Abstract
The vacuoles of pea (Pisum sativum) leaves and red beet (Beta vulgaris) storage root are major sites for the intracellular compartmentation of folates. In the light of these findings and preliminary experiments indicating that some plant multidrug resistance-associated protein (MRP) subfamily ATP-binding cassette transporters are able to transport compounds of this type, the Arabidopsis thaliana vacuolar MRP, AtMRP1 (AtABCC1), and its functional equivalent(s) in vacuolar membrane vesicles purified from red beet storage root were studied. In so doing, it has been determined that heterologously expressed AtMRP1 and its equivalents in red beet vacuolar membranes are not only competent in the transport of glutathione conjugates but also folate monoglutamates and antifolates as exemplified by pteroyl-l-glutamic acid and methotrexate (MTX), respectively. In agreement with the results of these in vitro transport measurements, analyses of atmrp1 T-DNA insertion mutants of Arabidopsis ecotypes Wassilewskia and Columbia disclose an MTX-hypersensitive phenotype. atmrp1 knock-out mutants are more sensitive than wild-type plants to growth retardation by nanomolar concentrations of MTX, and this is associated with impaired vacuolar antifolate sequestration. The vacuoles of protoplasts isolated from the leaves of Wassilewskia atmrp1 mutants accumulate 50% less [3H]MTX than the vacuoles of protoplasts from wild-type plants when incubated in media containing nanomolar concentrations of this antifolate, and vacuolar membrane-enriched vesicles purified from the mutant catalyze MgATP-dependent [3H]MTX uptake at only 40% of the capacity of the equivalent membrane fraction from wild-type plants. AtMRP1 and its counterparts in other plant species therefore have the potential for participating in the vacuolar accumulation of folates and related compounds.
Tetrahydrofolate (THF)4 and its derivatives, “folates,” are essential cofactors for one-carbon transfer reactions, for instance those crucial for nucleotide biosynthesis and amino acid metabolism. In humans and other animals who cannot synthesize folates de novo, these cofactors must be obtained from dietary sources, principally plant materials. The repercussions of dietary folate deficiencies range from an increased predisposition to megaloblastic anemia and birth defects to cardiovascular disease and certain cancers (1).
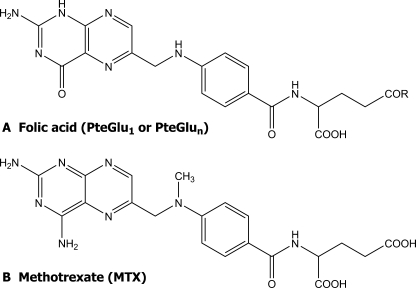
Folates are tripartite molecules, consisting of pteridine and p-aminobenzoate, which together constitute the pteroyl moiety, and one or more glutamate residues (Fig. 1A). In plants and most other organisms, the parent folate molecule, pteroyl monoglutamate (PteGlu1), is poly-γ-glutamylated to yield pteroyl polyglutamates (PteGlun) containing 1–7 additional glutamate residues (2).
FIGURE 1.
Structures of folic acid (A) and methotrexate (MTX) (B). R in structure A is OH in folate monoglutamate (pteroyl-l-glutamic acid, PteGlu1) and n glutamate residues in folate polyglutamates (PteGlun).
The experiments described here were directed at determining whether a multidrug resistance-associated protein (MRP)-type ATP-binding cassette (ABC) transporter might participate in vacuolar folate uptake. The reasons for conducting these studies were 2-fold. The first was recognition that a significant fraction of total cellular folate localizes to the vacuolar compartment of plant cells. Vacuoles purified from pea (Pisum sativum) leaves contain an average of 20% of the total cellular folate, compared with ∼50 and 10%, respectively, in mitochondria and chloroplasts (3). Approximately 50% of the principal vacuolar folate in this system, 5-methyl-THF, is polyglutamylated, whereas the principal mitochondrial and plastidial forms are polyglutamylated derivatives of 5-formyl-THF and 5–10-methenyl-THF, respectively (3). This is probably a general phenomenon in that between 20 and 60% of total tissue folate, mainly in the form of 5-methyl-THF, of which about 80% is polyglutamylated, can be recovered in the vacuolar fraction of red beet (Beta vulgaris) storage roots (3). A hitherto unsuspected role for plant vacuoles in folate storage is implicated, which in turn necessitates a mechanism or mechanisms for the transport of these compounds into this compartment. At the time of writing, the only plant folate transporters to have been identified are those that localize to plastid membranes (4, 5).
A second reason for these investigations was an appreciation that some of the ABC transporters from mammalian sources, specifically some members of the MRP subfamily, are competent in the transport of the chemotherapeutic antifolate methotrexate (MTX; Fig. 1B) and physiological folates (6–8).
The cellular pharmacology of MTX is reasonably well defined in mammals. It has been determined that cellular uptake occurs primarily by facilitated diffusion and that once inside the cell MTX acts as a potent competitive inhibitor of the enzyme dihydrofolate reductase, responsible for the regeneration of reduced THF. MTX thereby retards cell proliferation by depleting intracellular reduced folate pools essential for the biosynthesis of the purines required for DNA replication. Some mammalian MRPs contribute to the detoxification of MTX and compromise chemotherapy by participating in the active extrusion of this drug across the plasma membrane (8). Because MRPs represent the second largest subfamily of full molecule ABC transporters from plant sources, a potentially significant implication follows. If some of the MRPs from mammalian sources can transport MTX, a strict structural analog of PteGlu1, as well as PteGlu1 itself (7), there is a possibility that one or more members of the same subfamily from plant sources share this property.
Many early studies of plant MRP-type transport processes focused on the MgATP-energized, vanadate-inhibitable, un-coupler-insensitive uptake of two model glutathione (GSH) conjugates (GS-conjugates), N-ethylmaleimide-GS (NEM-GS) and S-(2,4-dinitrophenyl)-GS (DNP-GS), and the glutathionylated chloroacetamide herbicide, metolachlor-GS, by isolated vacuoles (9) and vacuolar membrane vesicles purified from plants (10). In this way it was recognized that the transporters responsible bear a close functional resemblance to the GS-conjugate transporting ATPases of mammalian cells and probably belong to the MRP subfamily of ABC transporters (11, 12).
Of the 15 unique MRPs encoded by the genome of Arabidopsis thaliana, five (AtMRPs 1–5) have been cloned and shown to encode functional transporters after heterologous expression in Saccharomyces cerevisiae ycf1Δ strains from which the gene encoding the endogenous vacuolar MRP, yeast cadmium factor 1(ycf 1), has been disrupted (reviewed in Ref. 13). All five are competent in the transport of GS-conjugates. In addition, several are able to transport other amphipathic anions, including glucuronate conjugates and linearized chlorophyll (tetrapyrrole) catabolites (13).
If plant MRPs are to contribute to the vacuolar sequestration of folates, the membrane localization of these transporters and the form in which the folates are transported and stored must be addressed. Regarding membrane localization, two members of the Arabidopsis MRP subfamily, AtMRP1 and AtMRP2 (AtABCC1 and AtABCC2, respectively, according to the new nomenclature described in Ref. 14), have been clearly localized to the vacuolar membrane in planta (15, 16). However, on the basis of pilot experiments, only AtMRP1 catalyzes appreciable folate and MTX transport in vitro. Although AtMRP4 (ABCC4) is also able to transport these compounds at high capacity (17), its exact membrane localization is unclear. On the one hand, it localizes to the plasma membrane rather than the vacuolar membrane when its partial translation product is fused with green fluorescent protein (18). On the other hand, the results of recent Arabidopsis organellar proteomic analyses and studies of constructs in which green fluorescent protein is C-terminally fused to the full-length translation product are consistent with localization to the vacuolar membrane (19). Pending further insight into the membrane localization of AtMRP4, the investigations described here are specifically directed at elucidating the role AtMRP1 might play in vacuolar folate uptake.
Two factors are crucial when considering the form in which folates are transported. The first is that although it has been established that a subset of human and rodent MRPs confer resistance to MTX and have the capacity to transport this and physiological folates, they only do so when these compounds are monoglutamylated; polyglutamylation essentially abolishes transport (7, 8). The second factor is that in plants, as in other organisms, folates are usually polyglutamylated with up to seven γ-linked glumate residues (2) that serve to enhance cofactor activity and stability. More than 50% of the extractable vacuolar pool of folates is polyglutamylated (3). When account is taken of the likelihood that the vacuole lacks the machinery for folate polyglutamylation, the enzyme folylpolyglutamate synthetase (FPGS) and the energy source ATP (3, 20, 21), the implication is clear. If plant MRPs do participate in the vacuolar localization of folates, they are responsible for delivery of only a subfraction of this pool, the monoglutamylated component, into this compartment or, unlike their mammalian counterparts, are able to transport polyglutamates as well as monoglutamates. A primary objective of the investigations reported here was to identify the transport form of vacuolar folates, namely whether they are transported as monoglutamates or polyglutamates, and to determine whether a vacuolar membrane-associated MRP-type functionality, as exemplified by AtMRP1, might be responsible.
To assess the general applicability of the properties of heterologously expressed AtMRP1 and Arabidopsis T-DNA knockout mutants for this transporter to native membranes from other plant sources, parallel experiments were performed on vacuolar membrane vesicles purified from red beet storage root. This system was considered to be particularly appropriate for these investigations for three reasons. First, red beet storage root is a rich source of high purity transport-competent vesicles derived from the membrane bounding the vacuole (22). Second, previous investigations have established that vacuolar membrane vesicles purified from this source contain an MRP-like functionality or functionalities capable of catalyzing the transport of GS-conjugates (10, 23, 24). Third, red beet storage root is listed as one of the richest natural sources of folates (25), a sizeable fraction of which localize to the vacuole (3). The folate content of red beet storage root is as high as those of green leaves, for instance those of spinach which is considered to be one of the richest sources of this class of vitamins (25).
MATERIALS AND METHODS
Chemicals—ATP, creatine kinase (type I from rabbit muscle), creatine phosphate, and N-ethylmaleimide (NEM) were purchased from Sigma. Mixed cellulose ester membrane filters (HAWP filters, 0.45-μm pore size) and hydrophilic polyvinylidene difluoride (Durapore) membrane filters (GVWP filters, 0.22-μm pore diameter) were purchased from Millipore Corp. [glycine-2-3H]Glutathione ([3H]GSH) (41.5 Ci/mmol) was purchased from PerkinElmer Life Sciences. [3′,5′,7,9-3H]Folic acid ([3H]PteGlu1) (42.8 Ci/mmol) and [3′,5′,7-3H]methotrexate ([3H]MTX) (12 Ci/mmol) were purchased from Moravek Biochemicals and Radiochemicals (Brea, CA). Radiolabeled polyglutamylated folate ([3H]PteGlun) was synthesized as described (26). Briefly, 2 nmol of [3H]PteGlu1 were incubated with 25 μg of Escherichia coli FPGS for 24 h at 37 °C in 50 μl of 50 mm Tris-HCl buffer (pH 8.6) containing 10 mm MgCl2, 50 mm KCl, 5 mm ATP, 25 μg of bovine serum albumin, and 20 mm l-glutamate. The reaction was terminated by boiling the mixture for 5 min and subsequent centrifugation at 20,000 × g for 10 min. The products of the reaction were purified by FBP-affinity chromatography as described below for the purification of [3H]PteGlu1, and quantitated and identified by HPLC with electrochemical detection (26). For the investigations described here, the [3H]PteGlun preparations consisted of 45% [3H]PteGlu3, 55% [3H]PteGlu4, and 5% [3H]PteGlu5. [3H]NEM-GS was synthesized by mixing reduced [glycine-2-3H]GSH with NEM at a 1.1:1.0 molar ratio in Hepes/Bistris propane buffer (pH 8.0) (27). All of the molecular biology reagents, including TRIzol, Superscript II RNase H– RT, and the DNeasy plant mini kits were purchased from Invitrogen and Qiagen. All of the other reagents were of analytical grade and purchased from Fisher, Research Organics, Inc., or Sigma.
Plant Materials—For the majority of the investigations of A. thaliana, ecotype Wassilewskia (WS) was employed except for the analyses of atmrp1-2 knock-out mutants, which were performed on ecotype Columbia (Col-0). Fresh red beet (Beta vulgaris) storage roots were purchased locally, stored at 4 °C, and used within 2 days of purchase.
Isolation of AtMRP1 T-DNA Insertion Mutants—For the experiments described here, two AtMRP1 insertion mutants, atmrp1-1 and atmrp1-2, respectively, from Arabidopsis ecotypes WS and Col-0 were obtained. The WS allele was isolated by PCR-based reverse genetic screens of the Feldmann collection of T-DNA insertion lines (28) using appropriate AtMRP1-specific and T-DNA left or right border-specific primer pairs. The Col-0 atmrp1-2 allele was identified using the SIGnAL Arabidopsis gene mapping tool “T-DNA Express,” and the corresponding T-DNA insertion seed stock was obtained from the Arabidopsis Biological Resource Center, Ohio State University. Both alleles were characterized genomically by standard procedures.
RT-PCR—To assess the steady state levels of AtMRP1 transcripts and therefore the severity of the knock-outs or knockdowns for the atmrp1 mutants, RNA was extracted from 14-day-old atmrp1-1 and atmrp1-2 mutant and wild-type WS and Col-0 plants using TRIzol reagent (Invitrogen) according to the manufacturer's recommendations. For RT-PCR of AtMRP1, 1-μg aliquots of the RNA samples were reverse-transcribed using Superscript II RNase H– RT (Invitrogen), and the first-strand cDNA products were PCR-amplified using the AtMRP1-specific primer pair AtMRP1-RT-F/AtMRP1-RT-R (5′-CGCAGAAATCCTCTTGGTCTTGA-3′ and 5′-CCGTTAGCTTCTCTGGTACGTTTG-3′, respectively). PCR amplification was for 3 min at 94 °C followed by 27 cycles of 30 s at 94 °C, 30 s at 62 °C, and 1 min at 72 °C. To assess the efficacy of RNA extraction, aliquots of the same RNA samples were also subjected to RT-PCR using the Arabidopsis Actin-8 gene primer pair Actin-F/Actin-R (5′-CCTGCTATGTATGTGGCTATT-3′ and 5′-CTGTGGTGGTGAAAGAGTAAC-3′, respectively) using the same thermal profile except that the annealing step was done at 58 °C.
Phenotypic Characterization of atmrp1-1 and atmrp1-2 Mutants—For the preliminary phenotypic screens, seeds of atmrp1-1 mutant and wild-type WS plants and of atmrp1-2 mutant and wild-type Col-0 plants were surface-sterilized with 0.05% (w/v) sodium hypochlorite/0.1% (w/v) Tween 20, washed exhaustively with sterile water, and germinated for 48 h at 4 °C on solid MS medium (pH 5.7) containing 1% (w/v) sucrose. Xenobiotics, elicitors of oxidative stress, and phytohormones were incorporated into the medium at the concentrations indicated. Thereafter, the plates were grown vertically under controlled environmental conditions (24 ± 2 °C; continuous cool fluorescent illumination; 70% relative humidity) for 12 days before measuring primary root length. For subsequent detailed screens of the sensitivity of growth to MTX at the whole plant level, surface-sterilized mutant and wild-type seeds were germinated for 48 h at 4 °C on solid MS medium containing 0.5 g/liter Mes, 0.9% (w/v) Difco-Bacto agar, 1% (w/v) sucrose, and the indicated concentrations of MTX in plant tissue culture vessels before transfer to a plant growth room for growth at 22 ± 2 °C and 70% relative humidity under a 16/8-h photoperiod for a further 14 days. In all of the phenotypic screens, precautions were taken to ensure that several independent mutant and wild-type seed batches were treated and screened in parallel under identical conditions.
Affinity Purification of [3H]PteGlu1—To remove breakdown products and/or contaminants that were suspected to interfere with transport, the radiolabeled stocks of [3H]PteGlu1 received from the suppliers were further purified by affinity chromatography before use. For this purpose, 20-μl aliquots of the [3H]PteGlu1 stocks (40–45 Ci/mmol) were added to 300 μl of 25 mm potassium phosphate buffer (pH 7.4) and applied to a column packed with folate-binding protein (FBP)-agarose (bed volume 0.5 ml) that had been equilibrated with the same buffer (26). After three successive washes with 2.5 ml of potassium phosphate buffer (pH 7.4) containing 1 m NaCl, 2.5 ml of potassium phosphate buffer alone, and 300 μl of 0.1 m HCl, the column was eluted with 1 ml of 0.1 m HCl. The final eluate containing purified [3H]PteGlu1 was combined with 0.1 ml of 10 mm 2-mercaptoethanol dissolved in 1 m Tris-base to yield a pH 7.4 solution that was used immediately or stored at –20 °C.
Heterologous Expression of AtMRP1 in Yeast and Purification of Vacuolar Membrane-enriched Vesicles—For studies of the transport capabilities of heterologously expressed AtMRP1, S. cerevisiae ycf1Δ strain DTY168 (MATα his6 leu2-3,-112 ura 3–52 ycf1::hisG) (29) was transformed with pYES3-AtMRP1 or empty pYES3 vector by the LiOAc/polyethylene glycol method (30) and selected for uracil prototrophy by plating on AHC medium containing tryptophan (31, 32). Vacuolar membraneenriched vesicles were purified from the transformants as described (31, 32).
Preparation of Red Beet Vacuolar Membrane Vesicles—Vacuolar membrane vesicles were purified from fresh red beet (B. vulgaris) storage roots by a combination of differential and density gradient centrifugation (10, 22). The final membrane pellets were resuspended in suspension buffer (5 mm Tris/Mes (pH 7.5), 1 mm Tris/EDTA, 0.5 mm dithiothreitol, 100 μg/ml butylated hydroxytoluene, and 10% (v/v) glycerol) to a final protein concentration of 1–2 mg/ml, frozen in liquid nitrogen, and stored at –80 °C.
Preparation of Vacuolar Membrane-enriched Vesicles from atmrp1-1 Mutant and Wild-type Arabidopsis WS Root Cultures—After germination and growth on solid MS medium supplemented with 1% (w/v) sucrose (pH 5.7) for 7 days, batches of 50–75 atmrp1-1 mutant or wild-type Arabidopsis WS seedlings were transferred to 125-ml culture flasks containing 50 ml of half-strength MS medium and cultured for 14 days with shaking at 130 rpm. Vacuolar membrane-enriched vesicles were purified from the root cultures by a combination of differential and density gradient centrifugation on 10/23% (w/w) sucrose step gradients as described (10).
Measurements of Uptake by Yeast, Red Beet, and Arabidopsis Vacuolar Membrane-enriched Vesicles—Measurements of the MgATP-dependent uptake of [3H]PteGlu1 (1.5 mCi/mmol), [3H]PteGlun (3.5 mCi/mmol), [3H]MTX (7.5 mCi/mmol), or [3H]NEM-GS (6 mCi/mmol) by vacuolar membrane-enriched membrane vesicles purified from pYES3-AtMRP1 or empty pYES3 vector-transformed yeast DTY168 cells, red beet storage root, or Arabidopsis root cultures were performed as described (10, 32). Uptake was initiated by the addition of 20 μg of yeast vacuolar membrane-enriched vesicles, 30 μg of red beet vacuolar membrane vesicles, or 50 μgof Arabidopsis vacuolar membrane-enriched vesicles to the uptake media and brief agitation on a vortex mixer. In all of the experiments described here, uptake was measured at 25 °C in 200-μl reaction volumes containing 3 mm ATP, 3 mm MgSO4, 10 mm creatine phosphate, 16 units/ml creatine kinase, 50 mm KCl, 400 mm sorbitol, 25 mm Tris/Mes buffer (pH 8.0), and the indicated concentrations of radiolabeled transport substrate. At the times indicated, uptake was terminated by the addition of 1 ml of ice-cold wash medium (400 mm sorbitol, 3 mm Tris/Mes (pH 8.0)) and vacuum filtration of the suspension through prewetted membrane filters. For the measurements of [3H]PteGlu1, [3H]PteGlun, or [3H]MTX uptake GV Durapore polyvinylidene difluoride (GVWP) filters (0.22-μm pore diameter) were used; for the measurements of [3H[]NEM-GS uptake mixed cellulose ester (HAWP) filters (0.45-μm pore diameter) were used. After two washes with 1-ml volumes of ice-cold wash medium, the filters were airdried, and radioactivity was determined by liquid scintillation counting in 5-ml volumes of BCS liquid scintillation mixture (Amersham Biosciences).
Measurements of Total Cellular and Vacuolar [3H]MTX Uptake by Protoplasts Isolated from atmrp1-1 Mutant and Wild-type Arabidopsis WS Plants—Wild-type WS and atmrp1-1 mutant plants were greenhouse-grown (24 ± 2 °C; 10/14 h photoperiod; 80% relative humidity) for 4–5 weeks, and protoplasts were prepared from rosette leaves as described (33). MTX uptake was estimated by incubating equal amounts of the wild-type and atmrp1-1 protoplast suspensions in uptake medium (10 mm CaCl2, 1 mm MgCl2, 0.5 m sorbitol and 10 mm Tris/Mes (pH 5.5)) containing 19 nm [3H]MTX at 25 °C for 2.5 h with gentle agitation on an orbital shaker. For the estimation of total protoplast (“cellular”) [3H]MTX content, the protoplasts were pelleted by centrifugation at 60 × g for 5 min and washed three times with fresh uptake medium before the removal of aliquots for liquid scintillation counting. For the estimation of vacuolar [3H]MTX content, intact vacuoles were isolated from the protoplasts by differential osmolysis and Ficoll flotation centrifugation through 10% (w/v) and 4% (w/v) Ficoll cushions prepared in vacuolar resuspension buffer containing 0.45 m sorbitol (34). Bovine serum albumin (5 mg/ml) was added to the 10% Ficoll solution to diminish adherence of membranes from other sources to the surfaces of the vacuoles during fractionation (35). Intact vacuoles were collected from the 0/4% Ficoll interface for liquid scintillation counting.
Protein Estimations—Protein was estimated by a modification of the method of Bradford (36)
RESULTS
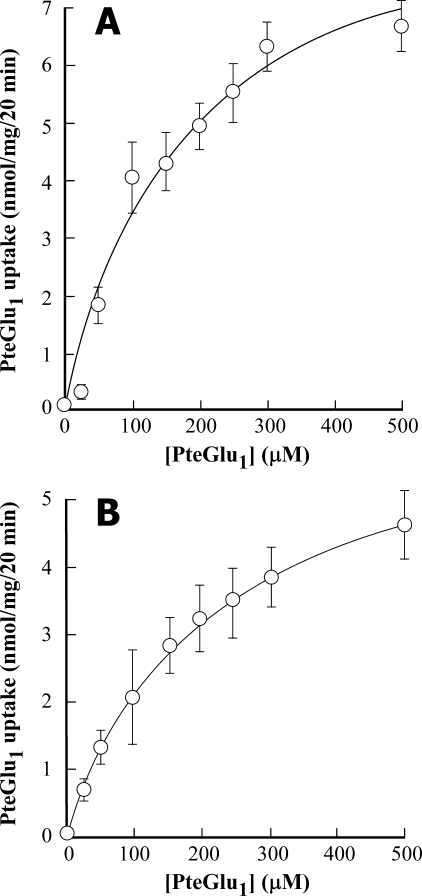
MgATP-dependent Transport of Folate Monoglutamate (Pteroyl-l-glutamate, PteGlu1)—Both heterologously expressed AtMRP1 and vacuolar membrane vesicles purified from red beet storage root are competent in the transport of PteGlu1. The concentration dependence of uptake of affinity-purified [3H]PteGlu1 by vacuolar membrane-enriched vesicles purified from pYES3-AtMRP1-transformed S. cerevisiae ycf1Δ strain DTY168 approximates Michaelis-Menten kinetics to yield Km and Vmax values of 188 ± 68 μm and 10.1 ± 1.8 nmol/mg/20 min, respectively (Fig. 2A). The corresponding values for native vacuolar membrane vesicles purified from red beet storage root are 195 ± 14 μm and 6.4 ± 0.2 nmol/mg/20 min, respectively (Fig. 2B). Crucial, however, if transport is to be measured reliably, is the purity of the [3H]PteGlu1 employed as transport substrate. For the experiments shown in Fig. 2 and all of the other experiments employing [3H]PteGlu1, the radiolabeled transport substrate as supplied was subjected to FBP-agarose affinity purification. If this precaution was not taken, the rates and extents of uptake were often underestimated. As exemplified by the results obtained with red beet vacuolar membrane vesicles, the rates and extents of MgATP-dependent uptake of FBP-agarose-purified [3H]PteGlu1 exceeded those of the unpurified compound by severalfold such that purification of the [3H]PteGlu1 stock was accompanied by a decrease in the Km from 403 ± 112 to 195 ± 15 μm concomitant with an increase in the Vmax from 2.3 ± 0.4 to 6.4 ± 0.2 nmol/mg/20 min (data not shown). The reason for this difference is not known, but it is suspected to result from contamination of the stock with breakdown products that interfere with uptake of the parent compound, [3H]PteGlu1, but which lack radiolabel and/or do not undergo appreciable MgATP-dependent uptake themselves. HPLC analysis of the [3H]PteGlu1 stock before and after affinity purification discloses additional species in the former that are absent from the latter.5
FIGURE 2.
Concentration dependence of MgATP-dependent [3H]PteGlu1 uptake by vacuolar membrane-enriched vesicles purified from pYES3-AtMRP1-transformed S. cerevisiae ycf1Δ strain DTY168 (DTY168/pYES3-AtMRP1) cells (A) and by vacuolar membrane vesicles purified from red beet storage root (B). MgATP-dependent [3H]PteGlu1 uptake by AtMRP1 (A) was calculated by subtracting the radioactivity taken up by vacuolar membrane-enriched vesicles purified from empty pYES3 vector-transformed strain DTY168 (DTY168/pYES3) cells from that taken up by the equivalent membrane fraction from DTY168/pYES3-AtMRP1 cells. The data were fitted to a single Michaelis-Menten function by nonlinear least squares analysis to yield Km and Vmax values of 188 ± 68 μm and 10.1 ± 1.8 nmol/mg/20 min, respectively, for heterologously expressed AtMRP1 (A) and 195 ± 15 μm and 6.4 ± 0.2 nmol/mg/20 min, respectively, for red beet vacuolar membrane vesicles (B). Values shown are means ± S.E. (n = 3).
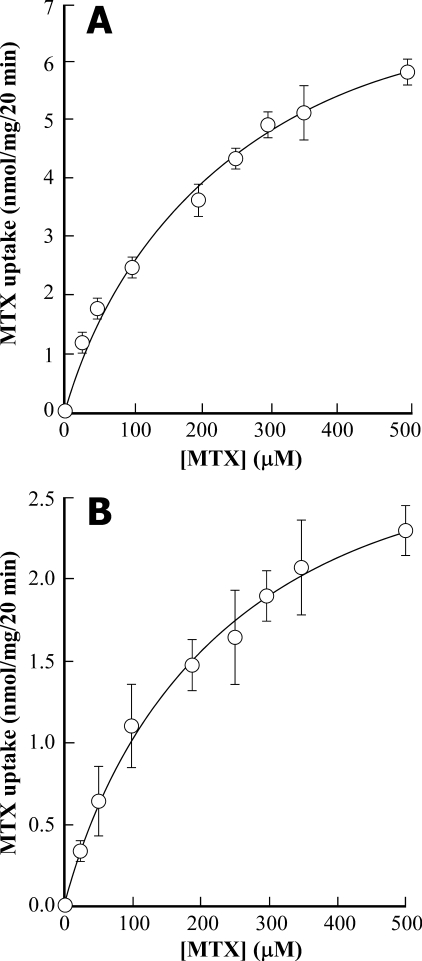
MgATP-dependent Transport of MTX—The kinetics of uptake of the antifolate MTX by yeast vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 or purified red beet vacuolar membrane vesicles are fundamentally equivalent to those of [3H]PteGlu1. In both membrane preparations, MgATP-dependent [3H]MTX uptake approximates Michaelis-Menten kinetics to yield Km and Vmax values of 243 ± 55 μm and 8.6 ± 0.9 nmol/mg/20 min, respectively, for heterologously expressed AtMRP1 (Fig. 3A) and 223 ± 37μm and 3.3 ± 0.2 nmol/mg/20 min, respectively, for red beet vacuolar membrane vesicles (Fig. 3B).
FIGURE 3.
Concentration dependence of MgATP-dependent [3H]MTX uptake by vacuolar membrane-enriched vesicles purified from DTY168/pYES3-AtMRP1 cells (A) and by vacuolar membrane vesicles purified from red beet storage root (B). The data were fitted to a Michaelis-Menten function to yield Km and Vmax values of 243 ± 55 μm and 8.6 ± 0.9 nmol/mg/20 min, respectively, for heterologously expressed AtMRP1 and 223 ± 37 μm and 3.3 ± 0.2 nmol/mg/20 min, respectively, for red beet vacuolar membrane vesicles. Values shown are means ± S.E. (n = 3).
Vanadate Inhibitability of [3H]PteGlu1 and [3H]MTX Transport—All four of the transport processes examined, MgATP-dependent uptake of [3H]PteGlu1 or [3H]MTX into yeast vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 or into red beet vacuolar membrane vesicles, are susceptible to inhibition by vanadate (Table 1). On this basis and the insensitivity of these processes to the V-ATPase inhibitor bafilomycin A1 or the protonophore gramicidin-D (data not shown), the transport measured appears to be largely attributable to the primary energization of ABC transporters by MgATP rather than secondary H+-coupled transport. The only qualitative difference between the kinetics of [3H]PteGlu1 and [3H]MTX transport by heterologously expressed AtMRP1 and the red beet membrane preparations is that ∼20% of the transport measured in the latter is insensitive to inhibition by vanadate. This necessitates subtraction of the uninhibitable component from the total uptake measured when enumerating the concentration of vanadate required to inhibit the inhibitable component by 50%. When this correction is applied, the I50 values for the inhibition of [3H]PteGlu1 and [3H]MTX uptake by vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 and red beet vacuolar membrane vesicles fall in the same range: 3.3 ± 0.5 and 6.7 ± 1.9 μm for [3H]PteGlu1 and [3H]MTX uptake by AtMRP1; 3.2 ± 1.0 and 9.6 ± 4.6 μm for [3H]PteGlu1; and [3H]MTX uptake by red beet (Table 1).
TABLE 1.
Sensitivity of MgATP-dependent [3H]PteGlu1, [3H]MTX, or [3H]NEM-GS uptake by yeast vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 and vacuolar membrane vesicles purified from red beet storage root to inhibition by vanadate
The rates of MgATP-dependent uptake of 100 μm concentrations of [3H]PteGlu1, [3H]MTX, or [3H]NEM-GS from assay media containing 0–80 μm vanadate were estimated as described in Figs. 2 and 4. Each data set was fitted to a single negative exponential function by nonlinear least squares analysis to yield estimates of the concentrations of vanadate required for 50% inhibition of net uptake (I50 values). Note that the I50 values calculated for red beet vacuolar membrane vesicles are those for the inhibitable component obtained by subtracting the uninhibitable component, which accounted for approximately 20% of total uptake, from total uptake. Values shown are means ± S.E. (n = 3).
|
Transport substrate
|
I50 (μm) for inhibition by vanadate
|
|
|---|---|---|
| Heterologously expressed AtMRP1 | Red beet vacuolar membrane vesicles | |
| PteGlu1 | 3.3 ± 0.5 | 3.2 ± 1.0 |
| MTX | 6.7 ± 1.9 | 9.6 ± 4.6 |
| NEM-GS | 2.3 ± 0.5 | 4.4 ± 0.5 |
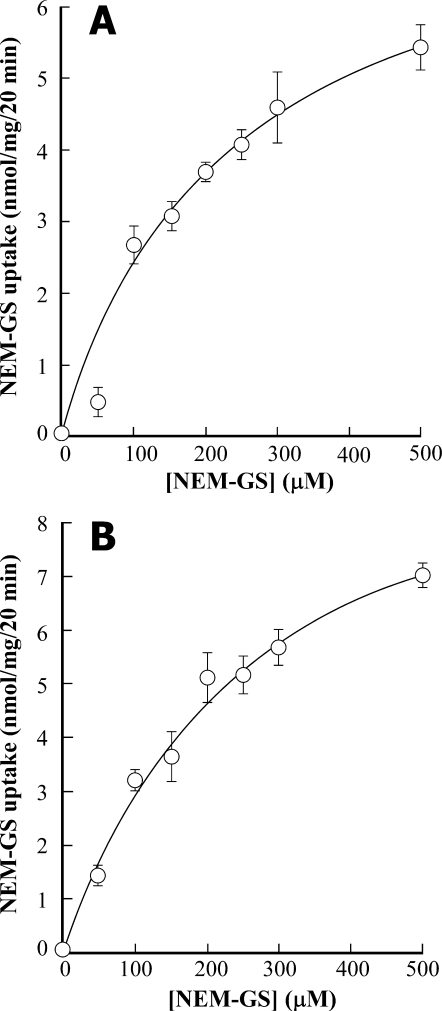
Transport of PteGlu1 and GS-conjugates by a Common MRP-type Functionality—AtMRP1 was first characterized in terms of its capacity to catalyze the vanadate-inhibitable, MgATP-dependent transport of GS-conjugates (32), a property it shares with many other MRP-type ABC transporters (13). Accordingly, heterologously expressed AtMRP1 and, as would be expected if similar transporters are to be found in native plant vacuolar membranes, red beet vacuolar membrane vesicles catalyze the vanadate-inhibitable uptake of [3H]NEM-GS. The Km and Vmax values for [3H]NEM-GS uptake by AtMRP1 and red beet are 292 ± 144 μm and 8.8 ± 2.2 nmol/mg/20 min and 258 ± 46 μm and 10.6 ± 0.9 nmol/mg/20 min (Fig. 4, A and B); the corresponding I50 values for inhibition by vanadate are 2.3 ± 0.5 and 4.4 ± 0.5 μm (after subtraction of the uninhibitable component in the latter case) (Table 1).
FIGURE 4.
Concentration dependence of MgATP-dependent [3H]NEM-GS uptake by vacuolar membrane-enriched vesicles purified from DTY168/pYES3-AtMRP1 cells (A) and by red beet vacuolar membrane vesicles (B). The data were fitted to a single Michaelis-Menten function to yield Km and Vmax values of 292 ± 144 μm and 8.8 ± 2.2 nmol/mg/20 min, respectively, for heterologously expressed AtMRP1, and 258 ± 46 μm and 10.6 ± 0.9 nmol/mg/20 min, respectively, for red beet vacuolar membrane vesicles. Values shown are means ± S.E. (n = 3).
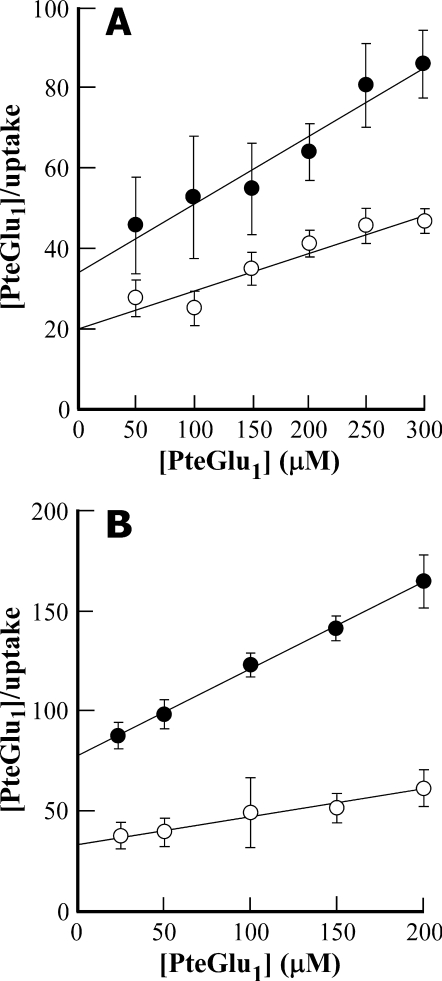
An intriguing finding, however, is that although NEM-GS interacts with the same MRP-type functionality as PteGlu1, it does so noncompetitively. Although inclusion of 100 μm NEM-GS in the uptake medium decreases the Vmax value for [3H]PteGlu1 uptake by vacuolar membrane-enriched vesicles purified from yeast heterologously expressing AtMRP1 from 10.1 ± 1.8 nmol/mg/20 min to 5.7 ± 0.8 nmol/mg/20 min, it has little or no effect on the Km value, which has values of 181 ± 52 and 188 ± 68 μm, respectively, in the presence and absence of NEM-GS (Fig. 5A). Qualitatively similar results are obtained when the same experiment is performed on red beet vacuolar membrane vesicles; the Vmax value for [3H]PteGlu1 uptake is decreased from 6.4 ± 0.2 nmol/mg/20 min to 2.3 ± 0.1 nmol/mg/20 min while leaving Km relatively unaffected at values of 195 ± 15 and 181 ± 12 μm (Fig. 5B) when NEM-GS is included in the uptake medium. Because under all four conditions [3H]PteGlu1 uptake closely approximates Michaelis-Menten kinetics to yield strictly linear Hanes-Woolf plots, the implication is that PteGlu1 and NEM-GS interact with a common functionality in both membrane preparations but at different sites. The interaction of heterologously expressed AtMRP1 or its equivalents in red beet with NEM-GS interferes with [3H]PteGlu1 transport without interfering with the binding of the latter to the transporter.
FIGURE 5.
Kinetics of inhibition of MgATP-dependent [3H]PteGlu1 uptake by vacuolar membrane-enriched vesicles purified from DTY168/pYES3-AtMRP1 cells (A) and red beet vacuolar membrane vesicles (B) by NEM-GS. Shown are Hanes-Woolf plots of [PteGlu1](μm)/uptake (nmol/mg/20 min) versus [PteGlu1](μm) in the absence (○) and presence (•) of 100 μm NEM-GS. The Km and Vmax values for AtMRP1-mediated [3H]PteGlu1 uptake (A) in the absence (○) and presence (•) of 100 μm NEM-GS were 188 ± 68 μm and 10.1 ± 1.8 nmol/mg/20 min, and 181 ± 52 μm and 5.7 ± 0.8 nmol/mg/20 min, respectively. The corresponding values for [3H]PteGlu1 uptake by red beet vacuolar membrane vesicles (B) were 195 ± 15 μm and 6.4 ± 0.2 nmol/mg/20 min, and 181 ± 12 μm and 2.3 ± 0.1 nmol/mg/20 min, respectively. Values shown are means ± S.E. (n = 3).
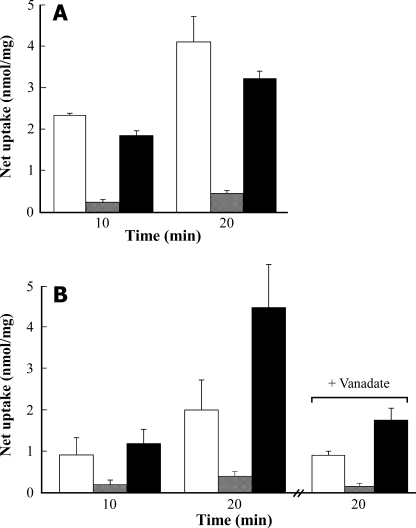
Polyglutamylated Folate as a Poor Transport Substrate—By comparison with the folyl monoglutamates, PteGlu1 and MTX, folyl polyglutamates (PteGlun) undergo only very low rates of MgATP-dependent uptake. Net uptake of 50 μm [3H]PteGlun into yeast vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 or red beet vacuolar membrane vesicles is 7–10- and ∼3-fold lower than the net uptake of 100 μm [3H]PteGlu1 measured after 10 or 20 min under the same conditions (Fig. 6, A and B). Moreover, whereas the inclusion of 50 μm PteGlun in the [3H]PteGlu1 uptake medium weakly but consistently inhibits uptake mediated by heterologously expressed AtMRP1, suggesting that PteGlu1 and PteGlun compete for common binding sites (Fig. 6A), the converse is seen in the red beet system. Addition of 50 μm PteGlun increases the net uptake of 100 μm [3H]PteGlu1 by red beet vacuolar membrane vesicles by factors of 1.3 and 2.2 after 10 and 20 min, respectively (Fig. 6B). The significance of this effect is not known, but the increase in [3H]PteGlu1 uptake seen when PteGlun is added to the assay medium is at least partially inhibited by vanadate (Fig. 6B), which is consistent with the participation of an ABC transporter.
FIGURE 6.
Net MgATP-dependent uptake of folate monoglutamate and folate
polyglutamates by vacuolar membrane vesicles purified from DTY168/pYES3-AtMRP1
cells (A) and purified red beet vacuolar membrane vesicles
(B). Net MgATP-dependent [3H]PteGlu1 (100
μm)(□) or [3H]PteGlun (50
μm) uptake
( ), or
[3H]PteGlu1 (100 μm) uptake from assay
media containing unlabeled PteGlun (50
μm)(▪) were measured after 10 and 20 min. In one set of
experiments on red beet vacuolar membrane vesicles, the vanadate
inhibitabilities of [3H]PteGlu1 and
[3H]PteGlun uptake and of
[3H]PteGlu1 uptake in the presence of unlabeled
PteGlun were determined after the addition of 100
μm vanadate to the assay medium. Values shown are means ±
S.E. (n = 3).
), or
[3H]PteGlu1 (100 μm) uptake from assay
media containing unlabeled PteGlun (50
μm)(▪) were measured after 10 and 20 min. In one set of
experiments on red beet vacuolar membrane vesicles, the vanadate
inhibitabilities of [3H]PteGlu1 and
[3H]PteGlun uptake and of
[3H]PteGlu1 uptake in the presence of unlabeled
PteGlun were determined after the addition of 100
μm vanadate to the assay medium. Values shown are means ±
S.E. (n = 3).
Isolation and Genomic Characterization of AtMRP1 Knockout Mutants—With the aim of gaining insight into the function of AtMRP1 in the intact plant, or at least processes for which its loss of function has discernible phenotypic consequences, two T-DNA insertion mutant alleles of AtMRP1, atmrp1-1 and atmrp1-2 from ecotypes Wassilewskia (WS) and Columbia (Col-0), respectively, were obtained. The former was isolated in in-house screens; the latter was obtained from the Arabidopsis Biological Resource Center Salk collection.
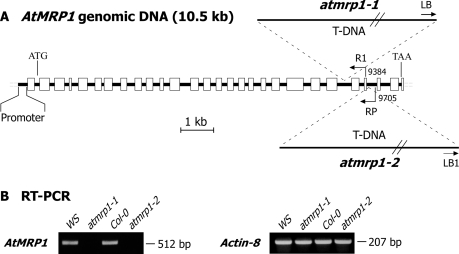
As confirmed by the results shown in Fig. 7, both T-DNA insertions, atmrp1-1 that maps to exon 23 of the genomic sequence of AtMRP1 and atmrp1-2 that maps to exon 25 (Fig. 7A), are associated with a knock-out or severe knockdown of expression (Fig. 7B). RT-PCR of total RNA extracted from atmrp1-1 or atmrp1-2 mutants and the corresponding wild types using the AtMRP1-specific primer pair AtMRP1-RT-F/AtMRP1-RT (see “Materials and Methods”) yields a strong signal that matches the 512-bp amplification product expected from the mature transcript from the wild-type RNA extracts but no signal from the mutant extracts (Fig. 7B).
FIGURE 7.
Schematic diagram depicting positions of T-DNA insertions in genome of Arabidopsis WS atmrp1-1 and Col-0 atmrp1-2 knock-out mutants (A), and results of RT-PCR analyses of AtMRP1 expression in wild-type and atmrp1-1 mutant WS plants and wild-type and atmrp1-2 mutant Col-0 plants (B). The DNA encompassing AtMRP1 starting at position 9384 and the left border of the T-DNA insertion of homozygous WS atmrp1-1 mutants was amplified using the primer pair AtMRP1-R1/T-DNA-LB (5′-TGCAAGTAGTGGTCGAATATTTTG-3′ and 5′-GATGCACTCGAAATCAGCCAATTTTAGAC-3′) to map the insertion in this allele to exon 23. The DNA encompassing AtMRP1 starting at position 9705 and the left border of the T-DNA insertion of homozygous Col-0 atmrp1-2 mutants was amplified using the primer pair AtMRP1–2 RP/T-DNA-LB1 (5′-GATTTCAAGGCTTTGGAGGTC-3′ and 5′-GCCTTTTCAGAAATGGATAAATAGCCTTGCTTCC-3′) to map this insertion to exon 25. RT-PCR of total RNA extracted from wild-type and atmrp1-1 mutant WS plants and from wild-type and atpmrp1-2 mutant Col-0 plants was performed using the primer pair AtMRP1-RT-F/AtMRP1-RT-R (see under “Materials and Methods”), which should yield a 512-bp amplification product from reverse-transcribed mature AtMRP1 transcript. To ensure equivalence between the extractions and sample loads, the same RNA samples were subjected to RT-PCR using the actin-F/actin-R primer pair (see under “Materials and Methods”), which yields a 207-bp amplification product.
MTX Hypersensitivity of atmrp1-1 and atmrp1-2 Knockout Mutants—Having established that both atmrp1-1 and atmrp1-2 are null mutants and that heterologously expressed AtMRP1 is competent in the MgATP-dependent transport of toxic amphipathic anions, such as MTX, as well as some GS-conjugable xenobiotics, screens were initiated to assess the sensitivity of these mutants by comparison with wild-type plants to the toxic effects of these agents.
In the first instance, seeds from atmrp1-1 and atmrp1-2 plants and their corresponding WS and Col-0 wild-type equivalents were germinated and grown in the light on sterile plates containing a broad range of concentrations of the antifolate MTX, CDNB (a cytotoxic generic glutathione S-transferase (GST) substrate), the GS-conjugable herbicides atrazine (a triazine derivative) or metolachlor (a chloroacetanilide), menadione (an elicitor of oxidative stress), or abscisic acid (ABA, a sesquiterpenoid stress hormone). In this way it was determined that although neither of these mutants is more susceptible than their corresponding wild types to any of the GS-conjugable xenobiotics tested or menadione or ABA, both are more sensitive to MTX in the growth medium. When germinated and grown vertically on MS medium supplemented with 1–10 μm atrazine or metolachlor, 5–60 μm CDNB, 5–100 μm menadione or 1–5 μm ABA, no difference between the mutant and wild-type seedlings was discernable; all showed similar degrees of root growth inhibition (data not shown). However, when the same experiments were conducted with MTX, differences between the atmrp1-1 and atmrp1-2 mutants and their corresponding wild types were evident in the nanomolar range.
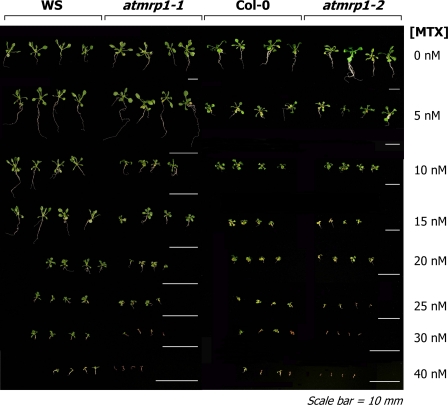
Expansion of the screens to the effects of MTX at the level of the intact seedling reinforced these findings. When grown on solid MS medium containing 0–40 nm MTX in plant tissue culture vessels under a 16/8-h photoperiod for a further 14 days after germination, significant, albeit small to moderate, differences between the atmrp1 mutants and their corresponding wild types are evident at both the root and shoot levels (Fig. 8). The wild-type and atmrp1 mutant seedlings are indistinguishable when grown on solid medium lacking MTX. However, starting at MTX concentrations of 10 nm in the case of ecotype WS and 15 nm in the case of ecotype Col-0, differences between the mutant and wild-type seedlings in terms of both root and shoot growth become evident such that although both the mutants and wild types are subject to growth retardation, the retardations are greater in both the atmrp1-1 and atmrp1-2 mutants at all MTX concentrations greater than 25 nm (Fig. 8). At the highest MTX concentrations examined, 30 and 40 nm, the mutants are more prone than the wild types to chlorosis, anthocyanin accumulation, and eventual necrosis (Fig. 8).
FIGURE 8.
Differential sensitivities of the growth of Arabidopsis WS atmrp1-1 mutant and wild-type plants (A) and Arabidopsis Col-0 atmrp1-2 and wild-type plants (B) to the inclusion of MTX in the growth medium. Seedlings were grown for 14 days on solid MS medium or MS medium supplemented with the concentrations of MTX indicated at 22 ± 2 °C and 70% relative humidity under a 16/8-h photoperiod.
Protoplasts from atmrp1-1 Mutants Are Defective in Vacuolar [3H]MTX Accumulation—In view of the capacity of heterologously expressed AtMRP1 for MgATP-dependent MTX transport in vitro and localization of this transport protein to the vacuolar membrane in planta, the most straightforward explanation for the increased susceptibility of atmrp1-1 and atmrp1-2 mutants to the toxic action of this antifolate is that by comparison with their wild-type counterparts they are impaired in the vacuolar sequestration of this compound. To test this proposal, two approaches were adopted. In the first, total cellular [3H]MTX uptake and the vacuolar levels achieved by protoplasts isolated from the leaves of atmrp1-1 mutant and wild-type WS plants were compared. In the second, the capacities of vacuolar membrane-enriched vesicles purified from atmrp1-1 mutant and wild-type WS liquid root cultures for MgATP-dependent [3H]MTX uptake in vitro were compared.
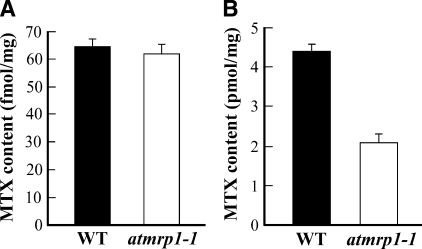
The results obtained from both approaches were consistent with a deficiency in vacuolar MTX sequestration in atmrp1-1 mutants. When incubated in culture medium containing 19 nm [3H]MTX for 2.5 h, protoplasts isolated from atmrp1-1 mutants and wild-type WS plants achieve similar total cellular levels of the antifolate (61.7 ± 3.1 and 63.8 ± 2.6 fmol/mg protein, respectively) (Fig. 9A). However, if aliquots of the same samples of protoplasts are gently disrupted by differential osmolysis to release intact vacuoles and the [3H]MTX contents of these are estimated, there is a marked difference depending on whether the vacuoles are fractionated from atmrp1-1 mutant or wild-type WS protoplasts. Vacuoles prepared from wild-type WS protoplasts achieve [3H]MTX contents of 4.4 ± 0.1 pmol/mg protein, whereas the [3H]MTX content of the corresponding fraction from atmrp1-1 mutant protoplasts is only 2.1 ± 0.2 pmol/mg protein (Fig. 9B). An ∼50% diminution of the vacuolar [3H]MTX content of atmrp1-1 mutant versus wild-type WS protoplasts despite comparable levels of total protoplast uptake indicates that the atmrp1-1 mutants are not impaired in total cellular MTX uptake or extrusion but are instead impaired in delivery of this compound into the vacuole.
FIGURE 9.
Comparison of the MTX contents of protoplasts (A) and intact vacuoles (B) isolated from atmrp1-1 mutant and wild-type (WT) Arabidopsis WS plants after incubation in media containing [3H]MTX. Equivalent amounts of protoplasts, estimated as total protein, isolated from rosette leaves of atmrp1-1 mutant and wild-type WS plants were incubated at 25 °C with gentle shaking in uptake medium containing 19 nm [3H]MTX. After 2.5 h, the protoplasts were sedimented by centrifugation at low speed and washed three times in the same medium lacking [3H]MTX. After removing aliquots of the suspended protoplasts for the estimation of total protoplast [3H]MTX content (A), samples of the suspensions were subjected to gentle disruption by differential osmolysis and Ficoll floatation centrifugation for the purification of intact vacuoles and estimation of their [3H]MTX content (B). Values shown are means ± S.E. (n = 3).
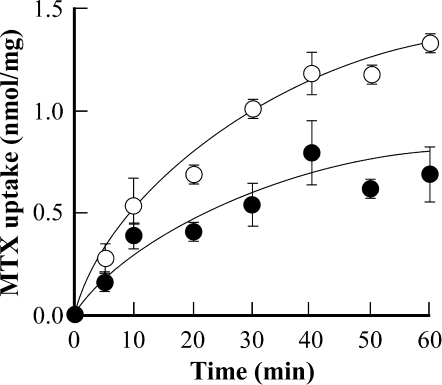
In agreement with this interpretation, the rates and extents of MgATP-dependent [3H]MTX uptake by vacuolar membrane-enriched vesicles purified from atmrp1-1 mutant root cultures when measured at an initial concentration of 50 μm are diminished by ∼60 and 40%, respectively, by comparison with the corresponding membrane fraction from wild-type WS root cultures (Fig. 10). Moreover, regardless of the source of the membrane vesicles, MgATP-dependent [3H]MTX uptake is more than 85% inhibited by the inclusion of 100 μm vanadate in the uptake medium (data not shown), which implies that not only the AtMRP1-dependent component but also the AtMRP1-independent component of uptake is largely attributable to ABC transporters and not to secondary H+-coupled, V-ATPase-energized uptake.
FIGURE 10.
Time course of MgATP-dependent [3H]MTX uptake by vacuolar membrane-enriched vesicles purified from liquid root cultures of atmrp1-1 (•) and wild-type Arabidopsis WS plants (○). MgATP-dependent MTX uptake from media containing 50 μm [3H]MTX was estimated as described in Fig. 3. Values shown are means ± S.E. (n = 3).
DISCUSSION
The findings presented establish that heterologously expressed AtMRP1 is not only competent in the MgATP-dependent transport of GS-conjugates but also monoglutamylated folates, as exemplified by PteGlu1 and MTX, and that this is a property shared by vesicles derived from the vacuolar membrane of red beet storage root. Moreover, it is shown that whereas both of the knock-out mutant alleles of AtMRP1, atmrp1-1 and atmrp1-2 from Arabidopsis ecotypes WS and Col-0, respectively, are indistinguishable from their wild-type counterparts when grown under standard conditions or on solid media containing GS-conjugable xenobiotics, they are more sensitive to nanomolar concentrations of MTX in the growth medium. In the case of the mutant allele that was characterized further, atmrp1-1, MTX hypersensitivity is associated with impaired vacuolar transport and sequestration of this antifolate. Evidently, AtMRP1 and its functional equivalent in the vacuolar membrane of red beet translocate folate monoglutamates and antifolates in vitro and the former contributes to antifolate tolerance in vivo.
Estimates of the internal volume of red beet vacuolar membrane vesicles prepared in the same manner as in this study yield a value of 10 μl/mg membrane protein (10, 37). Assuming a 1:1 mixture of right-side-out and inside-out vesicles, uptake of ∼5 nmol/mg protein after 60 min from a medium containing 100 μm [3H]PteGlu1 amounts to an accumulation ratio of 10. The corresponding value for the uptake of [3H]NEM-GS by the same preparation is 16. Given that the transport capabilities of yeast vacuolar membrane-enriched vesicles containing heterologously expressed AtMRP1 are similar to and often greater than those of red beet vacuolar membrane vesicles, it is apparent that transport is against a moderately steep concentration gradient in both preparations.
The substrate selectivity of AtMRP1 strictly parallels those of the MRPs from mammalian sources that have been implicated in the transport of folates. Human MRPs 1 and 3, for instance, are high capacity, low affinity MTX and folate transporters exhibiting little or no affinity toward polyglutamates (7). MRP3 and several other human MRPs confer resistance to MTX in transfected cultured cells (8). Equivalently, AtMRP1 is a high capacity, intermediate affinity folate and MTX transporter exhibiting little or no activity toward polyglutamates; it mediates very low rates of [3H]PteGlun transport, and the inclusion of PteGlun in the uptake medium only weakly inhibits [3H]PteGlu1 uptake. Knock-out mutants for AtMRP1 are hypersensitive to MTX in the growth medium and deficient in its vacuolar sequestration.
AtMRP1 and its functional equivalents in other plants have the characteristics of MgATP-dependent vacuolar pumps capable of contributing to the detoxification of non-glutathionylated amphipathic anions. That this should be the case, however, raises the following question: why does AtMRP1 appear not to confer tolerance toward CDNB or metolachlor, a generic cytotoxin and herbicide, respectively, whose GS-conjugates are also in vitro transport substrates (12, 32) if it confers tolerance toward the transport substrate MTX?
The answer to this question is not known, but functional redundancy or a lack thereof may be an important consideration. Because only a subset of plant MRPs have appreciable folate and antifolate transport activity, the problems of functional redundancy associated with screens involving GS-conjugable xenobiotics, namely the ability of most MRPs to transport GS-conjugates, apply less to MTX. An alternate or supplementary explanation is that, unlike the screens deploying GS-conjugable xenobiotics, the efficacy of MTX as a screening agent is probably not rate-limited by upstream enzymes, such as GSTs, whose activity in the case of GS-conjugable xenobiotics would set an upper limit on the rate of formation of transport-active derivatives. In lieu of findings to the contrary, one other possibility that cannot be ruled out is that the importance of vacuolar sequestration has simply been overstated in that conjugation of GS-conjugable xenobiotics, alone, might be sufficient for the detoxification of many xenobiotics regardless of whether the conjugates remain in the cytosol or are transported into the vacuole (38–40).
The MTX hypersensitivity profiles of atmrp1 mutants are reminiscent of the properties of atmrp2 knock-out mutants. Despite the capacity of heterologously expressed AtMRP2 for high rates of GS-conjugate transport, the highest reported to date for any MRP regardless of source (16, 41), the most striking phenotype of atmrp2 mutants is not associated with GS-conjugate transport but instead with the vacuolar sequestration of chlorophyll catabolites (40), amphipathic anions whose transport, as exemplified by the linearized tetrapyrrole Brassica napus nonfluorescent chlorophyll catabolite 1 (Bn-NCC-1), does not depend on upstream GST action (41).
Another striking similarity between AtMRP1 and AtMRP2 is that both interact with both GS-conjugates and non-glutathionylated amphipathic anions but not in a simple competitive manner. Heterologously expressed AtMRP2 catalyzes the transport of GS-conjugates, Bn-NCC-1 and glucuronides such as 17β-estradiol 17-(β-d-glucuronide) (E217βG), but not in a manner consistent with the interaction of these three classes of substrate with a common binding or transport site (41). For instance, DNP-GS and Bn-NCC-1 exert little or no effect on each other's transport via AtMRP2; both are transported at similar rates regardless of whether the other transport substrate is in the uptake medium (41). Rather than competing with each other for uptake by AtMRP2, DNP-GS stimulates the uptake of E217βG and vice versa (16). The kinetics of AtMRP2-mediated transport are consistent with a scheme in which DNP-GS, E217βG, and Bn-NCC-1 undergo transport through different AtMRP2-dependent pathways and that DNP-GS and E217βG promote each other's transport by binding sites distinct from but tightly coupled to the other's transport pathway (13, 16). The behavior of AtMRP1 is not as pronounced as that of AtMRP2 in this regard. Nonetheless, the binding and/or transport sites for NEM-GS and PteGlu1 behave as if distinct, but coupled. NEM-GS noncompetitively inhibits PteGlu1 transport and vice versa, implying that the two substrates interact with nonidentical binding sites on the same functionality. This is not a property peculiar to AtMRP1 because the equivalent transport processes in red beet vacuolar membrane vesicles behave similarly.
If MRP-type transporters contribute to the accumulation of vacuolar folates in planta, they likely do so by catalyzing uptake of the monoglutamylated rather than the polyglutamylated component. Because polyglutamylated folates represent such a sizeable fraction of the total vacuolar folate pool, a pressing question that remains to be answered is how polyglutamylated folates make their appearance in this compartment? Must alternate mechanisms for the delivery of polyglutamates into the vacuole be invoked if neither heterologously expressed AtMRP1 nor red beet vacuolar membrane vesicles catalyze appreciable PteGlun uptake and the vacuolar lumen is devoid of FPGS and ATP (3), so precluding intravacuolar polyglutamylation of the incoming monoglutamates?
A subsidiary consideration that has a bearing on whether AtMRP1 and its equivalents in other plant vacuolar membranes actually transport folate monoglutamates in vivo are their relatively low affinities for these compounds in vitro. The Km values for PteGlu1 transport by heterologously expressed AtMRP1 and red beet vacuolar membrane vesicles (188 and 195 μm, respectively) are far higher than the probable steady state cytosolic concentrations of these compounds in plant cells. Assuming a typical folate content of 2 nmol/g fresh weight (42) of which 5% or less is monoglutamylated (3), it is likely that the steady state cytosolic concentration of PteGlu1 seldom exceeds 1 μm if the cytosol amounts to ∼34 μl/g fresh weight (43, 44), and about 20% of the total pool is allocated to this compartment (3). In short, if AtMRP1 and its equivalents are to participate in vacuolar folate sequestration, the conditions for their assay in vitro are far from optimal and/or the transporters in question operate far from their maximal capacity in vivo.
Notwithstanding these uncertainties, there can be little doubt of the relevance of AtMRP1 for the detoxification of antifolates despite its correspondingly low in vitro Km value for MTX (243 μm) in that atmrp1 knock-out mutants are hypersensitive to nanomolar concentrations of this compound. Moreover, even when exposed to only nanomolar concentrations of MTX, there is a marked difference between the capacity of wild-type and atmrp1 knock-out mutant protoplasts for vacuolar sequestration of this antifolate, which might imply that even when operating suboptimally MRP-type transporters can make a significant contribution to the intracellular distribution of these and related compounds.
That said, there is still appreciable MTX uptake into the vacuoles of atmrp1-1 mutant protoplasts, and in vitro measurements of the uptake of [3H]MTX by vacuolar membrane-enriched vesicles purified from atmrp1-1 root cultures reveal considerable residual vanadate-inhibitable MgATP-dependent uptake. Knowing that heterologously expressed AtMRP4 is capable of high rates of PteGlu1 and MTX transport (17) and that at least a fraction of total cellular AtMRP4 may localize to the vacuolar membrane (19), there is a possibility that this transporter also contributes to the vacuolar uptake of folyl monoglutamates.
It is instructive to note that because physiological folates compete with antifolates for binding to their target enzymes, dihydrofolate reductase and FPGS, any factor that influences physiological folate pool size could affect antifolate efficacy. It is therefore conceivable that a diminution of vacuolar folate uptake, as might be the case for atmrp1 knock-out mutants, would confer MTX hypersensitivity by comparison with wild types not only because of a decrease in the capacity for vacuolar MTX sequestration but also because of a decrease in cellular folate pool size.
Of the many facets of vacuolar folate uptake that have yet to be resolved, one that is especially perplexing is the seeming localization of folate polyglutamates and γ-glutamyl hydrolase (GGH) to the same compartment (3). If this is correct and the activity of GGH in vitro approximates its activity in vivo, the vacuolar folate polyglutamate pools of Arabidopsis and red beet would be predicted to have half-lives of only ∼7 s and 5 min, respectively, under steady state conditions (3). This paradox has yet to be reconciled, but a possibility that cannot be ignored is that it is not monoglutamates but instead polyglutamates that are transported into the vacuole in vivo by a non-MRP-type functionality, and that the former are derived from the latter intravacuolarly through the action of a vacuolar GGH, i.e. there may be parallels between what happens in plants and what happens in mammals, where lysosomes do not store folates but instead import folate polyglutamates, hydrolyze them, and export monoglutamates (45, 46). Alternatively, vacuolar folate polyglutamates are protected from intravacuolar hydrolysis through their interaction with FBPs or by the presence of a potent GGH inhibitor in this compartment. Although there is no experimental evidence for or against either possibility, the latter is the less likely in that the inhibition of vacuolar GGH would not only diminish the deglutamylation of folate polyglutamates but also the deglutamylation of p-aminobenzoate polyglutamates, which would disrupt folate recycling (3). Another possibility, perhaps the most straightforward one, is that folate polyglutamates, or at least a sizeable fraction of this pool, and GGH do no reside in precisely the same vacuolar compartment but instead localize to different intravacuolar compartments or different subpopulations of vacuoles in the same cells or different cells within the same or different tissues.
This work was supported by United States Department of Energy Grant DE-FG02-91ER20055 (to P. A. R.) and in part by National Institutes of Health Grant RO1 GM071382 (to A. D. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: THF, tetrahydrofolate; ABA, abscisic acid; ABC, ATP-binding cassette; AtMRP, A. thaliana MRP; Bn-NCC-1, Brassica napus nonfluorescent chlorophyll catabolite 1; Bistris propane, 1,3-bis[tris(hydroxymethyl)methylamino]propane; CDNB, 1-chloro-2,4-dinitrobenzene; DNP-GS, S-(2,4-dinitrophenyl)-GS; E217βG, 17β-estradiol 17-(β-d-glucuronide); FBP, folate-binding protein; FPGS, folylpolyglutamate synthetase; GGH, γ-glutamyl hydrolase; GST, glutathione S-transferase; HPLC, high performance liquid chromatography; Mes, 2-(N-morpholino)ethanesulfonic acid; MRP, multidrug resistance-associated protein; MTX, methotrexate; NEM-GS, N-ethylmaleimide-GS; p-ABA, p-aminobenzoate; Pte, pteroyl; PteGlu1, pteroyl monoglutamate; PteGlun, pteroyl polyglutamate; RT, reverse transcription; WS, Wassilewskia; Col-0, Columbia.
V. Naponelli, A. D. Hanson, and J. Gregory, unpublished data.
References
- 1.Stover, P. J. (2004) Nutr. Rev. 62 S3–S12 [DOI] [PubMed] [Google Scholar]
- 2.Hanson, A. D., and Gregory, J. F., III (2002) Curr. Opin. Plant Biol. 5 244–249 [DOI] [PubMed] [Google Scholar]
- 3.Orsomando, G., Díaz de la Garza, R., Green, B. J., Peng, M., Rea, P. A., Ryan, T. J., Gregory, J. F., III, and Hanson, A. D. (2005) J. Biol. Chem. 280 28877–28884 [DOI] [PubMed] [Google Scholar]
- 4.Bedhomme, M., Hoffmann, M., McCarthy, E. A., Gambonnet, B., Moran, R. G., Rébeillé, F., and Ravanel, S. (2005) J. Biol. Chem. 280 34823–34831 [DOI] [PubMed] [Google Scholar]
- 5.Klaus, S. M., Kunji, E. R., Bozzo, G. G., Noiriel, A., de la Garza, R. D., Basset, G. J., Ravanel, S., Rebeille, F., Gregory, J. F., III, and Hanson, A. D. (2005) J. Biol. Chem. 280 38457–38463 [DOI] [PubMed] [Google Scholar]
- 6.Zeng, H., Liu, G., Rea, P. A., and Kruh, G. D. (2000) Cancer Res., 60 4779–4784 [PubMed] [Google Scholar]
- 7.Zeng, H., Chen, Z. S., Belinsky, M. G., Rea, P. A., and Kruh, G. D. (2001) Cancer Res. 61 7225–7232 [PubMed] [Google Scholar]
- 8.Kruh, G. D., Zeng, H., Rea, P. A., Liu, G., Chen, Z. S., Lee, K., and Belinsky, M. G. (2001) J. Bioenerg. Biomembr. 33 493–501 [DOI] [PubMed] [Google Scholar]
- 9.Martinoia, E., Grill, E., Tommasini, R., Kreuz, K., and Amrhein, N. (1993) Nature 364 247–249 [Google Scholar]
- 10.Li, Z. S., Zhao, Y., and Rea, P. A. (1995) Plant Physiol. 107 1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa, T., Li, Z.-S., Lu, Y.-P., and Rea, P. A. (1997) Biosci. Rep. 17 189–208 [DOI] [PubMed] [Google Scholar]
- 12.Rea, P. A., Li, Z. S., Lu, Y. P., Drozdowicz, Y. M., and Martinoia, E. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 727–760 [DOI] [PubMed] [Google Scholar]
- 13.Rea, P. A. (2007) Annu. Rev. Plant Biol. 58 347–375 [DOI] [PubMed] [Google Scholar]
- 14.Verrier, P. J., Bird, D., Burla, B., Dassa, E., Forestier, C., Geisler, M., Klein, M., Kolukisaoglu, U., Lee, Y., Martinoia, E., Murphy, A., Rea, P. A., Samuels, L., Schulz, B., Spalding, E. J., Yazaki, K., and Theodoulou, F. L. (2008) Trends Plant Sci. 13 151–159 [DOI] [PubMed] [Google Scholar]
- 15.Geisler, M., Girin, M., Brandt, S., Vincenzetti, V., Plaza, S., Paris, N., Kobae, Y., Maeshima, M., Billion, K., Kolukisaoglu, U. H., Schulz, B., and Martinoia, E. (2004) Mol. Biol. Cell 15 3393–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, G., Sánchez-Fernández, R., Li, Z. S., and Rea, P. A. (2001) J. Biol. Chem. 276 8648–8656 [DOI] [PubMed] [Google Scholar]
- 17.Rea, P. A., Sánchez-Fernández, R., Chen, S., Peng, M., Klein, M., Geisler, M., and Martinoia, E. (2003) in ABC Transporters from Bacteria to Humans (Cole, S. P., Kuchler, K., Higgins, C., and Holland, B., eds) pp. 335–355, Academic Press, Sheffield, UK
- 18.Klein, M., Geisler, M., Suh, S. J., Kolukisaoglu, H. U., Azevedo, L., Plaza, S., Curtis, M. D., Richter, A., Weder, B., Schulz, B., and Martinoia, E. (2004) Plant J. 39 219–236 [DOI] [PubMed] [Google Scholar]
- 19.Dunkley, T. P. J., Hester, S., Shadforth, I. P., Runions, J., Weimar, T., Hanton, S. L., Griffin, J. L., Bessant, C., Brandizzi, F., Hawes, C., Watson, R. B., Dupree, P., and Lilley, K. S. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 6518–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravanel, S., Cherest, H., Jabrin, S., Grunwald, D., Surdin-Kerjan, Y., Douce, R., and Rébeillé, F. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 15360–15365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farré, E. M., Tiessen, A., Roessner, U., Geigenberger, P., Trethewey, R. N., and Willmitzer, L. (2001) Plant Physiol. 127 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rea, P. A., and Turner, J. C. (1990) Methods Plant Biochem. 3 385–405 [Google Scholar]
- 23.Bartholomew, D. M., Van Dyk, D. E., Lau, S. M., O'Keefe, D. P., Rea, P. A., and Viitanen, P. V. (2002) Plant Physiol. 130 1562–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean, J. V., and Mills, J. D. (2004) Physiol. Plant. 120 603–612 [DOI] [PubMed] [Google Scholar]
- 25.Royal Society of Chemistry/MAFF (1991) McCance and Widdowson's The Composition of Foods, 5th Ed., pp. 237–290, Royal Society of Chemistry/Ministry of Agriculture, Fisheries, and Food, Cambridge, UK
- 26.Naponelli, V., Hanson, A. D., and Gregory, J. F., III (2007) Anal. Biochem. 371 127–134 [DOI] [PubMed] [Google Scholar]
- 27.Iliás, A., Urbán, Z., Seidl, T. L., Le Saux, O., Sinkó, E., Boyd, C. D., Sarkadi, B., and Váradi, A. (2002) J. Biol. Chem. 277 16860–16867 [DOI] [PubMed] [Google Scholar]
- 28.Feldmann, K. A. (1991) Plant J. 1 71–82 [Google Scholar]
- 29.Szczypka, M. S., Wemmie, J. A., Moye-Rowley, W. S., and Thiele, D. J. (1994) J. Biol. Chem. 269 22853–22857 [PubMed] [Google Scholar]
- 30.Gietz, R. D., and Schiestl, R. H. (1991) Yeast 7 253–263 [DOI] [PubMed] [Google Scholar]
- 31.Kim, E. J., Zhen, R. G., and Rea, P. A. (1995) J. Biol. Chem. 270 2630–2635 [DOI] [PubMed] [Google Scholar]
- 32.Lu, Y. P., Li, Z. S., and Rea, P. A. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 8243–8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, S., and Halkier, B. A. (2000) J. Biol. Chem. 275 22955–22960 [DOI] [PubMed] [Google Scholar]
- 34.Wilkins, T. A., Bednarek, S. Y., and Raikhel, N. V. (1990) Plant Cell 2 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders, J. A., and Conn, E. E. (1978) Plant Physiol. 61 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradford, M. M. (1976) Anal. Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- 37.Poole, R. J., Mehlhom, R. J., and Packer, L. (1985) in Biochemistry and Function of Adenosine Triphosphatase in Fungi and Plants (Marin, B., ed) pp. 114–118, Springer-Verlag, Berlin
- 38.Kreuz, K., Tommasini, R., and Martinoia, E. (1996) Plant Physiol. 111 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman, J. O. D., Blake-Kalff, M. M. A., and Davies, T. G. E. (1997) Trends Plant Sci. 2 144–151 [Google Scholar]
- 40.Frelet-Barrand, A., Kolukisaoglu, H. U., Plaza, S., Rüffer, M., Azevedo, L., Hörtensteiner, S., Marinova, K., Weder, B., Schulz, B., and Klein, M. (2008) Plant Cell Physiol. 49 557–569 [DOI] [PubMed] [Google Scholar]
- 41.Lu, Y. P., Li, Z. S., Drozdowicz, Y. M., Hortensteiner, S., Martinoia, E., and Rea, P. A. (1998) Plant Cell 10 267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyer, A., Collakova, E., Díaz de la Garza, R., Quinlivan, E. P., Williamson, J., Gregory, J. F., III, Shachar-Hill, Y., and Hanson, A. D. (2005) J. Biol. Chem. 28 26137–26142 [DOI] [PubMed] [Google Scholar]
- 43.Winter, H., Robinson, D., and Heldt, H. W. (1994) Planta 193 530–535 [Google Scholar]
- 44.Musgrave, M. E., Kuang, A., Brown, C. S., and Matthews, S. W. (1998) Ann. Bot. 81 503–512 [DOI] [PubMed] [Google Scholar]
- 45.Sirotnak, F. M., and Tolner, B. (1999) Annu. Rev. Nutr. 19 91–122 [DOI] [PubMed] [Google Scholar]
- 46.Barrueco, J. R., O'Leary, D. F., and Sirotnak, F. M. (1992) J. Biol. Chem. 267 15356–15361 [PubMed] [Google Scholar]