Abstract
Activation transcription factor-2 (ATF-2) is phosphorylated by various protein kinases, such as JNK/p38/ERK, calmodulin kinase IV, protein kinase A, and protein kinase C (PKC), in response to a variety of stimuli. However, the role of the phosphorylation of ATF-2 by PKC in vivo in the transcriptional control of genes that include the activation protein-1 (AP-1)/cyclic AMP-response element remains to be defined. Using antibodies against the phosphorylated serine residue (Ser(P)) at position 121 of ATF-2, we have demonstrated that PKC phosphorylates ATF-2 at Ser-121 and that phosphorylation of Ser-121 (to yield ATF-2pS121) becomes detectable at the late stage of the response of HeLa cells to 12-O-tetradecanoylphorbol-13-acetate (TPA) and is maintained for more than 2 h. By contrast, phosphorylation of ATF-2 at threonine residues 69 and 71 (Thr-69/71, to yield ATF-2pT69/71) and at Ser-340 and Ser-367 (to yield ATF-2pS340 and ATF-2pS367) is detectable as an immediate early response. Unlike levels of ATF-2pT69/71 and ATF-2pS340, the level of ATF-2pS121 increases in the nuclei of HeLa cells in response to TPA. A serine-to-alanine mutation at position 121 of ATF-2 represses the c-Jun-dependent transcription of AP-1/cyclic AMP-response element reporter genes and also the p300-mediated activation of a Gal4-reporter gene in response to TPA. Our results suggest that the phosphorylation of ATF-2 at Ser-121 plays a key role in the c-Jun-mediated activation of transcription that occurs in response to TPA.
The phosphorylation of transcription factors is critical for the expression of genes that are involved in the proliferation, differentiation, and survival of cells, and such phosphorylation can be induced by many types of stimuli, such as growth factors, stressors, and viral infection (1, 2). Moreover, phosphorylated transcription factors are not only involved in a variety of biological reactions in molecular association with other nuclear factors and cofactors, they are also involved in the degradation and oligomerization of proteins for transmission of signals to the nucleus (1, 2).
Activating transcription factor-2 (ATF-2)3 is a member of the family of ATF/cAMP-response element-binding proteins and is characterized by a basic zipper domain that consists of basic amino acids and a leucine zipper region that acts as a DNA-binding region. These members of the ATF-2 family have been identified to date, namely ATF-2, ATF-7 (ATFa), and CREBPa (3). ATF-2 regulates gene expression via the binding of ATF-2, as a homodimer, to the recognition sequences of the cAMP-response element (CRE) or via formation of a heterodimer with AP-1 (activation protein-1) that binds to CRE sequences. Thus, ATF-2 can interact with other members of the ATF family and with members of the AP-1 family (4). The heterodimerization of ATF-2 with c-Jun is critical for the nuclear localization of c-Jun-ATF-2 heterodimers and the transcriptional activity of this complex (5). Studies of ATF-2-null mice have shown that ATF-2 is essential to animal development (6, 7), and recent reports have shown that the gene for ATF-2 is a tumor-susceptibility gene associated with mammary tumors (8, 9) and also plays a role in suppression of skin cancer (10).
Stress-activated protein kinases, such as Jun NH2-terminal protein kinases 1 and 2 (JNK1 and JNK2) and p38, phosphorylate ATF-2 at Thr-69 and Thr-71 (to yield ATF-2pT69/71), which are close to its amino-terminal transcriptional activation domain, and in this way they enhance its transactivation capacity (11–13) in response to stresses such as UV light, osmotic stress, hypoxia, and inflammatory cytokines (14). It has been also proposed that activation of ATF-2 by p38/JNK might play a role in apoptosis (15). A recent study of “knock-in” mice showed that phosphorylation of both Thr-69 and Thr-71 of ATF-2 by p38 kinase is required for the initiation of anti-apoptotic activity in embryonic liver and heart in conjunction with ATF-7 (16).
Both insulin and serum can activate ATF-2 via a two-step mechanism that involves two distinct Ras effector pathways as follows: the phosphorylation (p-) of Thr-71 via the Ras-Raf-MEK-ERK pathway, and the subsequent phosphorylation of Thr-69 via the Ras-RalGDS-Ral-Src-p38 pathway (17). Phosphorylation of Thr-69 and Thr-71 stabilizes ATF-2 and modulates AP-1/CRE-dependent transcription (18, 19). Thus, ATF-2 is responsive to a variety of external and internal stimuli, which range from mitogenic signals that are mediated via ERK to stress signals that are mediated via p38 (11–14).
It was reported several years ago that induction of transcription of the c-jun gene in response to 12-O-tetradecanoylphorbol-13-acetate (TPA) is mediated by the upstream AP-1-like site (jun2 site) in the promoter of the c-jun gene (20–22). Moreover, a TPA-induced protein kinase, which is distinct from stress-activated protein kinases, phosphorylates c-Jun but not ATF-2 in HeLa cells (12, 23). However, phosphorylated amino acid residues in ATF-2 and the protein kinase responsible for the TPA-mediated phosphorylation of ATF-2 remain to be identified.
We reported previously that ATF-2 associates with p300 to regulate the differentiation-responsive element-dependent transcription of the c-jun gene during the retinoic acid-induced (RA-induced) differentiation of F9 cells (24, 25) (the differentiation-response element (DRE) resembles an AP-1-like sequence, a CRE-like sequence, and a TPA-responsive element). However, the molecular mechanisms underlying the kinase-mediated activation of transcription of the c-jun gene and the molecular signaling associated with activation of RA signals via PKC remain to be clarified.
We report here that the phosphorylation of the serine residue at position 121 of ATF-2, catalyzed by at least PKCα, is one aspect of the response to TPA, and this phosphorylation is involved in the regulated transcription of genes that include an AP-1-like or CRE-like sequence.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies—A series of human ATF-2 expression vectors derived from pcDNA3-FLAG (Invitrogen), pET15b (Novagen, Darmstadt, Germany), pGEX-4T-1 (GE Healthcare), and pFR-Luc (Stratagene, La Jolla, CA) were obtained from the RIKEN DNA Bank (Tsukuba, Ibaraki, Japan). Plasmids that encoded FLAG-ATF-2WT (wild type) and Gal4-ATF-2WT were used to generate the corresponding alanine substitution mutants, pcDNA-FLAG-ATF-2T69/71A, pcDNA-FLAG-ATF-2S121A, pcDNA-FLAG-ATF-2S340A, pcDNA-FLAG-ATF-2S367A, pGal4-ATF-2S121A, pGal4-ATF-2S340A, pGal4-ATF-2S367A and pGal4-ATF-2T69/71A by site-directed mutagenesis (24, 25). To generate His-tagged proteins and glutathione S-transferase (GST) fusion proteins, cDNAs that encoded ATF-2WT and ATF-2S121A and cDNAs that encoded ATF-2WT, ATF-2-(1–125), ATF-2S121A, ATF-2-(1–125)T69/71A, ATF-2-(1–125)-T69/71A, S121A, and ATF-2-(1–125)-S121A were generated by ligation of the full-length coding sequence, and PCR-amplified DNA that corresponded to a mutated site(s) into the respective vectors, inframe, and proteins fused to GST were purified as described previously (24, 25). Amino acid substitutions were verified by nucleotide sequencing. The CRE-luc construct was generated by amplification of CRE from CRE-tk-CAT (provided by Dr. M. R. Montiminy, Salk Institute, San Diego) and ligation to a Renilla reporter vector (Promega, Madison, WI). DRE-luc, mDRE-luc, and mCRE-luc were prepared as described elsewhere (25). pGal4-p300N, pGal4-p300M, pGal4-p300C, jun2-luc (encoding firefly luciferase), pGal4 tk-luc, pGal4-ATF-2WT, pCMV-FLAG-c-junWT, pCMV-FLAG-c-jun(S63A/S73A), pCMV-FLAG-PKCα, and pCMV-FLAG-PKCαKD (“kinase dead”) were kindly donated by Dr. Y. Shi (Harvard Medical School, Boston), Dr. H. van Dam (University of Leiden, Leiden, The Netherlands), Dr. S. Ohno (Yokohama City University, Yokohama, Japan), and Dr. K. Iwai (Osaka University, Osaka, Japan). The PKC inhibitor Gö6976, the p38 inhibitor SB202190, and the control compound SB202474 were obtained from Calbiochem. Rabbit polyclonal antibodies specific for human ATF-2 (N-96), for phosphorylated human ATF-2 (Thr(P)-69/71), for human PKCα, PKCβI, and PKCβII, for human β-actin, and horseradish peroxidase-IgG-F(ab)2 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and mouse monoclonal antibody against phosphorylated human ATF-2 (Thr(P)-71) was purchased from Cell Signaling Technology (Beverly, MA). Antibodies against ATF-2pS490/498 and FITC-IgG-F(ab′)2 were obtained from Colorado Bioscience (Aurora, CO) and BioSource International Inc. (Camarillo, CA), respectively. TRITC-IgG and FITC-IgG second antibodies and antibodies against FLAG (M2) were purchased from Sigma.
Preparation of Antibodies against Phosphorylated Serine Residues at Positions 121, 340, and 367 of ATF-2—Antibodies against phosphorylated peptides that included, respectively, a phosphorylated amino acid residue at positions 121, 340, and 367 of ATF-2 were raised in rabbits, which were immunized with keyhole limpet hemocyanin-conjugated phosphopeptides (NH2-TPIIRpSKIEEC-COOH (TB0608; 116–125), NH2-QTQSTpSGRRRC-COOH (TB0609; 335–344), and NH2-NRAAApSRC-COOH (TB0610; 362–368)). The antibodies were affinity-purified on columns prepared with the respective immobilized phosphopeptides according to the protocol from the manufacturer (MBL, Inc., Ina, Nagano, Japan).
Cell Culture—Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 units of penicillin plus streptomycin and glutamine (Invitrogen) at 100 μg per ml each (25). Cells were serum-starved in DMEM plus 0.5% FBS for 12–16 h at 37 °C in an atmosphere of 5% CO2 in air before stimulation. Then cells were treated with TPA (Sigma) at 60–200 nm for 6 h at 37 °C. Induction of the differentiation of F9 cells by RA was performed as described elsewhere (25). Primary mouse embryonal fibroblasts (MEF) derived from mice with null alleles for ATF-2 and ATF-7 or for ATF-2 only were generated from E11.5 and E12.5 embryos that were derived from crosses of heterozygous animals as follows: ATF-2+/-/ATF-7+/- × ATF-2+/-/ATF-7+/- (16).
Western Blotting—HeLa cells or HeLa-S cells were serum-starved, incubated with TPA (60–200 nm) for 6 h and lysed in RIPA buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 25 mm NaF, 25 mm β-glycerophosphate, and 0.1 mm Na3VO4) that contained a protease inhibitor mixture (Roche Diagnostics). After incubation for 30 min on ice and centrifugation at 15,000 rpm for 15 min, supernatants were saved and analyzed by SDS-PAGE (4–12% polyacrylamide) and Western blotting with the appropriate phosphorylation-specific antibodies (see above). In some experiments, cells were washed three times for 3 min each at 4 °C with CSK (cytoskeleton) buffer, which contained 10 mm PIPES, pH 6.8, 100 mm NaCl, 300 mm sucrose, 7 mm MgCl2, 1 mm EDTA, 0.5% Triton X-100, 1 mm Na3VO4, 50 mm NaF, and 20 mm β-glycerophosphate, and then lysed in RIPA buffer as described above. For peptide blocking, antibodies against ATF-2pS121 (1:100) were preincubated with or without 200 μg/ml Ser(P)-121 phosphorylated peptide (TB0608-1; MBL) or 200 μg/ml Ser-121 nonphosphorylated peptide (TB0608-2; MBL). The antibodies against ATF-2pS340 (1:100) were incubated with or without 200 μg/ml Ser(P)-340 phosphorylated peptide (TB0609-1; MBL) or 200 μg/ml Ser-340 nonphosphorylated peptide (TB0609-2; MBL). The antibodies against ATF-2pS367 (1:100) were incubated with or without 200 μg/ml Ser(P)-367 phosphorylated peptide (TB0610-1; MBL) or 200 μg/ml Ser-367 nonphosphorylated peptide (TB0610-2; MBL), for 60 min at room temperature. Phosphatase inhibitors (1 mm Na3VO4, 50 mm NaF, and 20 mm β-glycerophosphate) were included during blotting procedures.
Transfection and Assays of Luciferase Activity—From 2 to 5 × 104 HeLa cells, F9 cells, and MEF, respectively, were subcultured in 500 μl of DMEM plus 10% FBS in individual wells of 24-well plates (Greiner-Japan, Tokyo, Japan) and in collagen-coated or gelatin-coated wells of 24-well plates (IWAKI, Tokyo, Japan). The indicated amount of pcDNA-FLAG-ATF2WT or of one of its mutated derivatives and 0.4 μg of AP-1-luc or 0.2 μg of CRE-luc and pBSK-II(-) (to give a total final amount of DNA of 1 μg) were used for transfections with Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's protocol. Six hours after transfection, fresh DMEM plus 0.5% FBS was added, and incubation was continued for another 16 h. Cells were then exposed to 60 nm TPA for 6 h and assayed for luciferase activity with a Renilla (for CRE-luc) or a firefly (for AP-1-luc) luciferase reporter assay kit (Promega). Inhibitors Gö6976 (10 μm), SB202190 (20 μm), and SB202474 (20 μm), as indicated, were added to the culture medium 5 h before the treatment with RA or TPA. In mammalian two-hybrid assays, the luciferase reporter (pFR-Luc; 400 ng) was introduced into F9 cells, HeLa cells, or MEF with the pGal4-ATF-2 expression vector (100 or 200 ng) and the internal control pRL-CMV (100 ng), as well as pBSK-II(-) (to give a total final amount of DNA of 1 μg) by the Lipofectamine™ method (see above). Luminescence was measured immediately with a luminometer (AROVO™ Light, model 1420; PerkinElmer Life Sciences). Luciferase activity was normalized by reference to the protein concentration of the sample, as determined with a protein assay system from Bio-Rad with bovine serum albumin as the standard. At least three cell-culture wells were subjected to each treatment. All experiments were performed as least three times.
Immunofluorescence—HeLa cells were cultured in Iscove's modified DMEM (Invitrogen) plus 5% FBS. For stimulation of phosphorylation at Ser-121 of ATF-2, cells were treated with 200 nm TPA for 30 min. PKC-mediated phosphorylation of serine was blocked by treatment with 10 μm Gö6976 (PKC inhibitor; Calbiochem) for 30 min prior to the treatment with TPA. Confocal and Nomarski differential interference contrast images were obtained with a confocal laser scanning microscope with a 40× 1.00 NA oil immersion objective (Fluoview FV500; Olympus, Tokyo; see Ref. 26). Cells were treated in situ with 0.5% Triton X-100 at 4 °C for 3 min prior to fixation in 4% paraformaldehyde for 20 min (27), and they were permeabilized in phosphate-buffered saline (PBS) plus 0.1% saponin and 3% bovine serum albumin at room temperature (28). Cells were stained with primary antibody for 1 h, washed with PBS plus 0.1% saponin, and then stained with FITC- or TRITC-conjugated secondary antibody for 1 h. For DNA staining, cells were treated with 200 μg/ml RNase A for 30 min and 20 μg/ml propidium iodide (PI) for 30 min. Stained cells were mounted with ProLong™ antifade reagent (Molecular Probes, Eugene, OR). For peptide blocking, antibodies against ATF-2pS121 (1:100) were preincubated with or without 200 μg/ml Ser(P)-121 phosphorylated peptide (TB0608-1; MBL) or 200 μg/ml Ser-121 nonphosphorylated peptide (TB0608-2; MBL). The antibodies against ATF-2pS340 (1:100) were preincubated with or without 200 μg/ml Ser(P)-340 phosphorylated peptide (TB0609-1; MBL), 200 μg/ml Ser-340 nonphosphorylated peptide (TB0609-2; MBL) or 200 μg/ml Ser(P)-121 phosphorylated peptide (TB0608-1; MBL) for 30 min at room temperature. Phosphatase inhibitors (1 mm Na3VO4, 50 mm NaF, and 20 mm β-glycerophosphate) were included during in situ extraction and peptide blocking. One planar (xy) section slice (thickness, 1.0 μm) was examined in each replicate of each experiment. To ensure that there was no bleed through from the fluorescein signal into the red channel, fluorescein and/or PI and rhodamine were excited independently at 488 and 543 nm, respectively (29). The mean fluorescence intensity of phosphorylated ATF-2 in nuclei was measured with Fluoview version 4.3 software (Olympus).
Statistical Analysis—Statistical significance was determined by Student's t test for experiments with two groups and by an analysis of variance followed by Fisher's protected least significant difference test for experiments with more than two groups. A value of p < 0.05 was considered to represent statistical significance.
For phosphorylation of His6-ATF-2WT, His6-ATF-2S121A, and synthetic peptides by in vitro PKC; gene silencing with siRNA and real time reverse transcription-PCR; and assays of kinase activity in vitro, immunokinase assays and phosphatase treatment, see the supplemental material.
RESULTS
PKC Is Involved in AP-1/CRE-dependent Transcription during the Retinoic Acid-induced Differentiation of F9 Cells—We reported previously that phosphorylation of ATF-2 by PKC is critical for DRE-dependent transcription during the differentiation of F9 cells in response to RA or adenovirus E1A (24). Talmage and co-workers (30, 31) showed that PKCα and PKCβ exhibit different patterns of induced expression and that each plays a distinct role during such RA-induced differentiation. Moreover, RA triggers the transition of expression from PKCβ to PKCα in differentiated F9 cells. However, protein kinase Cα is not sufficient for induction of the full differentiation of F9 cells but is involved in pathways that lead to the expression of differentiation-associated genes (32). To investigate the roles of PKC in AP-1/CRE-dependent transcription during the response to RA, we used siRNAs to suppress the expression of genes for PKCα, PKCβI, and PKCβII. As shown in Fig. 1A, the expression of a CRE reporter gene was decreased 6- and 5-fold by siRNA specific for PKCα and by siRNA specific for PKCβI, respectively, during the response of F9 cells to RA. However, expression of the reporter gene was unaffected by siRNA directed against PKCβII and by the negative control siRNA (random siRNA). We observed a similar response by the RA-inducible AP-1 reporter gene with siRNAs against PKCα and against PKCβI but not against PKCβII (Fig. 1B). Each siRNA reduced the expression of the respective endogenous PKC, but the control siRNA did not (Fig. 1C). Thus, at least the signal cascade of PKCα and PKCβI appeared to be involved in the AP-1/CRE-related response of F9 cells that are exposed to RA. To investigate in further detail the effect of each siRNA on the RA-induced differentiation of F9 cells, as described above, we treated cells with 1 μm RA to induce differentiation and then monitored morphological changes. Compared with morphological changes in cells that had been pretreated with control siRNA (negative control), morphological changes in cells that had been pretreated with siRNAs against PKCα and against PKCβI were significantly delayed or absent. By contrast, changes in cells pretreated with siRNA against PKCβII were unaffected (Fig. 1D).
FIGURE 1.
Effects of siRNA directed against PKC on RA-dependent transcription of an AP-1/CRE-luciferase reporter gene in F9 cells. A, CRE reporter assay. 2 × 104 F9 cells were transfected with 20 (odd lanes) and 40 pmol (even lanes) of control siRNA (negative control, N.C.), PKCα-specific siRNA, PKCβI-specific siRNA, and PKCβII-specific siRNA, respectively, plus 200 ng of CRE-luc, and then they were exposed to RA, as described in the text. After exposure to RA for 24 h, cells were assayed for luciferase activity. Values from a representative experiment are given as means ± S.E. (n = 3). B, AP-1 reporter assay. 2 × 104 F9 cells were transfected similarly with 20 (odd lanes) and 40 pmol (even lanes) of siRNA, as described above, plus 200 ng of AP-1-luc. After exposure to RA for 24 h, cells were harvested for assays of luciferase activity. Values from a representative experiment are given as means ± S.E. (n = 3). C, inhibition of the expression of PKCs by the respective siRNAs. 2 × 104 F9 cells were transfected with 40 pmol of an active siRNA or the control siRNA together with reporter plasmids. After treatment of cells for 24 h with RA, cells extracts were analyzed by SDS-PAGE (4–12% acrylamide) and Western blotting (WB) with respective antibodies (Anti-), as indicated on the right and described in the text. Lane 1, without siRNA; lane 2, with siRNA; lane 3, with negative control; and lane 4, RA-treated F9 cells. D, knockdown of PKC suppresses RA-mediated morphological changes in F9 cells that are associated with differentiation. F9 cells that had been treated with indicated siRNAs directed against expression of PKCα, PKCβI, and PKCβII, as well as with the negative control, were incubated with 1 μm RA. The cells were photographed 72 h after the start of exposure to RA under a phase-contrast microscope (original magnification, ×125). E, quantitation of levels of transcripts of RA-inducible genes and of genes that are markers of cell differentiation. The relative levels of expression of genes for laminin B1 and collagen 4α1 in F9 cells that had been treated with indicated siRNAs, with or without treatment with RA (1 μm), were obtained by determining Ct values from the standard curve. All experiments were performed in triplicate (results are means ± S.D.), and negative controls without template RNA were always included in each experiment. All results were normalized by reference to the values for glyceraldehyde-3-phosphate dehydrogenase mRNA. F, effects of inhibitors of PKC and p38 on expression of AP-1/CRE reporter genes. We examined the effects of Gö6976 and SB202190 on the TPA-induced transcription of AP-1/CRE-reporter genes. CRE-luc, AP-1-luc, or the mutant derivative (mCRE-luc and mAP-1-luc) reporters (0.2 μg) and pBSK II(-) plasmid, included to give a total amount of DNA equal to 1 μg, were used for transfection of HeLa cells as indicated in the text. Starved cells were pretreated with Gö6976 (10 μm), SB202190 (20 μm), or the control compound SB202474 (20 μm) for 2 h prior to treatment with 150 nm TPA or DMSO (vehicle), as a control, for 6 h. Luciferase activities were measured. All experiments were performed three times, and average results with standard deviations are shown.
We next compared, by real time reverse transcription-PCR, the levels of expression of RA-inducible genes and of genes whose expression is a marker of differentiation and nondifferentiation after the exposure of cells to 1 μm RA. The levels of the induced expression of genes for laminin B1 and collagen type 4α1, two markers of endodermal differentiation, were 85–90% lower in cells that had been pretreated with siRNAs against PKCα and against PKCβI than in control cells 72 h after treatment with RA (Fig. 1E). These results indicated that the “knockdown” of PKCα and PKCβI inhibited the RA-induced differentiation of F9 cells.
We also studied the effects of the PKC inhibitor Gö6976 and the p38 inhibitor SB202190 on the transcription of AP-1/CRE reporter genes in F9 cells. Fig. 1F shows that pretreatment with an inhibitor for 2 h prior to the exposure of cells to RA resulted in 50–70% lower transcription of the respective reporter genes, as compared with transcription in the presence of the control reagent (SB202474). These results indicated that pathways involving PKC and p38 might both be involved in the transcription of AP-1/CRE reporter genes during the differentiation of F9 cells in response to RA.
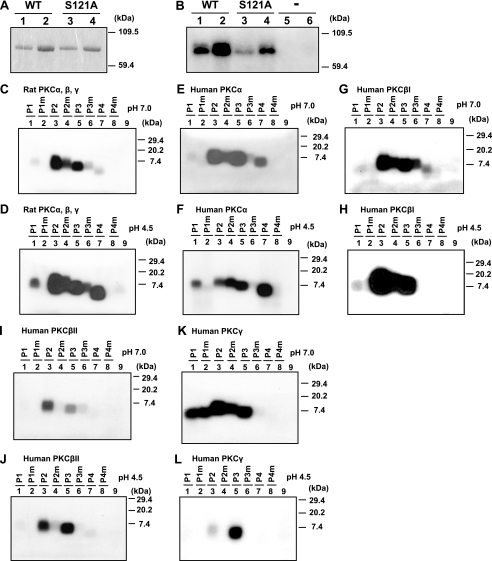
Phosphorylation of ATF-2 at Amino Acid Residue 121—To examine the phosphorylation of ATF-2 by PKC in vitro, we used recombinant ATF-2 and corresponding peptides that included the possible sites of phosphorylation in ATF-2. Sakurai et al. (33) reported that serine residues at positions 340 and 367 are critical for PKCβ-induced phosphorylation in vitro. By contrast, we showed previously that the serine residue at position 121 is crucial for PKCα-induced phosphorylation and p300-dependent transcription (24). However, the exact roles of these residues in ATF-2 remain unknown. We first examined the relative levels of phosphorylation by PKC of the recombinant wild-type His-ATF-2 protein (His6-ATF-2WT) and a serine-to-alanine mutant at residue 121 (His6-ATF-2S121A). As shown in Fig. 2, A and B, the extent of phosphorylation by rat PKC of His6-ATF-2S121A was 30–50% lower than that of His6-ATF-2WT. We also observed a similar relative reduction in phosphorylation of His6-ATF-2S121A by human PKCα and rabbit PKCα (data not shown). Our results indicated that the serine residue of position 121 (Ser-121) of ATF-2 is a possible site of phosphorylation by PKC. We next examined the phosphorylation of peptides that corresponded to positions 121, 340, and 367 of ATF-2, in vitro, at pH 7.0 and at pH 4.5 (Fig. 2, C–L). In the analysis of the phosphorylation of Ser-121 of ATF-2 by PKC, we included peptide P4, which did not include the two glutamic acid residues normally found at positions 124 and 125, adjacent to Ser-121, because these glutamic acid residues are negatively charged at neutral pH, and such charged glutamic acid residues might prevent access by PKC (34). We examined peptides P1 and P4 as substrate peptides at both pH 7.0 and pH 4.5. Conventional PKCs, such as α, βI, and βII, and PKCs from human and rat were all able to phosphorylate Ser-121 of human ATF-2 to some extent. The extent of phosphorylation of peptides that included Ser-121 was 5–10-fold lower than that of peptides that included Ser-340 or Ser-367. At acidic pH (pH 4.5), the phosphorylation of Ser-121 was significantly enhanced in some cases in the presence of the glutamic acid residues at positions 124 and 125, adjacent to Ser-121. We found that Ser-121 was clearly phosphorylated in vitro by all tested PKCs with the exception of PKCγ.
FIGURE 2.
PKC phosphorylates recombinant ATF-2 at Ser-121. A and B, His-ATF-2 and its mutant derivative (S121A; 1.0 and 0.5 μg) were incubated with 15 ng of rat PKC for 10 min at 30 °C. The phosphorylation reaction was performed as described in the text. A, proteins were then fractionated by SDS-PAGE (10% acrylamide). ATF-2 was visualized by staining with Coomassie Brilliant Blue. Lanes 1 and 2, 0.5 and 1.0 μg of wild-type (WT) His-ATF-2 protein; lanes 3 and 4, 0.5 and 1.0 μg of mutated His-ATF-2 (S121A). B, phosphorylation of His-ATF-2 and its mutant derivative (S121A) by PKC. Lanes 1 and 2, 0.5 and 1.0 μg of wild-type His-ATF-2; lanes 3 and 4, 0.5 and 1.0 μg of His-ATF-2 (S121A); lanes 5 and 6, no added protein substrate. C–L, phosphorylation of synthetic peptides by PKC. Individual peptides (1.25 pmol), encompassing the serine residues at positions 121 (Ser-121), 340 (Ser-340), and 367 (Ser-367), were incubated with 15 ng of rat protein kinase C (C and D), and with recombinant human protein kinase Cα (E and F), βI(G and H), βII (I and J), and γ (K and L), in the respective and appropriate PKC buffers (50 mm Tris-HCl buffer adjusted to pH 7.0 (C, E, G, I, and K) and 50 mm sodium citrate buffer adjusted to pH 4.5 (D, F, H, J, and L)) for 10 min at 30 °C. The PKC-catalyzed phosphorylation reactions were performed according to the instructions from the manufacturer of the enzymes (Upstate Biotechnology Inc.). The resultant phosphorylated peptides were fractionated by SDS-PAGE (18% polyacrylamide). In all panels, lane 1, Ser-121 peptide (amino acid residues 111–130; P1); lane 2, mutant derivative of Ser-121 peptide (S121A; P1m); lane 3, Ser-340 peptide (amino acid residues 332–351; P2); lane 4, mutant derivative of Ser-340 peptide (S340A; P2m); lane 5, Ser-367 peptide (amino acid residues 357–376; P3); lane 6, mutant derivative of Ser-367 peptide (S367A; P3m); lane 7, Ser-121 peptide (amino acid residues 111–123; P4); lane 8, mutant derivative of Ser-121 peptide (S121A; P4m); and lane 9, no peptide added as substrate.
Antibodies Directed against Phosphorylated Ser-121, Ser-340, and Ser-367—We generated antibodies that specifically recognized ATF-2 that had been phosphorylated at positions 121, 340, and 367, respectively (24, 33) (Fig. 3A). Bands of proteins of ∼68–70 kDa were detected after SDS-PAGE of extracts of HeLa cells with antibodies against ATF-2 (N-96; Fig. 3B). Similar mobilities of phosphorylated ATF-2 in HeLa cells were revealed by antibodies specific for Ser(P)-121, Ser(P)-340, and Ser(P)-367 (Fig. 3B). As shown in Fig. 3C, recognition of phosphorylated ATF-2 by antibodies against Ser(P)-121 was blocked by the addition of 200 μg/ml Ser(P)-121 peptide (TB0608-1) but not by addition of the nonphosphorylated peptide (TB0608-2). Moreover, the signals due to antibodies against Ser(P)-340 and Ser(P)-367 were blocked by peptides that corresponded to the respective phosphorylated peptides (TB0609-1 and TB0610-1) but not by those that corresponded to the nonphosphorylated peptides (TB0609-2 and TB0610-2). These results indicated that each preparation of antibodies was specific for the phosphorylation of the respective serine residue (Ser(P)-121, Ser(P)-340, and Ser(P)-367). In some cases, two bands corresponding to Ser(P)-121, Ser(P)-340, and Ser(P)-367, respectively, were evident, and the intensity of each band seemed to depend on the extent of phosphorylation and on cell type. We obtained similar results after Western blotting of lysates from cell lines derived from rabbit cornea and kidney and mouse teratocarcinoma (supplemental Fig. S1).
FIGURE 3.
Characterization of antibodies specific for phosphorylated ATF-2. A, schematic representation of ATF-2 and its domains, with sites of phosphorylation (as indicated) by JNK/p38/ERK, ATM, and PKC. N, amino terminus; C, carboxyl terminus; ZNF, zinc finger motif; AD, activation domain; NLS, nuclear localization signal; NES, nuclear exclusion signal; S/TQ, serine-, threonine-, and glutamine-rich region; DBD, DNA-binding domain; PR, proline-rich; a.a., amino acids. B and C, Western blotting of phosphorylated ATF-2 with antibodies against phosphopeptides that included Ser-121, Ser-340, Ser-367, and ATF-2 (N-96). B, lysates were prepared from HeLa-S cells as described in the text. The lysates were incubated with antibodies (anti-p-Ser121, anti-p-Ser340, anti-p-Ser367, anti-ATF-2 (N-96)) in the presence of goat IgG (to reduce nonspecific binding), and Western blotting (WB) was performed as described in the text. β-Actin was included as a control. Each sample was analyzed in duplicate (odd lanes, 20 μg; even lanes, 10 μg). C, in competition experiments, lysates were incubated with 200 μg of an antigenic phosphorylated or nonphosphorylated peptide, which corresponded to Ser(P)-121 (TB0608-1 and TB0608-2), Ser(P)-340 (TB0609-1 and TB0609-2), and Ser(P)-367 (TB0610-1 and TB0610-2), respectively, with respective primary antibodies against phosphorylated ATF-2. Other procedures were the same as described in B. Each sample was analyzed in duplicate (odd lanes, 10 μg; even lanes, 20 μg). β-Actin was included as a control. D, treatment with protein phosphatase-1A (PP1A) of phosphorylated ATF-2. HeLa cells, transfected with pcDNA-FLAG-ATF-2, were treated with TPA for 6 h, and then cell lysates, containing phosphorylated ATF-2, were prepared and immunoprecipitated with FLAG-specific antibodies. Immunoprecipitates were treated with protein phosphatase-1A (PP1A; lane 2) or control buffer in the absence (lanes 1 and 2) or presence (lane 3) of phosphatase inhibitor, as described in the text. Western blotting with indicated antibodies was performed as described in the text.
The recognition, in enzyme-linked immunosorbent assays, of phosphorylated peptides of ATF-2 by antibodies against Ser(P)-121, Ser(P)-340, and Ser(P)-367, respectively, was inhibited by the phosphorylated peptides, but not by the nonphosphorylated peptides, that included the respective sites of phosphorylation in ATF-2 (supplemental Fig. S2, A–C), confirming the specificity of each antibody. Each major band of protein of 68–70 kDa disappeared when the relevant phosphopeptide was included as a competitor. However, the bands of the lower molecular mass protein visualized with Ser(P)-367-specific antibodies did not disappear, suggesting that these bands might represent unrelated cross-reacting proteins. We treated nuclear extracts with protein phosphatase-1A (PP1A) and examined its effects by Western blotting. As shown in Fig. 3D, the bands of phospho-ATF-2 disappeared after such treatment. Thus, the respective bands were specific for phosphorylated ATF-2, and each individual residue of ATF-2 was indeed a site of phosphorylation in vivo. To confirm this conclusion, we performed immunoprecipitation and Western blotting to demonstrate the cross-reactivity of antibodies specific for ATF-2 in vivo (supplemental Fig. S2D). First, we used antibodies specific for each phospho-ATF-2 to immunoprecipitate the protein from lysates of HeLa cells, and we then performed immunoblotting with N-96 antibodies that are specific for general preparations of ATF-2. Each preparation of phospho-ATF-2-specific antibodies cross-reacted with ATF-2 in the reaction with N-96 antibodies in extracts of HeLa cells.
Sequential immunoprecipitations revealed that the intensity of the slowly migrating band of phosphorylated ATF-2, as visualized with antibodies against Ser(P)-121, fell by 40–50% when extracts had been treated with ATF-2pT69/71-specific antibodies (supplemental Fig. S2E). These results indicated that specificity, in terms of the present assays, for phosphorylated ATF-2pS121 overlapped by 40–50% that for ATF-2pT69/71.
Phosphorylation Assays—To confirm the results of phosphorylation of Ser-121 of ATF-2, we performed a kinase assay in vitro with GST-fused ATF-2-(1–125), namely the amino-terminal half protein, and its serine-to-alanine variant at position 121 as substrates. Each substrate was mixed with recombinant human PKC and then incubated with [γ-32P]ATP. As shown in Fig. 4A, GST-ATF-2-(1–125) was phosphorylated by PKCα, but the GST-ATF-2-(1–125)-S121A mutant of this fragment of ATF-2 was phosphorylated only 40–60% as efficiently as wild-type GST-ATF-2-(1–125).
FIGURE 4.
Phosphorylation assays with PKC. A, 1.0 μg of GST-ATF-2-(1–125)-WT, GST-ATF-2-(1–125)-S121A, GST-ATF-2-(1–125)-T69/71A, GST-ATF-2-(1–125)-T69/71AS121A, and GST were incubated with 12.5 ng of human PKCα and 5 μCi of [32P]ATP in kinase buffer for 10 min at 30 °C, as described in the text. Loading of equal amounts of GST-ATF-2-(1–125) derivatives and GST was confirmed by staining with Coomassie Brilliant Blue (CBB). B, 1 × 106 HeLa cells were transfected with 5 μg of pFLAG-PKCαWT or pFLAG-PKCαKD plus 3 μg of pRSV-LacZ. After treatment of cells with TPA for 6 h, cell lysates were immunoprecipitated with antibodies against FLAG and protein A + G. Immunocomplexes were incubated with 0.5 μg(lanes 6–10) or 0.25 μg(lanes 1–5) of GST-ATF-2-(1–125)-WT or GST-ATF-2-(1–125)-S121A and 5 μCi of [γ-32P]ATP in kinase buffer for 10 min at 30 °C. The resultant phosphorylated protein complexes were subjected to SDS-PAGE (10% polyacrylamide) and autoradiography. A Western blot with antibodies specific for FLAG is shown in the lowest panel. Levels of GST-ATF-2-(1–125) and GST were visualized by staining with Coomassie Brilliant Blue. C, kinetics of phosphorylation of ATF-2 in response to TPA in HeLa cells. Analysis of the phosphorylation of ATF-2. HeLa cells were treated with 150 nm TPA for 6 h as described in the text. Cells were harvested, and cell lysates were prepared at the indicated time points. Levels of the various forms of phosphorylated ATF-2, ATF-2 (N-96), and β-actin were analyzed by immunoblotting as described in the text.
We also performed an immunokinase reaction using immunoprecipitates obtained with PKCα-specific antibodies and GST-ATF-2-(1–125)-WT or GST-ATF-2-(1–125)-S121A as substrate for the kinase reaction. HeLa cells were transfected with pCMV-FLAG-PKCα and the plasmid that encoded the kinase-dead form, namely pCMV-FLAG-PKCαKD, and then after or without treatment with TPA, cell extracts were immunoprecipitated with FLAG-specific antibodies. Immunoprecipitates were incubated with GST-ATF-2-(1–125), as substrate, and [γ-32P]ATP, and then each reaction mixture was subjected to SDS-PAGE (Fig. 4B). As we had anticipated, GST-ATF-2-(1–125)-WT was phosphorylated by PKCα but not by PKCαKD (Fig. 4B, lanes 6–10). The serine-to-alanine mutant GST-ATF-2-(1–125)-S121A was not phosphorylated to a significant extent, as compared with the wild type, in response to TPA (Fig. 4B, lanes 2, 4, 7, and 9). Thus, we were able to conclude definitively that PKCα phosphorylates ATF-2 at Ser-121.
Induction of Phosphorylation of Serine 121 in ATF-2 in Response to TPA—As shown in Fig. 1, PKC plays a critical role in RA signaling during the differentiation of F9 cells and the phosphorylation of Ser-121 of ATF-2, as well as in the transcription of AP-1/CRE reporter genes. We next examine the molecular mechanism of TPA-induced phosphorylation of Ser-121 of ATF-2 by PKC and the effect of such phosphorylation on the transcription of AP-1/CRE reporter genes in HeLa cells, the so-called “TPA response.” To investigate the role of each site of phosphorylation in ATF-2, we stimulated HeLa cells with TPA and examined levels of phosphorylation of ATF-2, after induction, by Western blotting with antibodies specific for the various sites of phosphorylation of ATF-2. Fig. 4C shows that TPA induced the time-dependent phosphorylation of the threonine residues at positions 69 and 71 of ATF-2 (to yield ATF-2pT69/71). Such phosphorylation was detectable within 15 min, peaked at 30 min, then declined at 60 min, and returned to the basal level. A similar pattern was detected in the case of phosphorylation of Ser-340 and Ser-367 of ATF-2. By contrast, the phosphorylation of Ser-121 began 30 min after the start of exposure to TPA, increased gradually, and remained strong for more than 2 h.
In F9 cells, the phosphorylation of the threonine residues at positions 69 and 71 (Thr-69/71) was enhanced 24 h after the start of incubation with RA, and then the level of phosphorylated ATF-2 decreased (supplemental Fig. S1). Such temporary induction was detected similarly in the case of phosphorylation of Ser-340. The phosphorylation of Ser-367 also continued into the late phase of the induction of differentiation by RA. However, the phosphorylation of Ser-121 of ATF-2 was initially induced at 30 h and then increased gradually until 117 h. These results indicated clearly that, in both HeLa cells and F9 cells, induction of the phosphorylation of Ser-121 in ATF-2 is distinct and delayed as compared with the phosphorylation of other sites in ATF-2 in response to TPA and to RA.
The temporary induction of phosphorylation of Thr-69/71 might be due to the induction of JNK and p38, which respond to stress signals, or of ERK, which responds to mitogenic signals. PKC is also involved in phosphorylation during the RA-induced activation of AP-1/CRE genes in F9 cells (24, 30–32). To clarify the distinct roles of JNK/p38/ERK and PKC, we used selective inhibitors to identify the major upstream kinases that activate ATF-2. Pretreatment of HeLa cells with SB202190 caused an average of decline of ∼40% in TPA-induced CRE-mediated transcription, as compared with negative control pretreatments (supplemental Fig. S3). Gö6976, an inhibitor of PKC, depressed TPA-induced CRE-mediated transcription by more than 85%. In addition, the negative control compound SB202474 did not significantly alter the extent of TPA-induced transcription. Thus, it is likely that the PKC and JNK/p38/ERK pathways are both involved in TPA-induced transcription.
Nuclear Localization of Ser-121-phosphorylated ATF-2 and Ser-340-phosphorylated ATF-2—To examine the distribution of endogenous Ser-121-phosphorylated and Ser-340-phosphorylated ATF-2 in HeLa cells, we used the antibodies specific for the respective phosphorylated peptides. As shown in Fig. 5A, immunostaining with antibodies against Ser(P)-121 was blocked by the addition of 200 μg/ml Ser(P)-121 peptide (TB0608-1) but not by addition of the nonphosphorylated peptide (TB0608-2) or of the phosphorylated Ser-340 (Ser(P)-340) peptide (TB0609-1). Similarly, signals due to antibodies against ATF-2pS340 were blocked by peptides that corresponded to Ser(P)-340 (TB0609-1) but not by those that corresponded to nonphosphorylated Ser-340 (TB0609-2) and Ser(P)-121 peptides (TB0608-1) (Fig. 5B). Both forms of phosphorylated ATF-2 protein were detected throughout the nuclei but strong signals were observed only in regions of decondensed chromatin. Antibodies against Ser(P)-121 peptide yielded strong signals both inside and outside the nuclei. The signals that we obtained outside the nuclei seemed to be due to nonspecific cross-reacting proteins because these signals did not disappear after treatment of cells with the PKC inhibitor Gö6976, although, by contrast, the signals within nuclei disappeared completely after such treatment (see Figs. 5 and 6).
FIGURE 5.
Staining of ATF-2 phosphorylated at Ser-121 and at Ser-340. A, HeLa cells were double-stained with antibodies specific for ATF-2pS121 and with PI (for DNA). Peptide blocking was performed with the Ser(P)-121 peptide, the Ser-121 (nonphosphorylated) peptide, and the Ser(P)-340 peptide, as described in the text. Asterisks indicate cross-reactive staining in the perinuclear region. B, HeLa cells were double-stained with antibodies specific for ATF-2p340 and PI. Peptide blocking was performed with the Ser(P)-340 peptide, the Ser-340 (nonphosphorylated) peptide, and the Ser(P)-121 peptide, as described in the text. Scale bars, 10 μm.
FIGURE 6.
Effects of TPA on the nuclear accumulation of phosphorylated ATF-2. A, HeLa cells treated with DMSO (vehicle) alone or with 200 nm TPA for 30 min were double-stained with antibodies against ATF-2 (N-96), ATF-2pS121, ATF-2pS340, ATF-2pS490/498, or ATF-2pT69/71. Scale bars, 10 μm. B, HeLa cells, pretreated with DMSO alone or with 10 μm Gö6976 for 30 min, were incubated with or without 200 nm TPA for 30 min. Cells were stained with antibodies against ATF-2pS121, ATF-2pS340, or ATF-2pT69/71. Scale bars, 10 μm. Lower panel, mean intensities of fluorescence because of various phosphorylated forms of ATF-2 after immunostaining were measured in nuclei; bars indicate means ± S.D. Results shown are from a representative experiment (n = 89–134 cells), as indicated; three independent experiments gave similar results. The dots represent individual values of mean intensity in the nucleus. Asterisks indicate significant differences (***, p < 0.001 and N.S., not significant), as determined by Student's t test.
Co-localization of ATF-2pS121 and ATF-2pT69/71 in the Nuclei of HeLa Cells—Signals due to ATF-2pT69/71 were localized in regions of decondensed chromatin and mainly as nuclear foci (Fig. 6A). Signals due to ATF-2pT69/71 were detected in all regions of nuclei with the exception of the nucleoli. The entire nuclear region was immunostained with antibodies against ATF-2 (N-96), as was the cytoplasm (5). Treatment with TPA did not significantly alter the localization of signals due to ATF-2pT69/71 and N-96-immunostained ATF-2. Moreover, in most cases, the signals due to ATF-2pS121 partially overlapped the signals due to ATF-2pT69/71. Incubation with TPA resulted in increasingly intense signals due to ATF-2pS121, leading to even more conspicuous co-localization of ATF-2pS121 with ATF-2pT69/71. Signals due to ATF-2pS340 were co-localized to some extent with those due to ATF-2pT69/71; however, treatment with TPA did not alter the localization of the respective signals in nuclei. Phosphorylation of ATF-2 at positions 490 and 498 is critical for the stress response to DNA damage that involves ATM kinase (35). Signals due to ATF-2pS490/498 were evident throughout nuclei but were not co-localized with those due to ATF-2pT69/71. Treatment with TPA did not alter the localization of the respective signals in nuclei.
We also examined the specificity of signals due to Ser(P)-121, Ser(P)-340, and Thr(P)-69/71 by preincubating cells with the PKC inhibitor Gö6976 for 30 min. The intensity of signals increased significantly upon incubation with TPA, but signals were abolished by the pretreatment with Gö6976 and remained at basal levels (Fig. 6B). By contrast, signals due to ATF-2pS340 were unchanged by TPA, but pretreatment with Gö6976 resulted in signals below basal levels. Moreover, treatment with TPA slightly decreased the intensity of signals due to ATF-2pT69/71, and Gö6976 reduced these signals to basal levels. These data indicate that TPA affects the phosphorylation of ATF-2 at Ser-121 in the nucleus specifically, but it does not similarly affect ATF-2pS340 and ATF-2pT69/71. TPA increases the nuclear accumulation of ATF-2pS121 and decreases the level of ATF-2pT69/71. The dissimilar profiles of phosphorylation of ATF-2pS121 and ATF-2pT69/71 in response to TPA might be due to different respective actions of PKC.
Reporter Assays with MEF from ATF-2-Null Mice—The DRE locus is necessary and sufficient for the RA-induced expression of the c-jun gene in F9 cells (36, 37), and in a previous report, we described the transcription of DRE-containing genes that is induced by ATF-2 and p300 in F9 cells (24). In this study, we focused on the roles of the phosphorylation of individual serine residues at positions 121, 340, and 367 of ATF-2 in the regulated transcription of AP-1/CRE genes. ATF-2 and ATF-7 exhibit considerable sequence homology, in particular in their amino-terminal activation domains, and their DNA-binding/dimerization domains are almost identical. ATF-7 can form dimers with other members of the ATF family and with AP-1 proteins, and its expression is regulated via JNK and p38 (38, 39). ATF-2 and ATF-7 are expressed similarly in various adult and embryonic tissues.4 Thus, it is possible that ATF-2 and ATF-7 have overlapping functions in mammalian development. Therefore, in the following experiments, we used MEF from ATF-2-null mice (ATF-2-/-) (Fig. 7A) and from ATF-2-null × ATF-7-null mice (ATF-2-/-/ATF-7-/-) (Fig. 7B) to circumvent any compensatory effects by either partner.
FIGURE 7.
Mutation of ATF-2 at Ser-121 affects the TPA-dependent transactivation of Gal4-p300 and also AP-1-mediated transcription in ATF-2-/- MEF, ATF-2-/-/ATF-7-/- MEF, and HeLa cells. A, 400 ng of pGal4-luc reporter plus 400 ng of pG4-p300N and 200 ng of pcDNA-FLAG-ATF-2WT, pcDNA-FLAG ATF-2S121A, pcDNA-FLAG-ATF-2T69/71A, pcDNA-FLAG-ATF-2S367A, or pcDNA4 empty vector were used for transfection of 5 × 104 ATF-2-/- MEF. Six h after transfection, cells were incubated with DMEM plus 0.5% FBS for another 24 h. Then TPA (150 nm; even lanes) or DMSO (odd lanes) was added, and after incubation for 6 h, cells were harvested and assayed for luciferase activity. The average luciferase activities from three experiments are shown with standard deviations (results with asterisks differ significantly from the result for ATF-2WT without TPA; p = 0.004 (16) and 0.009, as indicated). B, 5 × 104 MEF from ATF-2-/-/ATF-7-/- null mice were co-transfected with 400 ng of AP-1 reporter construct and pcDNA-FLAG-ATF-2WT or a derivative, as indicated, plus 200 ng of pFLAG-c-Jun and 300 ng of pRSV-lacZ. Then 48 h after transfection, cells were harvested for assays of luciferase activity, as described in A (asterisks indicate p = 0.003). C, 5 × 104 HeLa cells were transfected with 400 ng of pG4-luciferase, 200 ng of pG4-ATF-2 WT or pG4-ATF-2S121A, and 400 ng of pCMV-FLAG-PKCα or pCMV-FLAG-PKCαKD. Then 12 h after transfection, cells were incubated with DMEM for another 12 h and with DMEM plus 0.5% FBS for another 24 h. Finally, TPA was added for 6 h, and cells were harvested for assays of luciferase activity. The average luciferase activities from three experiments are shown with standard deviations. (p = 0.017 relative to the value for the pG4-ATF-2WT.) pPKCα and pPKCαKD indicate the expression plasmids pCMV-FLAG-PKCα and kinase-dead pCMV-FLAG-PKCαKD (400 ng each).
First, to investigate the role of ATF-2pS121, we examined the effects of the transient expression of pCMV-FLAG-ATF-2WT and pCMV-FLAG-ATF-2S121A on an AP-1 reporter gene in ATF-2-/- MEF in response to TPA. As shown in Fig. 7A, both ATF-2S121A and ATF-2T69/71A decreased the TPA-dependent transactivation of pGal4-p300 by 20–30%, as compared with the wild-type ATF-2 (p = 0.004 and 0.009, respectively).
The product of the c-jun oncogene forms a complex with ATF-2 (1, 12) and ATF-7 (38, 39), both in vivo and in vitro, to activate the transcription of AP-1 target genes. Efficient activation of transactivational potential in response to TPA has been observed with Gal4-c-Jun chimeric constructs that include the transactivation domain of c-Jun (40, 41). Therefore, we next examined the effect of c-Jun on the activation of an AP-1 gene by ATF-2WT and ATF-2S121A, as well as by other derivatives. As shown in Fig. 7B, ATF-2S121A reduced the expression of the AP-1 reporter gene by 30–40% (p = 0.003). By contrast, other mutated constructs, namely ATF-2S340A, ATF-2S367A, and ATF-2T69/71A, did not affect the activity of the AP-1 reporter gene. To determine whether our derivatives of ATF-2 could act as transcriptional activators, we fused various forms of ATF-2 to the DNA-binding domain of Gal4 and examined their transactivation capacity in HeLa cells (Fig. 7C). In the absence of a c-Jun expression vector, only ATF-2S367A significantly enhanced the Gal4-reporter activity in MEF from ATF-2-null mice (ATF-2-/-) and from ATF-2-/-/ATF-7-/- mice (Fig. 7, B and C). We then transfected HeLa cells with the Gal4-site-containing reporter gene together with Gal4-ATF-2 mutants that corresponded to respective sites of phosphorylation and measured the luciferase activity because of Gal4-luciferase in the presence of the pPKCα or the kinase-dead pPKCαKD expression vector. Because the amino-terminal half of ATF-2 acts as a transcriptional activation domain and the carboxyl-terminal half of ATF-2 has an inhibitory effect on AP-1-dependent transactivation (12, 13, 42), we used a gene that encoded full-length ATF-2 fused to Gal4. We found that the reporter activity of Gal4-luciferase in HeLa cells transfected with ATF-2WT was significantly enhanced in the presence of PKCα. By contrast, the mutant derivative in which Ser-121 had been replaced by Ala-121 (S121A) repressed expression of the reporter activity (Fig. 7C). Although the transactivational activity with Gal4-ATF-2 was weak, we were able to observe a significant difference in reporter activity between ATF-2WT and ATF-2S121 (p = 0.017). Thus, we can conclude that phosphorylated ATF-2S121 plays a role in the regulation of both TPA- and c-Jun-dependent transcription in HeLa cells.
DISCUSSION
In this study, we found that the serine residue at position 121 of ATF-2 is phosphorylated by PKC and is essential for the nuclear accumulation of ATF-2 in response to TPA, as well as for the transactivation of AP-1 reporter genes. It has been demonstrated that TPA preferentially enhances c-Jun-dependent transcription but does not affect ATF-2-dependent transcription (11–13). Moreover, the TPA-induced protein kinase, which is distinct from stress-activated protein kinases, phosphorylates c-Jun but not ATF-2 (12). The Raf-MEK-ERK pathway triggers the monophosphorylation of ATF-2T71, providing an explanation for the fact that TPA, a strong activator of the Raf-MEK-ERK pathway, failed to activate an ATF-2-dependent reporter gene (12, 23). Moreover, experiments with kinase inhibitors demonstrated that ATF-2 is phosphorylated at Thr-71 by JNK1/2 and by ERK1/2 (22). In this study, we used an antibody specific for Ser-121 of ATF-2 to clarify the phosphorylation cascade that occurs in the response to TPA and the role of the c-Jun-dependent transactivation of AP-1/CRE reporter genes.
We showed previously that phosphorylation of ATF-2 at Ser-121 by PKC is relevant to RA-induced transactivation of AP-1 target genes in cooperation with p300 (24). The predicted sites of phosphorylation of ATF-2 by PKC in vitro were reported to be Ser-121 (24), Ser-340 (33), and Ser-367 (33). We previously performed phosphorylations in vitro with human recombinant ATF-2 as substrate and a mixture of rabbit PKCα, PKCβ, and PKCγ, which had been purified by biochemical methods (24). We assumed that phosphorylation of ATF-2 was mediated by PKCα because PKCα is the major kinase in F9 cells during RA-induced differentiation (24, 30–32). By contrast, Sakurai et al. (33) used biochemically purified protein kinase Cβ from rat brain to phosphorylate human recombinant ATF-2 protein, and they identified the phosphorylated residues as Ser-340 and Ser-367. We cannot explain the discrepancy between their report and ours. It is possible that a dipeptide that includes Ser-121, generated by digestion with l-1-tosylamido-2-phenylethyl chloromethyl ketone-trypsin, might have eluted in the void volume from the reversed-phase high pressure liquid chromatography column used by Sakurai et al. (33). Alternatively, rat PKCβ might not phosphorylate Ser-121 as efficiently as rabbit PKCα, PKCβ, and PKCγ. However, we know that this alternative possibility is not relevant because, in our hands, all three sites of phosphorylation of ATF-2 were phosphorylated by PKCα and PKCβ derived from human, rat, and rabbit, even though levels of phosphorylation were variable (data not shown). As shown in Figs. 2 and 3, the serine residue at position 121 of ATF-2 was clearly phosphorylated by PKCα, PKCβI, and PKCβII in vitro. The extent of phosphorylation of the Ser-121 peptide by PKC was 5–10-fold lower than that of the Ser-340 and Ser-367 peptides in vitro both at pH 7.0 and at pH 4.5 (Fig. 2). Moreover, the DNA binding activity of ATF-S121A and its capacity for formation of a heterodimer with c-Jun in vitro were similar to those of ATF-2WT and the T69A/T71A mutant of ATF-2 (Ref. 16; data not shown).
To determine whether or not PKCα can phosphorylate Ser-121 of ATF-2, we performed in vitro phosphorylation reactions with GST-ATF-2 and its mutant derivatives as substrates. GST-ATF2-(1–125)-S121A was phosphorylated ∼3-fold less efficiently by human PKCα than was GST-ATF-2-(1–125)-WT (Fig. 4A). The weak background signals in the experiments with the GST-ATF-2-(1–125) series were because of the nonspecific phosphorylation of Ser-9 of ATF-2 (data not shown). We also examined immunoprecipitates when TPA-treated cells were used as the source of enzyme. As shown in Fig. 4B, at least PKCα, but not “kinase-dead” PKCα, was able to phosphorylate ATF-2 at Ser-121. The siRNA “knockdown” experiments also showed that PKCα and PKCβI are involved in the RA-induced transcription of AP-1 genes in F9 cells (Fig. 1). Thus, although PKCα is clearly able to phosphorylate ATF-2, we cannot rule out the possibility that other subspecies of PKC that are sensitive to the PKC inhibitor Gö6976 might be involved in the phosphorylation of ATF-2 in vivo because the nuclear localization of ATF-2pS121 partially overlapped not only that of PKCα but also that of PKCβI, and these co-localized signals were not significantly enhanced but were maintained upon treatment of cells with TPA (data not shown). We did find evidence for the molecular association of Ser(P)-121 with PKCα and with PKCβI by immunoprecipitation and Western blotting (supplemental Fig. S4). However, we could barely detect the relevant bands when we performed immunoprecipitations with antibodies against Ser(P)-121 and then blotted with either PKCα- or PKCβI-specific antibodies, perhaps because of the low titers of the antibodies against the Ser(P)-121 peptide or because the sites of association might have hidden the antigenic sites from the antibodies.
As shown in Fig. 3, we prepared antibodies against Ser(P)-121, Ser(P)-340, and Ser(P)-367 peptides (anti-p-Ser121, anti-p-Ser340, and anti-p-Ser367). We detected a major band of protein of 68–70 kDa when we examined the specificity of each of these antibodies (Fig. 3B). Competition experiments with phosphopeptides that corresponded to each respective phosphorylated residue and a phosphatase experiment confirmed the antigenic specificity of each preparation of antibodies (Fig. 3, C and D). The nature of the newly prepared antibodies against the various PKC-phosphorylated sites in ATF-2 was confirmed by cross-reaction with antibodies against phosphorylated ATF-2 (supplemental Fig. S2D). The antigenic specificity of Ser(P)-121 slightly overlapped that of Thr(P)-69/71 but not that of Ser(P)-340 or of Ser(P)-367, as demonstrated by the results of an absorption experiment (supplemental Fig. S2E). The specificity of each antibody was also tested in competition experiments that involved immunostaining of cells. As shown in Fig. 5, we found that antibodies against Ser(P)-121 and Ser(P)-340 could be used successfully for cell immunostaining experiments.
We compared the extent of phosphorylation of Thr-71, as well as that of two threonine residues Thr-69/71, of ATF-2 with those of Ser-121 and Ser-340 after stimulation of HeLa cells with TPA (Fig. 4C). In HeLa cells, the phosphorylation of Ser-340, Ser-367, and Thr-69/71 in ATF-2 in response to TPA was transient. The phosphorylation of Thr-69/71 appeared at 15–30 min and decreased 45 min after the start of treatment with TPA. By contrast, the phosphorylation of Ser-121 was induced at 30 min and was then maintained for another 1.5 h. A similar delay in the phosphorylation of Ser-121 was evident during the RA-induced differentiation of F9 cells (Fig. 1). We also detected significant phosphorylation of Ser-121, Ser-340, and Ser-367 in rabbit kidney PK13 cells but only weak phosphorylation of these three sites in rabbit normal cornea RC4 cells. Moreover, exposure of these cells to UV light did not significantly alter the extent of phosphorylation of these residues in ATF-2, suggesting that these residues might not be involved, to any great extent, in the response to UV stress (supplemental Fig. S1A). To confirm a difference between the role of the phosphorylation of Ser-121 and that of Ser-340, we compared the nuclear localization of the two forms of phospho-ATF-2. As shown in Fig. 5, we detected signals due to ATF-2pS121 both inside and outside nuclei. Signals outside nuclei were probably because of noise caused by cross-reacting proteins because nuclear signals due to Ser(P)-121 were muted in the presence of the Ser(P)-121 peptide but not in the presence of the unphosphorylated Ser-121 peptide or of the Ser(P)-340 peptide. As shown in Fig. 6B, treatment with the PKC inhibitor Gö6976 eliminated only the nuclear signals due to Ser(P)-121 and did not eliminate cytoplasmic signals. Thus, signals inside nuclei were because of ATF-2pS121. Signals due to Ser(P)-340 were also specific and muted by the Ser(P)-340 peptide and not by either the Ser(P)-121 peptide or the Ser-340 peptide (Fig. 6B). It was therefore clear that signals due to ATF-2pS121 and ATF-2pS340, respectively, were different and distinct from one another.
The nuclear accumulation of ATF-2, upon stimulation of cells by TPA, was only recognized for ATF-2pS121 and not for ATF-2pS340 and ATF-2pS490/498. ATF-2pS490/498 is a marker of ATM-dependent phosphorylation, whereas ATF-2pT69/71 is a marker of the JNK/p38/ERK pathway (12, 23, 35). The nuclear accumulation of ATF-2pS121 disappeared in the presence of the PKC inhibitor Gö6976 (Fig. 6B). Some signals due to ATF-2pS121 were found in foci as were some of those due to ATF-2pT69/71. The total number of foci of ATF-2pS121 was increased by treatment with TPA. These data indicate that ATF-2pS121 is phosphorylated very weakly under steady-state conditions and that treatment with TPA increases the nuclear accumulation of ATF-2pS121. The PKC inhibitor Gö6976 eliminated any enhancement of signals due to ATF-2pS121 above basal levels. By contrast, the extent of phosphorylation of ATF-2S340 was maximal under steady-state conditions, being three times that of ATF-2pS121, but treatment with TPA did not increase the intensity of the former signals (Fig. 6B). In the presence of Gö6976, the signals due to strongly phosphorylated ATF-2pS340 that we detected in steady-state HeLa cells disappeared completely, suggesting that, at steady state, ATF-2pS340 is phosphorylated in HeLa cells, and its phosphorylation does not respond to TPA. Thus, the actions of ATF-2pS121 and ATF-2pS340 appear to be separate and different. Signals due to ATF-2pT69/71 were observed mainly as foci in nuclei (Fig. 6, A and B). The intensity of signals due to this form of ATF-2 did not increase significantly upon treatment of cells with TPA, and the inhibitor of PKC also failed to reduce these signals significantly. Thus, we can conclude that, in HeLa cells, PKC only phosphorylates ATF-2 to yield ATF-2pS121 in response to TPA and then ATF-2pS121 accumulates in the nuclei. Neither ATF-2pS340 nor ATF-2pT69/71 accumulated in the nuclei in response to TPA, but the inhibitor Gö6976 abolished the phosphorylation of Ser-340 and Thr-69/71. As a control we examined the effect of the p38 inhibitor SB202190 on the nuclear accumulation of ATF-2pS121, and we found no significant accumulation of ATF-2pS121 as in the case of ATF-2pT69/71, in nuclei after exposure of cells to SB202190 (supplemental Fig. S5). Liu et al. (5) reported similar observations.
The results of Western blotting of whole cell lysates confirmed that levels of ATF-2pS340, ATF-2pS367, and ATF-2pT69/71 were elevated within 15–30 min of the start of exposure of cells to TPA, whereas significant accumulation of ATF-2pS121 was evident only 30–120 min after the start of treatment of HeLa cells with TPA (Fig. 4C). Thus, we can conclude that only Ser-121 of ATF-2 is responsible for transport of ATF-2 to the nucleus in response to TPA-induced phosphorylation.
TPA does not affect the transactivation potential of ATF-2 to any significant extent in cells that do not express c-Jun, such as F9 cells (11–13). By contrast, in c-Jun-expressing cells, TPA affects the activation of ATF-2 in a c-Jun-dependent manner (5). In such cells, induction is probably mediated by c-Jun/ATF-2 heterodimers, suggesting that the composition of subunits determines the external signals to which the c-Jun promoter responds (5). Thus, the transactivational activity of ATF-2 is dependent on the contribution of c-Jun as a partner, which might reflect the cellular response to TPA.
Disruption of the intramolecular interaction between the bZIP domain and the amino-terminal transcriptional activation domain of ATF-2 is essential for the activation of ATF-2 in the nucleus because exogenously expressed ATF-2 has little transcriptional activation capacity unless it is co-expressed with co-activators (40–42). Hu et al. (43) reported that exogenously expressed ATF-2 accumulates in the cytoplasm and, moreover, that the deletion of 341 amino-terminal residues of ATF-2 enhances the nuclear localization of c-Jun/ATF-2 heterodimers. Thus, the 341 amino-terminal residues might influence the structure of ATF-2 to block transport to the nucleus. It seems that ATF-2 has two possible nuclear localization signal motifs and that these motifs might be masked by intramolecular interactions that involve the amino terminus and the bZIP domain (41). The c-Jun/ATF-2 dimerization might mask or otherwise inhibit the function of the ATF-2 nuclear export signals of ATF-2, so the complex is retained within the nucleus (5). We followed our demonstration that the phosphorylation of Ser-121 is critical for the TPA-induced nuclear accumulation of phosphorylated ATF-2 by examining the effect of a Ser-121 to Ala-121 mutation on the transactivation of AP-1/CRE genes.
Our reporter assays with an AP-1 reporter in ATF-2-/-/ATF-7-/- MEF showed that the ATF-2S121A mutant elicited lower AP-1 reporter activity than did ATF-2WT, ATF-2S340A, ATF-2S367A, and ATF-2T69/71A, respectively (Fig. 7B). Moreover, ATF-2S121A failed to transactivate a pG4-p300 reporter gene in response to TPA in ATF-2-/- MEF (Fig. 7A). Thus, at least PKCα affected the transactivation activity of ATF-2, whereas ATF-2S121A had no such activity (Fig. 7C).
Liu et al. (5) reported that the phosphorylation of c-Jun and of ATF-2 by mitogen-activated protein kinases (MAPK), such as JNK/p38/ERK, is not related to the dimerization and nuclear localization of ATF-2. In fact, we do not yet know the details of the signaling that occurs during the response to TPA- and PKC-mediated induction. We also do not know whether dimerization with c-Jun is critical for ATF-2-mediated transactivation, whether activated PKC moves directly to the nucleus to “switch on” AP-1/CRE target genes with the help of c-Jun, and whether PKC activates other kinases, such as Raf-1, to transmit a signal for cross-talk with the Raf-MEK-ERK system. It has been proposed that activated PKC in the cytoplasm might be transported directly to the nucleus, where it is involved in the chromatin-mediated regulation of TPA-responsive genes (44). We failed to detect any significant enhancement of the co-localization of ATF-2pS121 and PKCα after treatment of HeLa cells with TPA, but we did observe that ATF-2pS121 accumulated in nuclei upon treatment of cells with TPA. Thus, it seems plausible that phosphorylation of ATF-2 by PKC might occur in the cytoplasm and then phosphorylated ATF-2 might be transported to the nucleus with c-Jun.
This is the first report, to our knowledge, of the unequivocal identification of the residue in ATF-2 that is phosphorylated in HeLa cells in response to TPA. This phosphorylation is mediated a PKC, most likely, PKCα. Moreover, the individual replacement by alanine of Thr-69/71, Ser-340, and Ser-367 in ATF-2 did not affect the activation of a Gal4-luciferase reporter by the respective derivatives of pGal4-ATF-2 in the presence of PKCα, but the alanine-serine mutation of Ser-121 repressed the expression of the reporter (Fig. 7C). Thus, we can conclude that regulation by TPA of the role of ATF-2 is dependent on the phosphorylation of Ser-121 and on the accumulation of ATF-2pS121 in the nucleus, as well as on the formation of heterodimers with partners, such as c-Jun. With respect to the phosphorylation of ATF-2T69/71, we detected the co-localization of ATF-2pT69/71 and ATF-2pS121 in the nucleus. However, TPA did not stimulate the entry of ATF-2pT69/71pS121 into nuclei but only that of ATF-2pS121 (data not shown). Our results strongly suggest that signaling via Ser(P)-121 and signaling via Thr(P)-69/71 are both different and independent.
Supplementary Material
Acknowledgments
We thank Drs. N. Watanabe, K. Itakura, H. Ugai, Y. Shi, and R. Chiu for valuable comments and helpful discussions. We also thank Drs. S. Ohno, K. Iwai, H. van Dam, M. R. Montiminy, D. Mercola, W. Greitwieser, and N. Jones for reagents and “MEF from ATF-2 and/or ATF-7 knock-out” mice, and K. Inabe, M. Hirose and M. Nakajima for their skilled support.
This work was supported by Grant 19041069 from the Ministry of Education, Culture, Sports, Science and Technology of Japan and grants from the Naito Memorial Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Figs. S1–S5, and additional references.
Footnotes
The abbreviations used are: ATF-2, activation transcription factor-2; CRE, cAMP-response element; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; MEF, mouse embryonic fibroblasts; TPA, 12-O-tetradecanoylphorbol-β-acetate; PKC, protein kinase C; JNK, c-Jun NH2-terminal kinase; ERK, extracellular signal-regulated kinase; RA, retinoic acid; siRNA, small interfering RNA; FITC, fluorescein isothiocyanate; TRITC, tetramethylrhodamine isothiocyanate; PIPES, piperazine-N,N′-bis(2-ethanesulfonic acid); DRE, differentiation-response element; PI, propidium iodide; GST, glutathione S-transferase; ATM, ataxia telangiectasia mutated.
W. Breitwieser and N. Jones, personal communication.
References
- 1.van Dam, H., and Castellazzi, M. (2001) Oncogene 20 2453-2464 [DOI] [PubMed] [Google Scholar]
- 2.Shaulian, E., and Karin, M. (2002) Nat. Cell Biol. 4 E131-E136 [DOI] [PubMed] [Google Scholar]
- 3.Vlahopoulos, S. A., Logotheti, S., Mikas, D., Giarike, A., Gorgoulis, V., and Zoumpourlis, V. (2008) BioEssays 30 314-327 [DOI] [PubMed] [Google Scholar]
- 4.Eferl, R., and Wagner, E. F. (2003) Nat. Rev. Cancer 3 859-868 [DOI] [PubMed] [Google Scholar]
- 5.Liu, H., Deng, X., Shyu, Y. J., Li, J. J., Taparowsky, E. J., and Hu, C. D. (2006) EMBO J. 25 1058-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reimold, A. M., Grusby, M. J., Kosaras, B., Fries, J. W., Mori, R., Maniwa, S., Clauss, I. M., Collins, T., Sidman, R. L., and Glimcher, M. J. (1996) Nature 379 262-265 [DOI] [PubMed] [Google Scholar]
- 7.Maekawa, T., Bernier, F., Sato, M., Nomura, S., Singh, M., Inoue, Y., Tokunaga, T., Imai, H., Yokoyama, M., Reinold, A., Glimcher, L. H., and Ishii, S. (1999) J. Biol. Chem. 274 17813-17819 [DOI] [PubMed] [Google Scholar]
- 8.Maekawa, T., Shinagawa, T., Sano, Y., Sakuma, T., Nomura, S., Nagasaki, K., Miki, Y., Saito-Ohara, F., Inazawa, J., Kohno, T., Yokota, J., and Ishii, S. (2007) Mol. Cell. Biol. 27 1730-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maekawa, T., Sano, Y., Shinagawa, T., Rahman, Z., Sakuma, T., Nomura, S., Licht, J. D., and Ishii, S. (2008) Oncogene 27 1045-1054 [DOI] [PubMed] [Google Scholar]
- 10.Bhoumik, A., Fichtman, B., Derossi, C., Breitwieser, W., Kluger, H. M., Davis, S., Subtil, A., Meltzer, P., Krajewski, S., Jones, N., and Ronai, Z. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 1674-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, S., Campbell, D., Derijard, B., and Davis, R. J. (1995) Science 267 389-393 [DOI] [PubMed] [Google Scholar]
- 12.van Dam, H., Wilhelm, D., Herr, I., Steffen, A., Herrlich, P., and Angel, P. (1995) EMBO J. 14 1798-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livingstone, C., Patel, G., and Jones, N. (1995) EMBO J. 14 1785-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, L., and Karin, M. (2001) Nature 410 37-40 [DOI] [PubMed] [Google Scholar]
- 15.Gupta, M., Gupta, S. K., Balliet, A. G., Hollander, M. C., Fornace, A. J., Hoffman, B., and Liebermann, D. A. (2005) Oncogene 24 7170-7179 [DOI] [PubMed] [Google Scholar]
- 16.Breitwieser, W., Lyons, S., Flenniken, A. M., Ashton, G., Bruder, G., Willington, M., Lacaud, G., Kouskoff, V., and Jones, N. (2007) Genes Dev. 21 2069-2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouwens, D. M., de Ruiter, N. D., van der Zon, G. C., Carter, A. P., Schouten, J., van der Burgt, C., Kooistra, K., Bos, J. L., Maassen, J. A., and van Dam, H. (2002) EMBO J. 21 3782-3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs, S. Y., Tappin, I., and Ronai, Z. (2000) J. Biol. Chem. 275 12560-12564 [DOI] [PubMed] [Google Scholar]
- 19.Angel, P., Hattori, K., Smeal, T., and Karin, M. (1988) Cell 55 875-885 [DOI] [PubMed] [Google Scholar]
- 20.Herr, I., van Dam, H., and Angel, P. (1994) Carcinogenesis 15 1105-1113 [DOI] [PubMed] [Google Scholar]
- 21.Tseng, C. P., Kim, Y. J., Kumar, R., and Verma, A. K. (1994) Carcinogenesis 15 707-711 [DOI] [PubMed] [Google Scholar]
- 22.Morton, S., Davis, R. J., and Cohen, P. (2004) FEBS Lett. 572 177-183 [DOI] [PubMed] [Google Scholar]
- 23.Radler-Pohl, A., Sachsenmaier, C., Gebel, S., Auer, H. P., Bruder, J. T., Rapp, U., Angel, P., Rahmsdorf, H. J., and Herrlich, P. (1993) EMBO J. 12 1005-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki, H., Song, J., Eckner, R., Ugai, H., Chiu, R., Taira, K., Shi, Y., Jones, N., and Yokoyama, K. K. (1998) Genes Dev. 12 233-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, C., Li, H., Murata, T., Sun, K., Horikoshi, M., Chiu, R., and Yokoyama, K. K. (2002) Mol. Cell. Biol. 22 4815-4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasahara, K., Nakayama, Y., Nakazato, Y., Ikeda, K., Kuga, T., and Yamaguchi, N. (2007) J. Biol. Chem. 282 5327-5339 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, Y., Kawana, A., Igarashi, A., and Yamaguchi, N. (2006) Exp. Cell Res. 312 2252-2263 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi, N., and Fukuda, M. N. (1995) J. Biol. Chem. 270 12170-12176 [DOI] [PubMed] [Google Scholar]
- 29.Tada, J., Omine, M., Suda, T., and Yamaguchi, N. (1999) Blood 93 3723-3735 [PubMed] [Google Scholar]
- 30.Khuri, F. R., Cho, Y., and Talmage, D. A. (1996) Cell Growth & Differ. 7 595-602 [PubMed] [Google Scholar]
- 31.Cho, Y., Klein, M. G., and Talmage, D. A. (1998) Cell Growth & Differ. 9 147-154 [PubMed] [Google Scholar]
- 32.Kindregen, H. C., Rosenbaum, S. E., Ohno, S., and Niles, R. M. (1994) J. Biol. Chem. 269 27756-27777 [PubMed] [Google Scholar]
- 33.Sakurai, A., Maekawa, T., Sudo, T., Ishii, S., and Kishimoto, A. (1991) Biochem. Biophys. Res. Commun. 181 629-635 [DOI] [PubMed] [Google Scholar]
- 34.Szczepanowska, J., Ramachandran, U., Herring, C. J., Gruschus, J. M., Qin, J., Korn, E. D., and Brzeska, H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 4146-4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhoumik, A., Takahashi, S., Breitweiser, W., Shiloh, Y., Jones, N., and Ronai, Z. (2005) Mol. Cell 18 577-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitabayashi, I., Kawakami, Z., Chiu, R., Ozawa, K., Mastuoka, T., Toyoshima, S., Umezono, K., Evans, R. M., Gachelin, G., and Yokoyama, K. (1992) EMBO J. 11 167-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitabayashi, I., Eckner, R., Areany, Z., Chiu, R., Gachelin, G., Livingston, D. M., and Yokoyama, K. (1995) EMBO J. 14 3496-3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatton, B., Bocco, J. L., Goetz, J., Gaire, M., Lutz, Y., and Kedinger, C. (1994) Oncogene 9 375-385 [PubMed] [Google Scholar]
- 39.Bocco, J. L., Bahr, A., Goetz, J., Hauss, C., Kallunki, T., Kedinger, C., and Chatton, B. (1996) Oncogene 12 1971-1980 [PubMed] [Google Scholar]
- 40.Franklin, C. C., Sanchez, V., Wagner, F., Woodgett, J. R., and Kraft, A. S. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 7247-7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, X. Y., and Green, M. R. (1996) Genes Dev. 10 517-527 [DOI] [PubMed] [Google Scholar]
- 42.Sano, Y., Tokitou, F., Dai, P., Maekawa, T., Yamamoto, T., and Ishii, S. (1998) J. Biol. Chem. 273 29098-29105 [DOI] [PubMed] [Google Scholar]
- 43.Hu, C. D., Chinenov, Y., and Kerppola, T. K. (2002) Mol. Cell 9 789-798 [DOI] [PubMed] [Google Scholar]
- 44.Schmalz, D., Hucho, F., and Buchner, K. (1998) J. Cell Sci. 111 1823-1830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.