Abstract
BACKGROUND
Smoking cessation has been demonstrated to reduce the rate of loss of lung function and mortality among patients with mild to moderate chronic obstructive pulmonary disease (COPD). There is a paucity of evidence about the effects of smoking cessation on the risk of COPD exacerbations.
OBJECTIVE
We sought to examine whether smoking status and the duration of abstinence from tobacco smoke is associated with a decreased risk of COPD exacerbations.
DESIGN
We assessed current smoking status and duration of smoking abstinence by self-report. Our primary outcome was either an inpatient or outpatient COPD exacerbation. We used Cox regression to estimate the risk of COPD exacerbation associated with smoking status and duration of smoking cessation.
PARTICIPANTS
We performed a cohort study of 23,971 veterans who were current and past smokers and had been seen in one of seven Department of Veterans Affairs (VA) primary care clinics throughout the US.
MEASUREMENTS AND MAIN RESULTS
In comparison to current smokers, ex-smokers had a significantly reduced risk of COPD exacerbation after adjusting for age, comorbidity, markers of COPD severity and socio-economic status (adjusted HR 0.78, 95% CI 0.75–0.87). The magnitude of the reduced risk was dependent on the duration of smoking abstinence (adjusted HR: quit <1 year, 1.04; 95% CI 0.87–1.26; 1–5 years 0.93, 95% CI 0.79–1.08; 5–10 years 0.84, 95% CI 0.70–1.00; ≥10 years 0.65, 95% CI 0.58–0.74; linear trend <0.001).
CONCLUSIONS
Smoking cessation is associated with a reduced risk of COPD exacerbations, and the described reduction is dependent upon the duration of abstinence.
KEY WORDS: chronic obstructive pulmonary disease, exacerbation, smoking cessation
INTRODUCTION
COPD exacerbation is an important cause of morbidity and a leading driver of health-care costs.1 Recently published reviews about strategies to reduce exacerbations have focused primarily on pharmacologic therapies that reduce COPD exacerbations, including inhaled corticosteroids, long-acting beta-agonists and anticholinergics, but have commented little about the benefits of smoking cessation, perhaps because of a lack of published information.2 From a public health perspective, smoking cessation is the single most effective therapy for COPD and is associated with a decrease in symptoms, reduction in prevalent symptoms3 and improved health status.4 Smoking cessation is also the only therapy that has been clearly demonstrated to improve both the rate of lung loss and survival among patients with mild to moderate COPD.5 Unlike for all pharmacologic therapies for COPD, the cost-effectiveness of smoking cessation on a population basis has been rigorously evaluated and found to be cost-effective to cost-saving over time.6–9 We examined whether smoking status and the duration of abstinence from tobacco smoke were associated with a decreased risk of COPD exacerbations.
METHODS
Setting and Subjects
We used data collected for the Ambulatory Care Quality Improvement Project (ACQUIP), a multi-center, randomized trial of a quality improvement intervention. The ACQUIP intervention had no documented effect. ACQUIP recruited patients enrolled in seven geographically diverse VA general internal medicine (GIM) clinics: Seattle, WA; West Los Angeles, CA; Birmingham, AL; Little Rock, AR; San Francisco, CA; Richmond, VA; White River Junction, VT. Patients were eligible to participate in ACQUIP if they had made at least one visit to a participating GIM clinic in the previous 12 months, had an assigned primary care provider, had a scheduled follow-up visit and had a valid mailing address. For this study, we included those patients who were enrolled in ACQUIP between December 1996 and October 1999 and who returned the health inventory checklist that included the exposure measure and 24 common health conditions. The Human Subjects Division of the University of Washington approved the ACQUIP study and analyses presented here.
Data Collection
ACQUIP collected mailed surveys to assess participant’s medical conditions. Patients who did not respond were mailed up to three additional reminders and questionnaires. As part of ACQUIP, we assessed inpatient and outpatient visits through weekly interrogations of the VA computerized medical record system. Likewise, all outpatient prescriptions received from a VA facility were obtained electronically from the electronic medical record system. Data on exposure and covariates were determined at the time of enrollment to the study, defined as the day that a patient’s health inventory was processed (index date). In addition to self-reported conditions, co-existing illnesses were collected using the electronic administrative data that included all International Classification of Diseases 9th Edition (ICD-9) diagnoses and health-care utilization from the VA (through September 2001) and Medicare (through December 1999).
Exposure and Outcome of Interest
As part of the health inventory checklist, we asked patients to affirm whether they were either current, former or never smokers. Patients who were former smokers were requested to estimate the duration of tobacco cessation, categorized as less than 1 year, 1 to 5 years, 5 to 10 years, or 10 or more years. Patients who smoked were asked to acknowledge the intensity of tobacco smoke exposure in 10-cigarette increments up to 40 or more cigarettes/day. We defined the outcome of interest as either an inpatient primary ICD-9 discharge diagnosis of COPD (491.x 492.x, 493.2 and 496.x) or an outpatient diagnosis of COPD accompanied by a prescription dispensed for either prednisone or an antibiotic used to treat outpatient respiratory infections within 2 days of the clinic visit.
Covariates
Demographic and clinical covariates, including health behaviors, were obtained from ACQUIP surveys and VA databases at baseline. Self-reported demographic and health behavior characteristics included alcohol consumption, marital status, highest level of education and employment status. We used the previously validated Seattle Index of Comorbidity (SIC) as a measure of overall medical co-morbidity.10 Higher SIC scores predict mortality and health-care utilization in the ACQUIP population. We used several markers as a proxy to COPD severity. These included first having had a previous COPD exacerbation in the 12 months prior to the index date that was defined as above for our outcome measure. A second marker of severity of COPD in the 12 months prior to the index date was the number of canisters of either albuterol and/or ipratropium bromide dispensed from VA pharmacies. Third, we assessed whether patients had received prescriptions for nebulized bronchodilators in the previous 12 months.
Statistical Analysis
We used Cox proportional-hazard models to estimate the risk and time to first COPD exacerbation associated with smoking status and duration of smoking cessation. Patients were followed until their first exacerbation, censored for death and right truncated at the end of follow-up. The proportional hazards assumption was checked graphically and by Schoenfeld residuals and was found to be valid for all models. The relationships between smoking status and duration of smoking cessation and COPD exacerbations were first examined unadjusted. Based on known risk factors for COPD exacerbations, we added the primary exposure, smoking status or duration of smoking cessation, followed by known covariates entered individually or en bloc, including age, smoking intensity, markers of COPD and COPD severity (previous COPD exacerbations in the 12 month prior to the index date, the number of canisters filled for bronchodilators, including albuterol and ipratropium bromide, and/or the having filled a prescription for a nebulized bronchodilator), the Seattle Index of Comorbidity (SIC score) and sociodemographic characteristics. All analyses were conducted with SPSS v. 13 or STATA v. 8.
RESULTS
We identified 23,971 subjects who reported either current or past tobacco consumption at baseline. The cohort had a median follow-up time of 3.87 years [interquartile range (IQR): 2.72–4.29 years] and a maximum follow-up of 4.57 years. Patient characteristics are summarized in Table 1. Consistent with VA populations, the majority of the cohort was older and had an average of SIC scores of 4.28 (±1.95). There were 8,067 individuals who reported they were current smokers and 15,904 who reported they were former smokers. Smokers had a tendency to be younger (p < 0.0001), non-Caucasian, unmarried and had fewer co-morbid conditions. Current smokers were more likely to have graduated high school, but less likely to have graduated college. In addition, individuals who had quit smoking for more than 10 years had received a lower mean number of beta-agonist and ipratropiurm bromide canisters and were less likely to have received and inhaled corticosteroid in the 12-month period prior to returning surveys. Furthermore, in the 12 months prior to the index date, there was an inverted U-shaped distribution of prior COPD exacerbations, with those who quit smoking between 0–1 years and 1–5 years having a significantly higher proportion of exacerbations than current smokers or individuals who had quit smoking for more than 10 years.
Table 1.
Characteristics of Participants Stratified by Smoking Status and Duration of Reported Abstinence
| AUDIT-C categories | ||||||
|---|---|---|---|---|---|---|
| Current smoker | Quit less than a year | Quit 1–5 years ago | Quit 6–10 years ago | Quit more than 10 years ago | p-value | |
| N | 8,067 | 1,301 | 2,321 | 2,119 | 10,163 | |
| % | 33.6 | 5.4 | 9.7 | 8.8 | 42.4 | |
| Characteristics | ||||||
| Age | ||||||
| <50 | 31.2 | 25.8 | 19.0 | 13.6 | 6.4 | <0.001 |
| 50/59 | 27.5 | 25.7 | 22.6 | 20.8 | 12.9 | |
| 60/69 | 26.3 | 30.7 | 33.3 | 34.9 | 31.9 | |
| 70/79 | 13.7 | 16.4 | 22.8 | 27.7 | 41.5 | |
| ≥80 | 1.4 | 1.4 | 2.4 | 3.1 | 7.2 | |
| Mean(SD) | 56.5(11.6) | 57.9(11.9) | 60.5(11.8) | 62.8(11.0) | 67.5(9.9) | <0.001 |
| Male | 96 | 96.5 | 96.9 | 96.7 | 98.0 | <0.001 |
| Caucasian | 71.5 | 70.2 | 73.6 | 77.1 | 82.1 | <0.001 |
| Married | 43.1 | 49.6 | 55.4 | 61.1 | 67.7 | <0.001 |
| Education | ||||||
| Less than high school | 27.5 | 24.0 | 27.8 | 26.5 | 30.7 | <0.001 |
| High school graduate | 61.8 | 62.9 | 58.8 | 60.6 | 53.0 | |
| College graduate | 10.7 | 13.1 | 13.4 | 13.1 | 16.3 | |
| SABA— or IPRA-neb | 1.65 | 3.15 | 3.4 | 2.74 | 1.72 | <0.001 |
| ICS | 6.9 | 11.6 | 11.5 | 9.6 | 7.1 | <0.001 |
| Number of SABA MDI | ||||||
| None | 79.7 | 75.3 | 77.0 | 80.7 | 86.3 | <0.001 |
| <5 | 7.4 | 8.2 | 7.2 | 6.2 | 4.9 | |
| 5–9 | 4.3 | 5.8 | 5.3 | 4 | 3.1 | |
| ≥10 | 8.7 | 10.8 | 10.5 | 9.2 | 5.7 | |
| Number of Ipra MDI | ||||||
| None | 87.4 | 83.4 | 83.3 | 87.7 | 92.4 | <0.001 |
| <5 | 4.1 | 4.5 | 4.5 | 3.0 | 2.2 | |
| 5–9 | 3.0 | 3.9 | 3.2 | 2.5 | 1.8 | |
| ≥10 | 5.5 | 8.2 | 9.0 | 6.8 | 3.7 | |
| Prior COPD exacerbations | 4.7 | 7.9 | 7.2 | 5.4 | 3.4 | <0.001 |
| Prior COPD | 34.9 | 35.4 | 35.6 | 30.3 | 23.2 | <0.001 |
| SIC (mean) | 5.4 | 3.5 | 3.8 | 3.7 | 3.7 | <0.001 |
| MI | 16.0 | 18.1 | 21.6 | 21.9 | 20.5 | <0.001 |
| Diabetes | 15.5 | 16.5 | 20.8 | 23.6 | 24.9 | <0.001 |
| Stroke | 9.9 | 9.6 | 12.4 | 10.7 | 10.7 | 0.009 |
| Cancer | 8.7 | 10.3 | 13.2 | 12.6 | 13.8 | <0.001 |
| CHF | 6.4 | 8.6 | 9.8 | 9.9 | 9.1 | <0.001 |
| Lung | 28.2 | 28.7 | 29.9 | 25.4 | 19.6 | <0.001 |
| Pneumonia | 13.5 | 14.9 | 14.7 | 14.3 | 14.5 | 0.298 |
| AUDIT-C | <0.001 | |||||
| Never | 36.2 | 39.7 | 49.7 | 47.9 | 48.9 | |
| 1–3 | 27.9 | 36.1 | 29.9 | 33.7 | 32.9 | |
| 4–5 | 13.5 | 11.7 | 10.9 | 11.2 | 11.8 | |
| 6–7 | 9.0 | 6.2 | 4.9 | 3.8 | 3.5 | |
| 8–9 | 6.1 | 3.8 | 2.8 | 1.8 | 1.8 | |
| 10+ | 7.3 | 2.5 | 1.8 | 1.7 | 1.0 | |
Smoking Status and Risk of COPD Exacerbation
During the follow-up period, there were 1,931 COPD exacerbations that occurred at a median of 277 days (median IQR: 121–553 days). Compared to current smokers, former smokers were at a significantly decreased risk of COPD exacerbation [Table 2; unadjusted hazard ratio (HR) 0.84; 95% confidence interval (CI) 0.77–0.93]. Adding the number of cigarettes smoked per day did not have a significant effect on the point estimates [adjusted HR 0.82; 95% CI (0.75–0.90)], nor did age, prior diagnoses of COPD or markers of COPD severity (use of nebulized bronchodilators, number of beta-agonist and ipratropium bromide canisters and prior COPD exacerbations; adjusted HR 0.74, 95% CI 0.67–0.82). Likewise, the addition of comorbidity and sociodemographic characteristics did not have significant effects on the overall point estimate (Table 2, adjusted HR 0.78, 95% CI 0.75–0.87).
Table 2.
Risk of COPD Inpatient or Outpatient Exacerbations
| Smoking status | Total no. | No. events | Person-years | Rate (per 1000) | Hazard ratio-unadjusted | Hazard ratio-adjusted |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Current smoker | 8,067 | 723 | 26,365 | 27.4 | Referent | Referent |
| Former smoker | 15,904 | 1,208 | 52,959 | 22.8 | 0.84 (0.77, 0.93) | 0.78 (0.75, 0.87) |
| Model 2 | ||||||
| Current smoker | 8,067 | 723 | 26,365 | 27.4 | Referent | Referent |
| Quit <1 year | 1,301 | 153 | 4,046 | 37.8 | 1.35 (1.13, 1.61) | 1.04 (0.87, 1.26) |
| Quit 1–5 years | 2,321 | 258 | 7,542 | 34.2 | 1.26 (1.09, 1.45) | 0.93 (0.79, 1.08) |
| Quit 6–10 years | 2,119 | 190 | 7,010 | 27.1 | 1.00 (0.86, 1.18) | 0.84 (0.70, 1.00) |
| Quit >10 years | 10,163 | 607 | 34,361 | 17.7 | 0.66 (0.59, 0.73) | 0.65 (0.58, 0.74) |
Duration of Smoking Cessation and Risk of COPD Exacerbation
In unadjusted models, the duration of smoking cessation was strongly associated with an inverse U-shaped distribution of risk for COPD exacerbation when compared to current smokers (Table 2: less than 1 year, HR 1.35, 95% CI 1.13–1.61; 1–5 years, HR 1.26, 95% CI 1.09–1.45; 5–10 years, HR 1.00, 0.86–1.18; ≥10 years HR 0.66, 95% CI 0.59–0.73). Similarly, adjusting for the number of cigarettes smoked per day did not have a significant effect on the point estimates (less than 1 year, HR 1.31, 95% CI 1.10–1.56; 1–5 years, HR 1.21, 95% CI 1.05–1.40; 5–10 years, HR 0.94, 95% CI 0.80–1.10; ≥10 years, HR 0.64, 95% CI 0.57–0.71). However, after adjusting for age, prior diagnoses of COPD and markers of COPD severity, there was a strong dose-response relationship between the risk of COPD exacerbation and duration of smoking cessation [adjusted HR (95% CI): less than 1 year, HR 0.97 (0.81–1.16); 1–5 years, HR 0.89 (0.77–1.03); 5–10 years, HR 0.79 (0.67–0.94); ≥10 years HR, 0.62 (0.55–0.70); linear trend p < 0.001]. Adding co-morbidity and sociodemographic characteristics to the overall model did not significantly affect the point estimates (Table 3: adjusted HR: less than 1 year, 1.04 (0.87–1.26); 1–5 years, 0.93 (0.79–1.08); 5–10 years, 0.84 (0.70–1.00); 10 or more years, 0.65 (0.58–0.74); linear trend <0.001].
Table 3.
Risk of COPD Exacerbations Associated with Previously Acknowledged COPD
| Smoking status | Total no. | No. Events | Person-years | Rate | Hazard ratio-unadjusted | Hazard ratio-adjusted |
|---|---|---|---|---|---|---|
| History of previous COPD | ||||||
| Model 1 | ||||||
| Current smoker | 2,817 | 603 | 8,099 | 74.5 | Referent | Referent |
| Former smoker | 4,292 | 1,038 | 11,950 | 86.9 | 1.16 (1.05, 1.29) | 0.84 (0.75, 0.95) |
| Model 2 | ||||||
| Current smoker | 2,817 | 603 | 8,099 | 74.5 | Referent | Referent |
| Quit <1 year | 460 | 137 | 1,184 | 115.7 | 1.50 (1.25, 1.81) | 1.07 (0.87, 1.30) |
| Quit 1–5 years | 827 | 227 | 2,254 | 100.7 | 1.34 (1.15, 1.56) | 0.94 (0.80, 1.11) |
| Quit 6–10 years | 643 | 168 | 1,779 | 94.4 | 1.27 (1.07, 1.51) | 0.92 (0.76, 1.11) |
| Quit >10 years | 2,362 | 506 | 6,733 | 75.2 | 1.01 (0.90, 1.14) | 0.72 (0.63, 0.83) |
| No previous history of COPD | ||||||
| Model 1 | ||||||
| Current smoker | 5,250 | 120 | 18,266 | 6.6 | Referent | Referent |
| Former smoker | 11,612 | 170 | 41,009 | 4.1 | 0.64 (0.51, 0.81) | 0.55 (0.41, 0.74) |
| Model 2 | ||||||
| Current smoker | 5,250 | 120 | 18,266 | 6.6 | Referent | Referent |
| Quit <1 year | 841 | 16 | 2,862 | 5.6 | 0.84 (0.50, 1.42) | 0.95 (0.54, 1.66) |
| Quit 1–5 years | 1,494 | 31 | 5,288 | 5.9 | 0.90 (0.61, 1.34) | 0.86 (0.56, 1.32) |
| Quit 6–10 years | 1,476 | 22 | 5,231 | 4.2 | 0.65 (0.41, 1.02) | 0.53 (0.32, 0.89) |
| Quit >10 years | 7,801 | 101 | 27,629 | 3.7 | 0.56 (0.43, 0.74) | 0.44 (0.31, 0.61) |
Risk of COPD Exacerbations Stratified by Previous COPD Diagnoses
In comparison to current smokers among the 7,109 individuals who had reported a physician diagnosis of COPD, in unadjusted models, there was a 16% increased risk of exacerbations associated with former smokers (HR 1.16, 95% CI 1.05–1.29); however, after adjustment, there was a 16% decreased risk of exacerbation associated with reporting being a former smoker (Table 3: adjusted HR 0.84, 95% CI 0.75–0.95). Similarly, in unadjusted analyses among individuals who had self-reported a physician diagnosis of COPD, there was a strong increased risk of COPD exacerbation when compared to current smokers. However, similar to analyses described above, the longer the duration of smoking cessation, the less the overall risk of exacerbation (less than 1 year, HR 1.50, 95% CI 1.25–1.81; 1–5 years, HR 1.34, 95% CI 1.15–1.56; 5–10 years, HR 1.27, 95% CI 1.07–1.51; ≥10 years, HR 1.01, 95% CI 0.90–1.14). After adjustment, there was a strong dose-response reduction in the risk of COPD exacerbation associated with duration of smoking duration (less than 1 year, HR 1.07, 95% CI 0.87–1.30; 1–5 years, HR 0.94, 95% CI 0.80–1.11; 6–10 years, HR 0.92, 95% CI 0.76–1.11; ≥10 years, HR 0.72, 95% CI 0.63–0.83; linear trend p < 0.001).
Among those without previously acknowledged COPD, the overall risk of COPD exacerbations among former smokers was approximately 40% less than current smokers (HR 0.64, 95% CI 0.51–0.81), and this association persisted after adjustment (HR 0.55, 95% CI 0.41–0.74). Likewise, the duration of smoking cessation was also strongly associated with a decreased risk of exacerbation in both unadjusted and adjusted models (Table 3).
Sensitivity Analyses to Those Treated for COPD with Ipratropium Bromide
We restricted the cohort to those 1,767 individuals who had a self-reported diagnosis of COPD, an ICD-9 diagnosis of COPD and received at least one prescription of ipratropium bromide. Of these individuals, there were 904 COPD exacerbations with a median time to event of 207.9 days (IQR 73–434 days). As with previous analyses, in unadjusted analyses, there was an increased risk of exacerbation associated with the report of being a former smoker (unadjusted HR 1.29, 95% CI 1.12–1.48). However, unlike previous analyses, after adjustment for age, number of cigarettes smoked per day, markers of COPD severity, comorbidity and sociodemographic characteristics, the overall point estimate remained modestly elevated, although not statistically significant (adjusted HR 1.13, 95% CI 0.96–1.34). In these analyses, there was also no overall reduction in risk associated with the duration of smoking cessation in both unadjusted (less than 1 year, HR 1.41, 95% CI 1.11–1.80; 1–5 years, HR 1.17, 95% CI 0.96–1.43; 5–10 years, HR 1.26, 95% CI 1.00–1.58; ≥10 years, HR 1.33, 95% CI 1.13–1.57) and adjusted analyses (less than 1 year, HR 1.18, 95% CI 0.90–1.53; 1–5 years, HR 1.09, 95% CI 0.87–1.36; 5–10 years, HR 1.16, 95% CI 0.87–1.50; ≥10 years HR 1.13, 95% CI 0.93–1.39) (Fig. 1).
Figure 1.
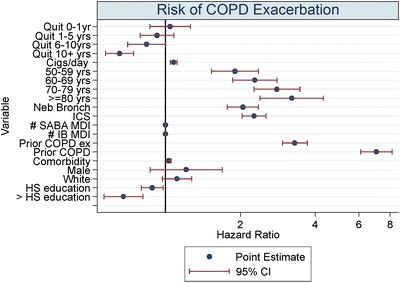
Risk of COPD exacerbation.
Discussion
Smoking tobacco causes up to 90% of prevalent COPD in the United States and continues to be a public health concern, with the current US prevalence of smoking at 21%.11,12 Despite recent improvements in the rate of smoking in the US, the rate of decline has slowed notably.12 Moreover, the worldwide prevalence of smoking remains high, particularly in countries with developing economies.13–15 Among patients with COPD, smoking cessation is the only intervention that modifies the rate of lung loss16 and only one of two therapies that have been demonstrated to reduce mortality.5,17 Unlike all other therapies that can be offered among patients with COPD, the cost-effectiveness of smoking cessation programs is unquestioned despite having relatively low proportions of successful quitters.5,16 In addition to proven benefits of smoking cessation on the rate of lung function decline, the Lung Health Study demonstrated that the smoking cessation intervention resulted in the development of fewer symptoms, including dyspnea, cough, sputum production and wheezing.3 We demonstrated that relative to current smokers, ex-smokers had a significant reduction in their risk of COPD exacerbation and that this effect was dependent on the duration of remaining tobacco free. This effect was apparent among those with and without prior recognition of COPD. In the context of the recent attention paid to pharmacological treatments to reduce exacerbation and improve symptoms, a greater emphasis is needed to address smoking cessation.
Our results have a potential basis in the known biology of tobacco smoke and COPD exacerbation risk. Tobacco smoke is a potent stimulant of the inflammatory response, and chronic inflammation has been suggested to contribute to COPD pathogenesis.18 The inflammatory response to tobacco smoke appears to be greater among people who are susceptible to developing COPD.19,20 Inflammation has been suggested as an important factor that may predispose individuals to increased risk of exacerbations. Recent data suggest that higher CRP concentrations, a general marker of inflammation, were associated with an increased risk of COPD exacerbations and mortality.21 In addition, similar studies have observed that markers of the host response to tobacco smoke may be important predictors of COPD exacerbations and symptoms.22–24 Inhaled corticosteroids that modify these markers of inflammation have been demonstrated to reduce the risk of COPD exacerbation.25 Interestingly, among patients with severe COPD, inflammation has been demonstrated to persist years after smoking cessation,26,27 and there are few data about whether modification in the rate of airflow obstruction is similar to those individuals with mild to moderate disease.
In our unadjusted models, we observed an increased risk of exacerbation associated with individuals who had reported stopping smoking for up to 5 years. This unadjusted observation fits with previous studies and may be consistent with the hypothesis of improved ciliary function leading to increased sputum production, clearance and symptoms.28–31 However, there is also the possibility of an indication bias leading to this finding in that individuals who have smoked for long periods of time often have some factors predisposing to future exacerbations, such as increasing dyspnea or prior exacerbations. Previous COPD exacerbation is a strong predictor of future exacerbations and may account for some of the unadjusted findings.32 This is further supported by the fact that after adding markers of COPD severity, including previous COPD exacerbations, we no longer find an increased risk of exacerbation associated with smoking cessation.
The decreased risk of exacerbation was greatest among individuals who did not have previously acknowledged COPD. There are a number of potential explanations for this finding. The effects of smoking cessation on the rate of lung function decline, and symptoms are only known for patients with mild to moderate disease.16 Because most patients with COPD present with symptoms relatively late in the disease course, it is not known whether smoking cessation would modify the rate of lung destruction and residual inflammation associated with severe disease. Because information provided about lung function and other health conditions may not facilitate quitting tobacco,33 our data provide additional support for recommending smoking cessation in all individuals regardless of the presence or absence of disease.
There are a number of explanations for our findings that do not necessarily imply a biological rationale. Smoking cessation may be accompanied by other healthy behaviors, such as improved adherence to medical therapy or curtailing heavy alcohol use that may reduce the risk of exacerbation. This would not however diminish our findings in that any positive accompanying health behavior would likely be welcome. In addition, the duration of smoking cessation may lead providers to attribute presentations for exacerbations to diagnoses other than COPD. In addition, individuals who stopped smoking earlier in their disease course may have less severe disease and therefore be more likely to benefit from the effects of smoking cessation. There remains an important gap in our current knowledge in that all of the randomized evidence to support the effect between smoking cessation on the rate of lung function decline and symptoms is based on those individuals with mild to moderate disease. As to whether smoking cessation confers the same benefits among severely impaired individuals remains unknown. Furthermore, our sensitivity analyses were concerning in that individuals who were most likely to have moderate to severe disease did not have a decreased risk of exacerbations associated with duration of smoking cessation. Our study, however, cannot address this particular concern.
This study had several strengths. First, we studied patients from multiple centers, minimizing the chance that the patterns of diagnosis or treatment by any single physician or group of clinicians exerted undue influence on our results. Second, the cohort was drawn from a complete primary care clinic population, reducing the likelihood of selection bias, such as is often found in randomized trials. Third, we had excellent reporting of smoking status and the ability to follow patients continuously in the VA system.
Still, this study had some potential limitations. First, some degree of ascertainment bias is likely present, as we were unable to assess clinic visits and hospital admissions to non-VA facilities. Second, we recognize that our definition of COPD did not include spirometric assessment and thereby allows for both potential misclassification of outcome and cohort definitions. However, we were interested in an entire population of clinic patients because many individuals are unaware that they may have COPD. Third, our assessment of exposure was performed only once. This may have led to under-reporting of tobacco consumption because of social desirability.34 In addition, some patients may have stopped or re-started smoking in the interval follow-up period, and previous studies have shown that the duration of abstinence is associated with the risk of recidivism, although after 6–12 months, the rate of permanent smoking abstinence plateaus.35 The early relapse rate may in part explain the effects noted in those who had quit for less than 1 year. Finally, we did not have any biological confirmation of smoking status, such as with cotinine or carbon monoxide measurements.
COPD exacerbations are an important cause of morbidity among patients with COPD. Recent strategies have focused on ways to decrease the rate of COPD exacerbations, primarily through pharmacological therapy or interventions. From a public health perspective, smoking cessation is an effective and cost-saving strategy, yet there are many payers of health care who do not pay for or only pay for very limited smoking cessation programs.36,37 Access to smoking cessation programs is relatively easy, with a number of quit lines and other telephone-based smoking cessation programs that do not necessarily require clinician referrals. Our data suggest that smoking cessation reduces the risk of COPD exacerbations. Greater efforts are needed to promote and facilitate access to smoking cessation programs and to understand the biologic changes that occur after successful smoking cessation.
Acknowledgments
David Au had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was funded by the Department of Veterans Affairs: IAC 05–206 and IIR-99-376. Dr. Bryson is funded by a VA Career Development Award (RCS 03-177).
Conflict of Interest None disclosed.
Footnotes
The views expressed in the manuscript reflect those of the authors and not necessarily those of the Department of Veterans Affairs.
References
- 1.Data Fact Sheet: Chronic Obstructive Pulmonary Disease, National Heart Blood and Lung Institiute. NIH Publication Number 2003;30–5229.
- 2.Calverley PM. Reducing the frequency and severity of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:121–4. [DOI] [PubMed]
- 3.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999;106:410–6. [DOI] [PubMed]
- 4.Bolliger CT, Zellweger JP, Danielsson T, et al. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine Tob Res. 2002;4:433–9. [DOI] [PubMed]
- 5.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9. [DOI] [PubMed]
- 6.Faulkner MA, Lenz TL, Stading JA. Cost-effectiveness of smoking cessation and the implications for COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:279–87. [DOI] [PMC free article] [PubMed]
- 7.Hoogendoorn M, Welsing P, Rutten-van Molken MP. Cost-effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Curr Med Res Opin. 2008;24:51–61. [DOI] [PubMed]
- 8.Johansson PM, Tillgren PE, Guldbrandsson KA, Lindholm LA. A model for cost-effectiveness analyses of smoking cessation interventions applied to a Quit-and-Win contest for mothers of small children. Scand J Public Health. 2005;33:343–52. [DOI] [PubMed]
- 9.Tsevat J. Impact and cost-effectiveness of smoking interventions. Am J Med. 1992;93:43S–7S. [DOI] [PubMed]
- 10.Fan VS, Au D, Heagerty P, Deyo RA, McDonell MB, Fihn SD. Validation of case-mix measures derived from self-reports of diagnoses and health. J Clin Epidemiol. 2002;55:371–80. [DOI] [PubMed]
- 11.The Health Consequences of Smoking: a Report of the Surgeon General. Washington DC: Department of Health and Human Services, Centers for Disease Control and Prevention. 2004;42–61. [PubMed]
- 12.Cigarette Smoking Among Adults-United States. 2003 doi:10.1001/jama.294.2.172. JAMA 2005;294:172–3. [PubMed]
- 13.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–53. [DOI] [PubMed]
- 14.Ait-Khaled N, Enarson D, Bousquet J. Chronic respiratory diseases in developing countries: the burden and strategies for prevention and management. Bull World Health Organ. 2001;79:971–9. [PMC free article] [PubMed]
- 15.Fagerstrom K. The epidemiology of smoking: health consequences and benefits of cessation. Drugs. 2002;62(Suppl 2):1–9. [DOI] [PubMed]
- 16.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. Jama. 1994;272:1497–505. [DOI] [PubMed]
- 17.Anthonisen NR. Long-term oxygen therapy. Ann Intern Med. 1983;99:519–27. [DOI] [PubMed]
- 18.American Thoracic Society/European Respiratory Society Task Force. Standards for the Diagnosis and Management of Patients with COPD [Internet]. New York: American Thoracic Society.
- 19.Turato G, Zuin R, Miniati M, et al. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166:105–10. [DOI] [PubMed]
- 20.Saetta M, Di Stefano A, Turato G, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–6. [DOI] [PubMed]
- 21.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. [DOI] [PubMed]
- 22.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–20. [DOI] [PMC free article] [PubMed]
- 23.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128:1995–2004. [DOI] [PMC free article] [PubMed]
- 24.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. [DOI] [PMC free article] [PubMed]
- 25.Wilt TJ, Niewoehner D, MacDonald R, Kane RL. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639–53. [DOI] [PubMed]
- 26.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. [DOI] [PubMed]
- 27.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–45. [DOI] [PubMed]
- 28.Cummings KM, Giovino G, Jaen CR, Emrich LJ. Reports of smoking withdrawal symptoms over a 21 day period of abstinence. Addict Behav. 1985;10:373–81. [DOI] [PubMed]
- 29.Bluman LG, Mosca L, Newman N, Simon DG. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113:883–9. [DOI] [PubMed]
- 30.Yamashita S, Yamaguchi H, Sakaguchi M, Yamamoto S, Aoki K, Shiga Y, Hisajima Y. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760–6. [DOI] [PubMed]
- 31.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Cremoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–4. [DOI] [PubMed]
- 32.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131:20–8. [DOI] [PubMed]
- 33.Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res. 2007;9:21–32. [DOI] [PubMed]
- 34.Boyd NR, Windsor RA, Perkins LL, Lowe JB. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern Child Health J. 1998;2:77–83. [DOI] [PubMed]
- 35.Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-xanalysis. Tob Control. 2006;15:280–5. [DOI] [PMC free article] [PubMed]
- 36.Stxate Medicaid coverage for tobacco-dependence treatments-United States. 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1194–7. [PubMed]
- 37.Curry SJ, Keller PA, Orleans CT, Fiore MC. The Role of Health Care Systems in Increased Tobacco Cessation. Annu Rev Public Health 2008. [DOI] [PubMed]


