Abstract
Patients with diastolic heart failure (HF) i.e. clinical HF with normal or near normal left ventricular ejection fraction (LVEF) may experience unstable angina pectoris (UAP) due to epicardial atherosclerotic coronary artery disease (CAD) and/or to subendocardial ischemia, even in the absence of CAD. However, the risk of UAP among ambulatory diastolic HF patients has not been well studied. We examined incident hospitalizations due to UAP among 916 diastolic HF (LVEF >45%) patients without significant valvular heart disease and 6800 systolic HF (LVEF ≤45%) patients in the Digitalis Investigation Group trial. During a 38-month median follow-up, 12% (797/6,800) of systolic HF patients (incidence rate, 435/10,000 person-years) and 15% (138/916) of diastolic HF patients (incidence rate, 536/10,000 person-years) were hospitalized for UAP (adjusted hazard ratio for diastolic HF, 1.22; 95% confidence interval, 1.02–1.47; p=0.032). There was a graded increase in incident hospital admissions for UAP with increasing LVEF. Hospitalizations for UAP occurred in 11% (520/4,808; incidence rate, 407/10,000 person-years), 14% (355/2556; incidence rate, 496/10,000 person-years) and 17% (60/352; incidence rate, 613/10,000 person-years) of HF patients, respectively, with LVEF <35%, 35–55%, and >55%. Compared with HF patients with LVEF <35%, the adjusted hazard ratios (95% confidence intervals) for UAP hospitalization in those with LVEF 35–55% and >55% were respectively 1.17 (1.02–1.34; p=0.028) and 1.57 (1.20–2.07; p=0.026). In conclusion, in ambulatory chronic HF patients, higher LVEF was associated with increased risk of hospitalizations due to UAP. As in patients with systolic HF, those with diastolic HF should be routinely evaluated for myocardial ischemia and managed accordingly.
Keywords: heart failure, diastolic, systolic, UAP, hospitalization
Diastolic heart failure (HF) is common and often associated with hypertensive heart disease and left ventricular (LV) hypertrophy, which may lead to subendocardial ischemia and unstable angina pectoris (UAP), even in the absence of atherosclerotic coronary artery disease (CAD).1–7 In addition, among diastolic HF patients with CAD, myocardium is likely to be viable rather than infarcted. Systolic HF patients, on the other hand, may be likely to have less viable myocardium due to prior myocardial infarction and may therefore be at lower risk for UAP.5,8,9 These observations suggest that the incidence of UAP may be increased in diastolic HF. However, the risk of hospitalizations due to UAP in ambulatory patients with chronic diastolic HF is unknown. The objective of this study, therefore, was to determine the incidence of hospitalization due to UAP in patients with diastolic HF compared to those with systolic HF.
Methods
Study design and patients
This is a post-hoc retrospective analysis of the Digitalis Investigation Group (DIG) trial.10,11 Of 7788 participants in the DIG trial, 6800 had systolic HF (LVEF ≤45%) and 988 had diastolic HF (LVEF >45%). Of the 988 diastolic HF patients, 72 had valvular heart disease as the primary etiology of their HF and were excluded from this analysis. Most patients were receiving angiotensin-converting enzyme inhibitors and diuretics. Data on beta-blocker use were not collected. However, many patients had prior myocardial infarction11 and may have been receiving beta blockers for this indication.12,13
Assessment of left ventricular ejection fraction
LV ejection fraction (LVEF) was measured upon enrollment into the DIG trial. An LVEF obtained during the 6 months prior to randomization was accepted if the patient remained stable during that period.14 LVEF was assessed using two-dimensional echocardiography, radionuclide ventriculography or contrast left ventriculography, without core laboratory adjudication. When more than one technique was used to measure LVEF, results of angiographic or radionuclide measurements were given priority over those from echocardiography.
Outcomes
Hospitalization due to UAP was a pre-specified secondary outcome in the DIG trial and was the primary outcome for this analysis. The diagnoses leading to hospitalizations were classified by DIG investigators but were not centrally adjudicated. Vital status was collected up to December 31, 1995 and was ascertained for 99% of the patients.
Statistical analysis
We calculated incidence rates for UAP hospitalization for patients with systolic and diastolic HF, and used Kaplan-Meier and bivariate and multivariable Cox regression analyses to estimate the association of diastolic HF with hospitalization due to UAP. To test if there was a graded relationship between LVEF and UAP hospitalization, we categorized patients into three LVEF groups: <35%, 35–55% and >55% and repeated the above analyses. We also repeated our analysis using LVEF as a continuous variable. To assess for heterogeneity in the association between LVEF and UAP hospitalization, we conducted subgroup analyses using multivariable Cox regression and tested for first-order interactions. All statistical tests were evaluated using a two-tailed 95% confidence level, and a p value <0.05 was required to reject the null hypothesis. All data analyses were performed using SPSS version 14.15
Results
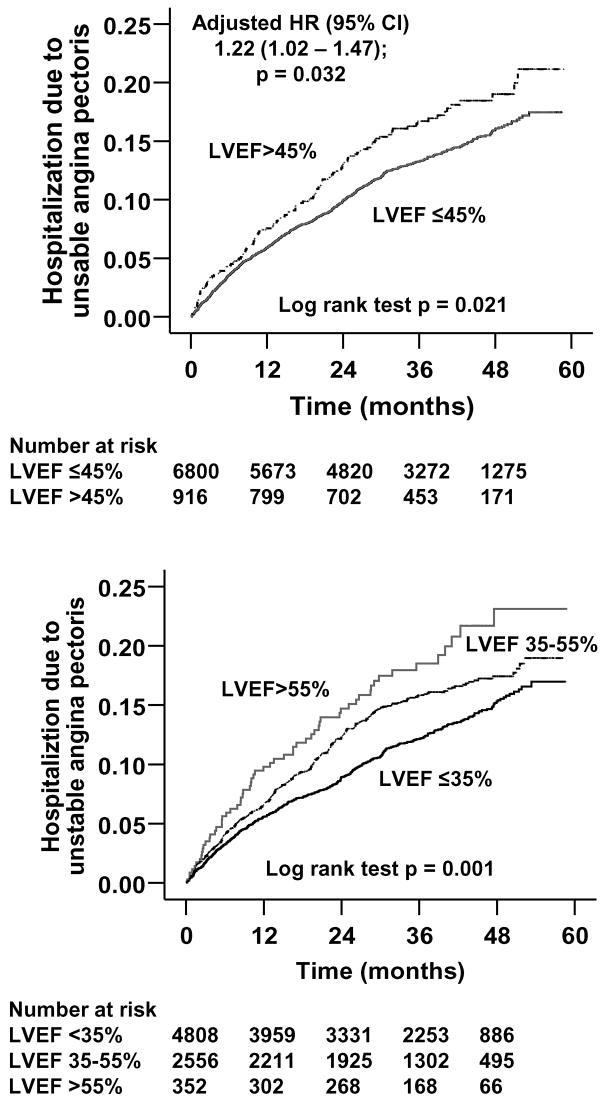
Baseline patient characteristics are displayed in Table 1. Patients with diastolic HF were older, more likely to be women, and to have hypertensive heart disease. Kaplan-Meier plots for time to first UAP hospitalization are shown in Figure 1. During a median follow-up of 38 months, UAP hospitalizations occurred in 12% (797/6,800) of systolic HF patients (incidence rate, 435/10,000 person-years) and 15% (138/916) of diastolic HF patients (incidence rate, 536/10,000 person-years). Adjusted hazard ratio for UAP hospitalization for diastolic HF, when compared with systolic HF was 1.22 (95% confidence interval, 1.02–1.47; p=0.032; Table 2).
Table 1.
Baseline patient characteristics
| Variables | Left ventricular ejection fraction
|
P | |
|---|---|---|---|
| ≤45% (n=6800) | >45% (n=916) | ||
| Age (years) | 63.4 (±10.9) | 66.6 (±10.2) | <0.0001 |
| Female | 1519 (22.3%) | 367 (40.1%) | <0.0001 |
| Non-white | 991 (14.6%) | 130 (14.2%) | 0.803 |
| Duration of heart failure (months) | 30.2 (±36.8) | 26.1 (±33.2) | 0.005 |
| Etiology of heart failure | |||
| Coronary ischemic | 4803 (70.6%) | 557 (60.8%) | |
| Hypertensive | 583 (8.6%) | 222 (24.2%) | <0.0001 |
| Others | 1414 (20.8%) | 137 (15.0%) | |
| Prior myocardial infarction | 4419 (65.0%) | 480 (52.4%) | <0.0001 |
| Current angina pectoris | 1821 (26.8%) | 280 (13.3%) | 0.049 |
| Hypertension | 3084 (45.4%) | 561 (61.2%) | <0.0001 |
| Diabetes mellitus | 1933 (28.4%) | 281 (30.7%) | 0.161 |
| Chronic kidney disease* | 3042 (44.7%) | 441 (48.1%) | 0.052 |
| New York Heart Association functional class | |||
| I | 907 (13.3%) | 176 (19.5%) | |
| II | 3670 (54.0%) | 532 (58.1%) | <0.0001 |
| III–IV | 2223 (32.7%) | 205 (22.4%) | |
| Laboratory findings at randomization | |||
| Serum creatinine (mg/dL) | 1.28 (±0.37) | 1.26 (±0.39) | 0.002 |
| Pulmonary congestion (current) | 1008 (14.8%) | 95 (10.4%) | <0.0001 |
| Cardiothoracic ratio >0.5 | 4194 (61.7%) | 450 (49.1%) | <0.0001 |
| Ejection fraction (%) | 28.5 (±8.8) | 55.1 (±7.9) | <0.0001 |
| Medications at randomization | |||
| Pre-trial digoxin use | 3017 (44.4%) | 312 (34.1%) | <0.0001 |
| Digoxin by randomization | 3397 (50.0%) | 461 (50.3%) | 0.833 |
| Angiotensin-converting enzyme inhibitors | 6422 (94.4%) | 795 (86.8%) | <0.0001 |
| Non-potassium sparing diuretics | 5325 (78.3%) | 691 (75.4%) | 0.051 |
| Nitrates | 2898 (42.6%) | 374 (40.8%) | 0.319 |
Chronic kidney disease was defined as glomerular filtration rate <60 ml/1.73 m2 body surface area
Figure 1.
Kaplan-Meier plots demonstrating cumulative risk of hospitalizations due to unstable angina pectoris LVEF = left ventricular ejection fraction
Table 2.
Hospitalization due to unstable angina pectoris (UAP) by left ventricular ejection fraction (LVEF)
| Number of events | Total follow up in years | Rate
|
Rate difference
|
Hazard ratio (95% confidence interval)
|
||
|---|---|---|---|---|---|---|
| Per 10000 person-year of follow up | Unadjusted | Adjusted* | ||||
| LVEF ≤45% (N=6800) | 797 | 18331 | 435 | Reference | Reference | Reference |
| LVEF >45% (N=916) | 138 | 2573 | 536 | +101 | 1.24 (1.03 – 1.48); p=0.022 | 1.22 (1.02 – 1.47); p=0.032 |
| LVEF <35% (N=4808) | 520 | 12766 | 407 | Reference | Reference | Reference |
| LVEF 35–55% N=(2556 | 355 | 7159 | 496 | +89 | (1.07 – 1.40); p=0.003 | (1.02 – 1.34); p=0.028 |
| LVEF >55% (N=352) | 60 | 979 | 613 | +206 | 1.51 (1.16 – 1.97); p=0.003 | 1.57 (1.20 – 2.07); p=0.026 |
| LVEF as a continuous variable (N=7788) | --- | --- | --- | --- | 1.009 (1.004 – 1.014); p<0.0001 | 1.008 (1.002 – 1.013); p=0.005 |
Adjusted for age, female sex, non-white race, body mass index, duration and etiology of heart failure, past myocardial infarction, current angina, hypertension, diabetes, pre-trial use of digoxin, digoxin use during trial, use of angiotensin-converting enzyme inhibitors, combined use of hydralazine and nitrates, use of non-potassium sparing diuretics, potassium-sparing diuretics, dyspnea at rest, dyspnea on exertion, activity limitation, New York Heart Association class, elevated jugular venous pressure, third heart sound, pulmonary râles, lower extremity edema, presence of 6 or more symptoms or signs, heart rate, blood pressure (systolic and diastolic), serum creatinine and potassium levels, pulmonary congestion and cardiothoracic ratio >0.5 by chest x-ray.
There was a graded increase in hospital admissions due to UAP with increasing LVEF. UAP hospitalizations occurred in 11% (520/4,808), 14% (355/2556) and 17% (60/352) of patients respectively with LVEF <35%, 35–55%, and >55% (Table 2). Incidence rates per 10,000 person-years of follow up were 407, 496, and 613 hospital admissions due to UAP, respectively, in HF patients with LVEF <35%, 35–55%, and >55% (Table 2). Compared to patients with LVEF <35%, the adjusted hazard ratios (95% confidence intervals) for UAP hospitalization for those with LVEF 35–55% and >55% were respectively 1.17 (1.02–1.34; p=0.028) and 1.57 (1.20–2.07; p=0.026). Each percent increase in LVEF was associated with a significant 0.8% increase in the risk of hospitalization for UAP (Table 2).
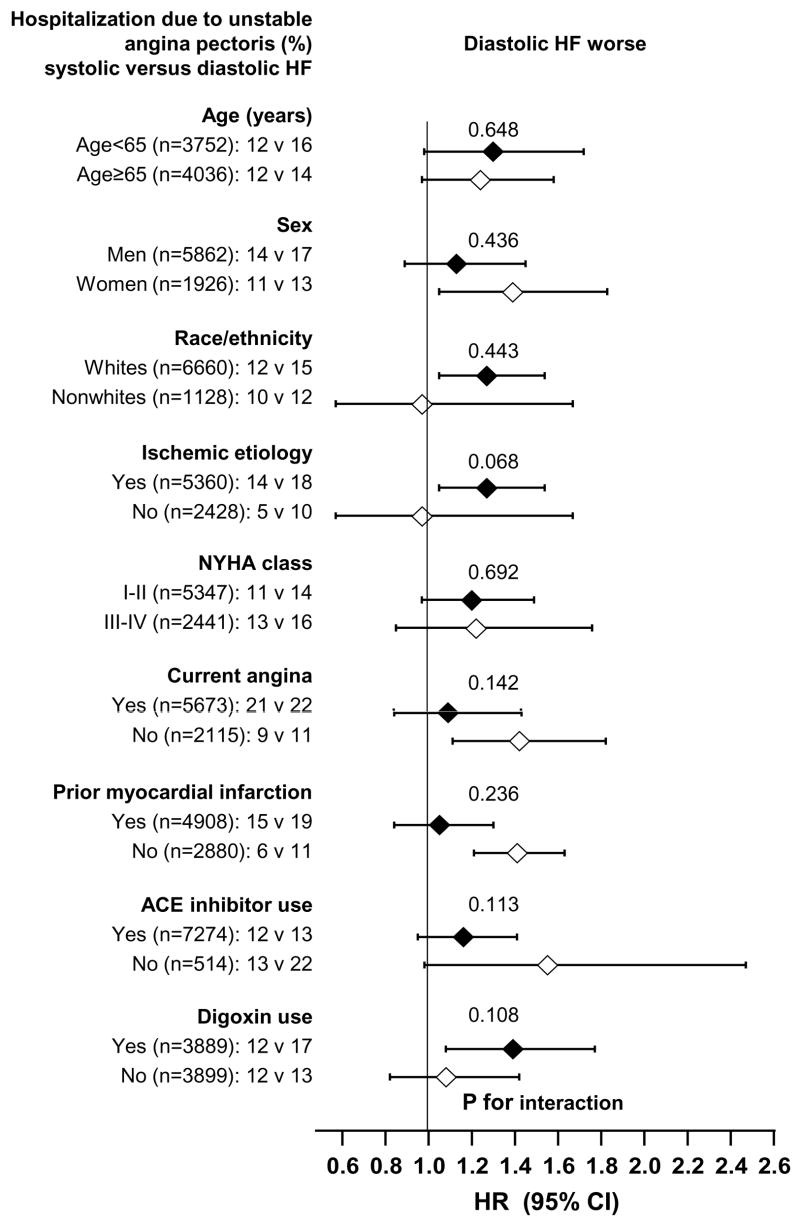
Associations between other baseline patient characteristics and hospitalization due to UAP are displayed in Table 3. The associations of diastolic HF and hospitalization due to UAP in various subgroups of patients are displayed in Figure 2. There were no significant interactions between LVEF and any of these subgroups.
Table 3.
Other predictors of hospitalization due to unstable angina pectoris
| Variables | Hazard ratio (95% confidence interval)
|
|
|---|---|---|
| Unadjusted | Adjusted* | |
| Women | 1.34 (1.17 – 1.54); p<0.0001 | 1.40 (1.22 – 1.62); p<0.0001 |
| Coronary ischemic etiology | 2.57 (2.15– 3.07); p<0.0001 | 1.71 (1.35 – 2.16); p<0.0001 |
| Prior myocardial infarction | 2.23 (1.90 – 2.61); p<0.0001 | 1.49 (1.21 – 1.83); p<0.0001 |
| Current angina | 2.60 (2.29 – 2.96); p<0.0001 | 2.08 (1.82 – 2.37); p<0.0001 |
| Diabetes | 1.46 (1.28 – 1.67); p<0.0001 | 1.32 (1.15 – 1.51); p<0.0001 |
| Prior digoxin use | 0.77 (0.68 – 0.88); p<0.0001 | 0.79 (0.69 – 0.91); p=0.001 |
| Current dyspnea at rest | 1.39 (1.20 – 1.61); p<0.0001 | 1.26 (1.09 – 1.47); p=0.002 |
Adjusted for the same covariates as in Table 2
Figure 2.
Hazard ratios (HR) and 95% confidence intervals (CI) for subgroups of patients with diastolic versus systolic heart failure (HF) ACE=angiotensin-converting enzyme; NYHA=New York Heart Association
Discussion
Our data indicate that over half of ambulatory patients with chronic mild to moderate diastolic HF enrolled in the DIG trial had CAD, and that compared with systolic HF patients, those with diastolic HF were at increased risk for hospitalization due to UAP. In addition, female sex, CAD, prior myocardial infarction, current angina, and diabetes were associated with increased UAP hospitalizations. These findings are important because diastolic HF patients may not be routinely evaluated and treated for myocardial ischemia. Furthermore, the prevalence of diastolic HF is expected to increase over the next several decades. Our data suggest that this trend could also lead to an increase in hospitalizations for UAP.
A possible explanation for the increased risk of UAP in diastolic HF patients is that these patients may be more susceptible to myocardial ischemia, in particular subendocardial ischemia.16–19 Diastolic HF is often associated with concentric LV hypertrophy, which may in turn be associated with relatively inadequate growth of the coronary arteries, reduction in coronary flow reserve, and increases in coronary medial thickness and perivascular fibrosis.20 The ensuing decreased capillary density and increased capillary to myocyte oxygen diffusion distance make hypertrophied myocardium more susceptible to ischemia, even in the absence of epicardial coronary atherosclerosis or stenosis.3
CAD, prior myocardial infarction and baseline angina were significantly associated with increased risk of incident UAP (Table 3). Diastolic HF patients with myocardial ischemia, with or without CAD, may have more viable myocardium than those with systolic HF, thus predisposing them to an increased risk for ischemia and UAP. A recent report by Nijland et al supports this possibility.21 In that study, HF patients with prior myocardial infarction who had viable myocardium in the infarct zone experienced more subsequent hospitalizations for UAP than those without viable myocardium (20% vs. 5%, p=0.006). Moreover, myocardial viability was the only predictor of UAP.
Our study has several potential limitations. UAP hospitalizations were not centrally adjudicated. Because beta-blocker use in HF patients has evolved considerably since the DIG trial was completed, the results of our analysis may not be generalizable to contemporary HF patients. However, the likely lower use of beta-blockers by DIG participants, in retrospect, has allowed us to study the association of LVEF with incident UAP in the natural history of HF. Greater use of beta-blockers in today’s systolic HF patients might further reduce UAP hospitalizations in this group, resulting in an even wider gap in UAP admissions between systolic and diastolic HF patients. Since CAD was more prevalent in systolic HF patients in the DIG trial, it is possible that more systolic HF patients were receiving beta-blockers, thus accounting for their lower incidence of UAP. However, at the time of the DIG trial, use of beta-blockers in systolic HF patients was low.22–25 Therefore, our results are unlikely to be explained entirely by a differential use of beta-blockers in systolic and diastolic HF patients. Finally, HF patients in this study were relatively young, predominantly men, and had normal sinus rhythm. The applicability of these findings to elderly HF patients, particularly older women and patients with atrial fibrillation is uncertain.
Over half of all community-dwelling HF patients have normal or near normal LVEF.4,7,26 Although these patients are often considered less likely to have CAD than those with systolic HF, the true burden of CAD in this population may be underestimated. The findings of the current analysis indicate that diastolic HF patients are at greater risk for hospitalization for UAP than those with systolic HF, and suggest that treatment of CAD in diastolic HF patients could result in fewer hospitalizations for UAP. Prospective studies are needed to test this hypothesis.
Acknowledgments
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100). Dr. Dell’Italia was supported by Specialized Center for Clinically Oriented Research (SCCOR) in Cardiac Dysfunction Grant P50HL077100 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland, and a grant from the Department of Veterans Affairs, Washington, DC
Footnotes
Conflict of Interest: None
References
- 1.Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986;74:964–972. doi: 10.1161/01.cir.74.5.964. [DOI] [PubMed] [Google Scholar]
- 2.Brush JE, Jr, Cannon RO, 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319:1302–1307. doi: 10.1056/NEJM198811173192002. [DOI] [PubMed] [Google Scholar]
- 3.Iriarte M, Caso R, Murga N, Faus JM, Sagastagoitia D, Molinero E, Lopez de Argumedo M, Boveda J. Microvascular angina pectoris in hypertensive patients with left ventricular hypertrophy and diagnostic value of exercise thallium-201 scintigraphy. Am J Cardiol. 1995;75:335–339. doi: 10.1016/s0002-9149(99)80549-9. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 5.Auerbach MA, Schoder H, Hoh C, Gambhir SS, Yaghoubi S, Sayre JW, Silverman D, Phelps ME, Schelbert HR, Czernin J. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation. 1999;99:2921–2926. doi: 10.1161/01.cir.99.22.2921. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Roseman JM, Duxbury AS, Allman RM, DeLong JF. Correlates and outcomes of preserved left ventricular systolic function among older adults hospitalized with heart failure. Am Heart J. 2002;144:365–372. doi: 10.1067/mhj.2002.124058. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A. Association of diastolic dysfunction and outcomes in ambulatory older adults with chronic heart failure. J Gerontol A Biol Sci Med Sci. 2005;60:1339–1344. doi: 10.1093/gerona/60.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mohammad A, Mahy IR, Norton MY, Hillis G, Patel JC, Mikecz P, Walton S. Prevalence of hibernating myocardium in patients with severely impaired ischaemic left ventricles. Heart. 1998;80:559–564. doi: 10.1136/hrt.80.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schinkel AF, Bax JJ, Boersma E, Elhendy A, Roelandt JR, Poldermans D. How many patients with ischemic cardiomyopathy exhibit viable myocardium? Am J Cardiol. 2001;88:561–564. doi: 10.1016/s0002-9149(01)01741-6. [DOI] [PubMed] [Google Scholar]
- 10.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. Beta blockers and the primary prevention of nonfatal myocardial infarction in patients with high blood pressure. Am J Cardiol. 1990;66:12G–14G. doi: 10.1016/0002-9149(90)90386-f. [DOI] [PubMed] [Google Scholar]
- 13.Held P. Effects of beta blockers on ventricular dysfunction after myocardial infarction: tolerability and survival effects. Am J Cardiol. 1993;71:39C–44C. doi: 10.1016/0002-9149(93)90085-q. [DOI] [PubMed] [Google Scholar]
- 14.The Digitalis Investigation Group. Protocol: Trial to evaluate the effect of digitalis on mortality in heart failure. Bethesda, MD: National Heart, Lung, and Blood Institute; 1991. pp. 1–36. [Google Scholar]
- 15.SPSS. SPSS for Windows, Rel. 14. Chicago, IL: SPSS Inc., Chicago, IL; 2006. [Google Scholar]
- 16.Marcus ML, Koyanagi S, Harrison DG, Doty DB, Hiratzka LF, Eastham CL. Abnormalities in the coronary circulation that occur as a consequence of cardiac hypertrophy. Am J Med. 1983;75:62–66. doi: 10.1016/0002-9343(83)90120-1. [DOI] [PubMed] [Google Scholar]
- 17.Isoyama S. Interplay of hypertrophy and myocardial ischemia. In: Lorell BH, Grossman W, editors. Diastolic Relaxation of the Heart. Boston: Kluwer Academic Publishers; 1992. pp. 203–211. [Google Scholar]
- 18.Iriarte M, Murga N, Sagastagoitia D, Molinero E, Morillas M, Salcedo A, Estella P, Etxebeste J. Congestive heart failure from left ventricular diastolic dysfunction in systemic hypertension. Am J Cardiol. 1993;71:308–312. doi: 10.1016/0002-9149(93)90796-f. [DOI] [PubMed] [Google Scholar]
- 19.Strauer BE. Significance of coronary circulation in hypertensive heart disease for development and prevention of heart failure. Am J Cardiol. 1990;65:34G–41G. doi: 10.1016/0002-9149(90)90957-3. [DOI] [PubMed] [Google Scholar]
- 20.Tomanek RJ, Wessel TJ, Harrison DG. Capillary growth and geometry during long-term hypertension and myocardial hypertrophy in dogs. Am J Physiol. 1991;261:H1011–1018. doi: 10.1152/ajpheart.1991.261.4.H1011. [DOI] [PubMed] [Google Scholar]
- 21.Nijland F, Kamp O, Verhorst PM, de Voogt WG, Visser CA. In-hospital and long-term prognostic value of viable myocardium detected by dobutamine echocardiography early after acute myocardial infarction and its relation to indicators of left ventricular systolic dysfunction. Am J Cardiol. 2001;88:949–955. doi: 10.1016/s0002-9149(01)01968-3. [DOI] [PubMed] [Google Scholar]
- 22.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 23.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 24.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]