, we observed that LPS-induced expression of several inflammatory mediators was significantly increased in lung and heart of gp91phox−/− mice but not p47phox−/− mice. These data are consistent with the observation that inflammatory manifestations of CGD are more common in the X-linked form (gp91phox deficiency), in which the patients’ phagocytes completely lack ROS production (50), than in the autosomal-recessive form (most often p47phox deficiency) (51), in which the cells are able to produce minute but detectable amounts of ROS (52). Therefore, it is possible that small differences in the levels of NOX-ROS have a noticeable impact on cellular function and biologic phenotype.
Finally, our results showed that genetic deficiency of gp91phox or p47phox did not protect from LPS-induced septic death. This observation is consistent with a previous report that deficiency of gp91phox did not protect mice from LPS-induced lethality (53). It is still possible that certain cells, tissues, or organs were protected, which could have been obscured by alternative mechanisms or causes of oxidative stress. However, we found no protection from LPS-induced production of inflammatory mediators and activation of NFκB and AP-1 in several organs of both gp91phox−/− and p47phox−/− mice compared to WT mice.
In conclusion, the data presented in this paper strongly suggest that NADPH oxidase-derived ROS and redox-sensitive cell signaling pathways do not promote but rather limit LPS-induced acute inflammatory responses in vivo. More research is needed to fully elucidate the underlying mechanisms.
ACKNOWLEDGEMENT
This publication was made possible by Grant Number P01 AT002034 (BF/WJZ) from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM or the National Institutes of Health.
LIST OF ABBREVIATIONS
- AP-1
activator protein-1
- bw
body weight
- CGD
chronic granulomatous disease
- ERK
extracellular receptor-activated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ICAM-1
intercellular adhesion molecule-1
- IKK
IκB kinase
- IL
interleukin
- LBP
LPS-binding protein
- LPS
lipopolysaccharide
- JNK
c-Jun kinase
- MCP-1
monocyte chemoattractant protein-1
- MIP
macrophage-inflammatory protein
- MKK
mitogen-activated protein kinase kinase
- MPO
myeloperoxidase
- NFκB
nuclear factor κB
- NOX-ROS
NADPH oxidase-derived ROS
- PMN
polymorphonuclear neutrophils
- Redox
reduction-oxidation
- ROS
reactive oxygen species
- sICAM-1
soluble intercellular adhesion molecule-1
- sVCAM-1
soluble vascular cell adhesion molecule-1
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule-1
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 2.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 4.Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med. 2002;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 5.Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: antioxidant therapy. Curr. Pharm. Des. 2005;11:3141–3158. doi: 10.2174/1381612054864894. [DOI] [PubMed] [Google Scholar]
- 6.Kolls JK. Oxidative stress in sepsis: a redox redux. J Clin Invest. 2006;116:860–863. doi: 10.1172/JCI28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 8.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 11.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 12.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 13.Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 14.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 15.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 16.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 17.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation inresponse to lip opolysaccharide in NADPH oxidase-deficient mice. Infect Immun. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 19.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 21.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappaB. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 23.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J. Clin. Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadikot RT, Zeng H, Yull FE, Li B, Cheng D-s, Kernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, Blackwell TS, Christman JW. p47phox Deficiency Impairs NF-kappa-B Activation and Host Defense in Pseudomonas Pneumonia. J. Immunol. 2004;172:1801–1808. doi: 10.4049/jimmunol.172.3.1801. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J. 2002;16:1713–1720. doi: 10.1096/fj.02-0331com. [DOI] [PubMed] [Google Scholar]
- 26.Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 2004;72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu S, Eriksson M. Oxidative injury and survival during endotoxemia. FEBS Lett. 1998;438:159–160. doi: 10.1016/s0014-5793(98)01290-3. [DOI] [PubMed] [Google Scholar]
- 29.Brandes RP, Koddenberg G, Gwinner W, Kim D, Kruse HJ, Busse R, Mugge A. Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension. 1999;33:1243–1249. doi: 10.1161/01.hyp.33.5.1243. [DOI] [PubMed] [Google Scholar]
- 30.Victor VM, Rocha M, De la Fuente M. N-acetylcysteine protects mice from lethalendotoxemia by regulating the redox state of immune cells. Free Radic Res. 2003;37:919–929. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shaul V, Lomnitski L, Nyska A, Zurovsky Y, Bergman M, Grossman S. The effect of natural antioxidants, NAO and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol Lett. 2001;123:1–10. doi: 10.1016/s0378-4274(01)00369-1. [DOI] [PubMed] [Google Scholar]
- 32.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CS, Lee DS, Song CH, An SJ, Li S, Kim JM, Kim CS, Yoo DG, Jeon BH, Yang HY, Lee TH, Lee ZW, El-Benna J, Yu DY, Jo EK. Roles of peroxiredoxin II in the regulation of proinflammatory responses to LPS and NADPH oxidase limits rather than enhances inflammation 25 protection against endotoxin-induced lethal shock. J. Exp. Med. 2007;204:583–594. doi: 10.1084/jem.20061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 36.Bowie A, O'Neill LAJ. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 37.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 38.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 39.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinauer M, Deck M, Unanue E. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J. Immunol. 1997;158:5581–5583. [PubMed] [Google Scholar]
- 42.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect. Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boczkowski J, Lisdero CL, Lanone S. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999;13:1637–1647. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- 44.Ritter C, Andrades M, Frota Junior ML, Bonatto F, Pinho RA, Polydoro M, Klamt F, Pinheiro CT, Menna-Barreto SS, Moreira JC, Dal-Pizzol F. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med. 2003;29:1782–1789. doi: 10.1007/s00134-003-1789-9. [DOI] [PubMed] [Google Scholar]
- 45.Nakai K, Kadiiska MB, Jiang JJ, Stadler K, Mason RP. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc Natl Acad Sci U S A. 2006;103:4616–4621. doi: 10.1073/pnas.0510352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faggioni R, Gatti S, Demitri MT, Delgado R, Echtenacher B, Gnocchi P, Heremans H, Ghezzi P. Role of xanthine oxidase and reactive oxygen NADPH oxidase limits rather than enhances inflammation 27 intermediates in LPS- and TNF-induced pulmonary edema. J. Lab. Clin. Med. 1994;123:394–399. [PubMed] [Google Scholar]
- 47.Yang S, Panoskaltsis-Mortari A, Shukla M, Blazar BR, Haddad IY. Exuberant inflammation in nicotinamide adenine dinucleotide phosphate-oxidase-deficient mice after allogeneic marrow transplantation. J Immunol. 2002;168:5840–5847. doi: 10.4049/jimmunol.168.11.5840. [DOI] [PubMed] [Google Scholar]
- 48.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172:1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marriott HM, Jackson LE, Wilkinson TS, Simpson AJ, Mitchell TJ, Buttle DJ, Cross SS, Ince PG, Hellewell PG, Whyte MK, Dockrell DH. Reactive oxygen species regulate neutrophil recruitment and survival in pneumococcal pneumonia. Am J Respir Crit Care Med. 2008;177:887–895. doi: 10.1164/rccm.200707-990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bylund J, MacDonald KL, Brown KL, Mydel P, Collins LV, Hancock RE, Speert DP. Enhanced inflammatory responses of chronic granulomatous disease leukocytes involve ROS-independent activation of NF-kappa B. Eur J Immunol. 2007;37:1087–1096. doi: 10.1002/eji.200636651. [DOI] [PubMed] [Google Scholar]
- 51.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Dahlgren C, Karlsson A, Bylund J. Measurement of respiratory burst products generated by professional phagocytes. Methods Mol Biol. 2007;412:349–363. doi: 10.1007/978-1-59745-467-4_23. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson SC, Grobmyer SR, Shiloh MU, Brause JE, Potter S, MacMicking JD, Dinauer MC, Nathan CF. Lethality of endotoxin in mice genetically deficient in the respiratory burst oxidase, inducible nitric oxide synthase, or both. Shock. 1999;11:253–258. doi: 10.1097/00024382-199904000-00005. [DOI] [PubMed] [Google Scholar]