Abstract
Decorin and biglycan are class I small leucine-rich proteoglycans (SLRPs) involved in regulation of collagen fibril and matrix assembly. We hypothesize that tissue-specific matrix assembly, such as in the cornea, requires a coordinate regulation involving multiple SLRPs. To this end, we investigated the expression of decorin and biglycan in the cornea of mice deficient in either SLRP gene and in double-mutant mice. Decorin and biglycan exhibited overlapping spatial expression patterns throughout the corneal stroma with differential temporal expression. Whereas decorin was expressed at relatively high levels in all developmental stages, biglycan expression was high early, decreased during development, and was present at very low levels in the mature cornea. Ultrastructural analyses demonstrated comparable fibril structure in the decorin- and biglycan-null corneas compared with wild-type controls. We found a compensatory up-regulation of biglycan gene expression in the decorin-deficient mice, but not the reverse. Notably, the corneas of compound decorin/biglycan-null mice showed severe disruption in fibril structure and organization, especially affecting the posterior corneal regions, corroborating the idea that biglycan compensates for the loss of decorin. Fibrillogenesis assays using recombinant decorin and biglycan confirmed a functional compensation, with both having similar effects at high SLRP/collagen ratios. However, at low ratios decorin was a more efficient regulator. The use of proteoglycan or protein core yielded comparable results. These findings provide firm genetic evidence for an interaction of decorin and biglycan during corneal development and further suggest that decorin has a primary role in regulating fibril assembly, a function that can be fine-tuned by biglycan during early development.
The characteristic architecture of different connective tissues is established through tissue-specific regulation of collagen fibrillogenesis and matrix assembly. Multiple steps are involved in collagen fibrillogenesis, including the nucleation of collagen assembly, assembly of immature fibril intermediates, and linear and lateral fibril growth of preformed intermediates (1-5). Each step is independently regulated through interactions of extracellular macromolecules with fibrils. Heterotypic interactions involving different fibril-forming collagens regulate fibril nucleation during development (1, 6). In contrast, interactions involving fibrils and small leucine-rich proteoglycans (SLRPs)2 are implicated in the regulation of linear and lateral growth of mature fibrils from preformed intermediates (5, 7-10). The focus of this work is to elucidate the differential regulatory role(s) of decorin and biglycan in fibrillogenesis.
SLRPs compose a family of five classes of structurally related, but genetically distinct molecules. The members within each class exhibit high protein homology and primary structure identity (11). Decorin and biglycan are class I SLRPs; and fibromodulin, keratocan, and lumican are class II SLRPs. Gene-targeting studies with mice deficient in these SLRPs demonstrate a cooperative relationship between SLRP pairs in each class (8-10, 12-16). This current work addresses the hypothesis that decorin and biglycan have coordinate roles in the regulation of fibrillogenesis and matrix assembly during development of mature, functional tissues.
The developing cornea is an excellent model system to elucidate the interactions and regulatory roles of SLRPs in fibrillogenesis. The corneal stroma contains small diameter fibrils with regular packing. Unlike most other connective tissues, during maturation corneal fibrils do not undergo lateral growth producing a heterogeneous distribution of fibril diameters. Rather, in the cornea a homogeneous distribution of small diameter fibrils is found throughout development and maturation. Fibril intermediate assembly is initiated, involving collagen I/V interactions. The intermediates undergo linear growth to generate mature fibrils, but no lateral growth. These later steps involve SLRP interactions with stromal collagen fibrils (9, 17-19). These structural features are essential for corneal transparency. SLRPs expressed by corneal keratocytes include decorin, biglycan, lumican, keratocan, and osteoglycin (9, 20-24). Mouse lines null in these SLRPs provide powerful models for analysis of the regulatory roles of SLRPs in fibrillogenesis. To date, only lumican-null mice have been shown to have structural defects in corneal fibrils. This involves a spatially restricted loss of lateral growth regulation in the posterior stroma (8, 9, 25, 26). An interaction between lumican and keratocan with lumican regulating keratocan expression has been documented (16). Decorin is distributed throughout the stroma and is the most abundant SLRP in the cornea. However, the fibril phenotype in the mature decorin-null cornea is normal (27). Notably, a point mutation in exon 10 of the human decorin gene is associated with congenital stromal corneal dystrophy, an autosomal dominant disease that leads to corneal opacities and abnormal vision (28, 29). In both families so far analyzed, the mutation causes the generation of a truncated decorin protein core lacking the terminal 33 amino acids, the so called ear repeat (30). Thus, it is likely that the presence of an abnormal protein core might interfere with the proper function of decorin in collagen organization in a dominant negative fashion. Recent studies on the roles of decorin and biglycan in regulation of tendon fibrillogenesis indicate that specific interactions between these two class I SLRPs are involved in regulation of lateral fibril growth (15). These data indicate that analyses of the regulatory function of one SLRP require integration with parallel studies of other members of the class and/or family.
In the current study, we analyzed the regulatory role(s) of decorin and biglycan in corneal stroma fibrillogenesis. The results indicate a coordinate interaction between biglycan and decorin during development involving differential temporal expression patterns. In the absence of decorin and with no compensatory increase in biglycan there is dysfunctional regulation of lateral fibril growth. Abnormally large diameter, irregular fibrils were assembled that are incompatible with corneal function. Analyses of compound decorin/biglycan-null mouse corneas indicate that decorin is the “dominant” regulator of lateral fibril growth in the corneal stroma and that this biological process is fine-tuned by biglycan expression early in development.
EXPERIMENTAL PROCEDURES
Animals—Gene-targeted mice deficient in decorin (27) or biglycan (31), as well as compound decorin/biglycan-null mice and wild-type controls were utilized. The compound nulls were obtained by cross-breeding of the single null mice with all animals in a C57BL background. Corneas from mice at postnatal day (P) 4, P10, P30, P60, and P90 were used. Only males were analyzed except for P4 and P10 mice where sex was not determined. All animal studies were performed in compliance with IACUC approved animal protocols.
Immunoblots—Corneas were dissected from decorin- and biglycan-null and wild-type controls at P10, P30, P60, and P90. Corneas were homogenized in a 20-fold excess (w/v) of extraction buffer (4 m guanidine HCl, 50 mm sodium acetate, pH 5.8) containing proteinase inhibitors (Thermo Scientific). The extracts were dialyzed against 150 mm Tris-HCl, 150 mm NaCl, pH 7.3, and hydrolyzed with chondroitinase ABC (Seikagaku Biobusiness Corporation) or endo-β-galactosidase (Sigma) as previously described (15). Total protein concentration was determined using a BCA protein assay kit (Pierce). Immunoblotting was done as previously described (15) with 5-20 μg of total protein loaded depending on the experiment. Anti-decorin (LF113) was used at 1:1000, both anti-biglycan (LF159) and anti-fibromodulin (LF-149) were used at 1:200. These antibodies were provided by Dr. L. Fisher, National Institutes of Health, NICDR (32). Anti-lumican was provided by Dr. Ake Oldberg, Lund University, Sweden, and used at 1:1000. Anti-keratocan was provided by Dr. Hassell, University of South Florida, Tampa, FL, and used at 1:200. Goat anti-rabbit IgG-peroxidase (Amersham Biosciences) was used as secondary antibody at 1:3000 with an ECL (Amersham Biosciences) detection system. After transfer, the SDS-PAGE gels were stained for type I collagen. Actin reactivity in each sample was detected using an anti-actin antibody (Chemicon).
Immunofluorescence Microscopy—Immunolocalization of decorin and biglycan in frozen sections from corneas at different developmental stages was done as previously described (9, 15). Anti-mouse decorin antibody was used at 1:200 and anti-mouse biglycan antiserum at 1:100. The secondary antibody was goat anti-rabbit IgG-Alexa Fluor 568 (Molecular Probes) at 1:400. The nuclei were counterstained using 4′,6-diamidino-2-phenylindole in the mounting medium (Vector Labs). Antibody incubations and image acquisition were done concurrently for the wild-type and knock-out sections, using identical procedures and settings to facilitate comparison.
ELISAs—Microtest™ 96-well ELISA plates were purchased from BD Biosciences (Bedford, MA). Equal protein amounts (serial dilution), extracted from mouse cornea and subsequently de-glycanated, were used to coat wells overnight at room temperature in carbonate buffer (0.15 m Na2CO3, 0.15 m NaHCO3). The following day plates were washed 3 times with PBS and blocked in 1% bovine serum albumin/PBS for 1 h. The antibodies used were anti-decorin (LF113) and anti-biglycan (LF159) both at a 1:1000 dilution in 1% bovine serum albumin/PBS for 1 h. Secondary antibody, anti-rabbit horseradish peroxidase, was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA) and incubated in 1% bovine serum albumin/PBS for 1 h. Chemiluminescence substrate SIGMAFAST™ OPD was purchased from Sigma. Plates were read at 450 nm for 1 s with a VICTOR3TM Multilabel Counter (PerkinElmer Life Sciences). Standard curves for decorin and biglycan protein core were generated for quantification of these proteins in the samples and also to evaluate the difference in antibody affinity between anti-decorin and anti-biglycan. Statistical analysis (Student's t test) was performed using Sigma-Plot version 9.1.
Transmission Electron Microscopy—Processing for transmission electron microscopy was as previously described (8, 33). Briefly, corneas were fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 m sodium cacodylate, pH 7.4, with 8.0 mm CaCl2, post-fixed with 1% osmium tetroxide and en bloc stained with 2% uranyl acetate in 50% ethanol. The samples were then dehydrated in an ethanol series followed by propylene oxide. The corneas were infiltrated and embedded in a mixture of Embed 812, nadic methyl anhydride, dodecenylsuccinic anhydride, and DMP-30 (Electron Microscopy Sciences, PA). A Reichert UCT ultramicrotome and a diamond knife were used to prepare 90-nm sections. The sections were stained with 2% aqueous uranyl acetate followed by 1% phosphotungstic acid, pH 3.2. Anterior and posterior regions in corneas were examined and photographed at 80 kV using a Tecnai 12 transmission electron microscope with a Gatan 2K Ultrascan bottom mount CCD camera.
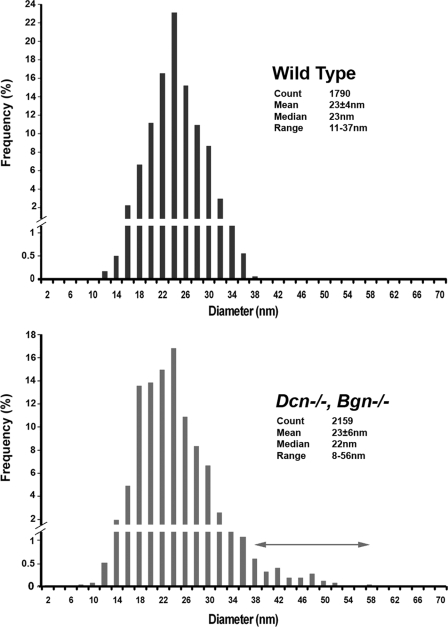
Fibril Diameter Analyses—Five corneas from three to five different animals at P60 were analyzed for each genotype: Dcn-/- (decorin-deficient), Bgn-/- (biglycan-deficient), Dcn-/-, Bgn-/- (compound decorin/biglycan-deficient), and wild-type (WT) control. Digital images were taken from non-overlapping regions of the central portion of anterior and posterior areas of the cornea at ×28,610. Images (25-35 anterior and posterior/group) were randomized and fibril diameters were measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN) in a masked manner. A total area of 0.211 μm2 per image at a final magnification of ×161,990 was analyzed. For each group, the number of animals, and number of different images (animals/images (fibril count, minimum-maximum in nm)) is as follows: WT anterior, 3/25 (count 1929, min 10.46, max 35.63); WT posterior, 3/25 (count 1790, min 10.46, max 37.72); Dcn-/- anterior, 4/25 (count 1987, min 8 nm, max 39 nm); Dcn-/- posterior, 4/25 (count 1642, min 10 nm, max 36 nm; Bgn-/- anterior, 5/25 (count 1734, min 11 nm, max 43 nm); Bgn-/- posterior, 5/25 (count 1704, min 10 nm, max 37 nm); double Dcn-/-, Bgn-/- anterior, 4/25 (count 2186, min 3 nm, max 67 nm); double Dcn-/-,Bgn-/- posterior, 4/25 (count 2159, min 7 nm, max 57 nm).
In Vitro Fibrillogenesis Assay—Native type I collagen was extracted from mouse tail in 0.5 m acetic acid containing protease inhibitors and purified as previously described (34). Type I collagen was purified by a series of differential salt precipitations from both neutral and acid pH and proteoglycans were removed using ion exchange chromatography on DEAE-Sephacel. Purified collagen type I was dialyzed into HCl, pH 2.0, and stored at 4 °C. Purified recombinant decorin and biglycan proteoglycans and protein cores were prepared as previously described (35). In vitro collagen fibrillogenesis assays were done using a collagen concentration of 100 μg/ml for all assays as previously described (34, 35). For each experiment type I collagen in HCl, pH 2.0, was mixed with 10× PBS, 0.1 n NaOH, and PBS to a final concentration of 100 μg/170 μl of PBS, pH 7.2. An aliquot (170 μl) was mixed with 30 μl of proteoglycans, protein cores, or buffer alone on ice to yield a final collagen concentration of 100 μg/ml. Collagen fibrillogenesis was analyzed at a stoichiometry ranging from 1:6 and 10:1, collagen to proteoglycan or core protein. Stoichiometry was based on the molar ratio of proteoglycans or core protein to type I collagen (300 kDa). Proteoglycan and protein core concentrations were determined from protein concentrations using a molecular mass of 38.7 kDa for the biglycan core and 38.4 kDa for the decorin core. The samples were transferred to a Synergy HT Multi-Detection Microplate Reader (Bio Tek). The absorbance at 313 nm was monitored over a period of 1 h at 34 °C. Fibril structure was analyzed using negative stained preparations and transmission electron microscopy as previously described (34).
RESULTS
Differential Temporal Expression with Overlapping Spatial Expression of Decorin and Biglycan in the Developing Mouse Cornea—To determine whether the regulation of corneal stromal fibrillogenesis involved coordinate regulation by decorin and biglycan, their expression patterns were analyzed in corneas as a function of development. Analyses of protein core expression utilizing semi-quantitative immunoblots demonstrated that decorin expression was relatively constant during development and maintained a high level of expression in the mature corneal stroma (P90). However, biglycan expression was highest early (P10), decreased with development, and was present at only very low levels in the mature stroma (Fig. 1). These data indicate a differential expression of these class I SLRPs. The stromal content of decorin and biglycan in wild-type mice was determined at P10, utilizing ELISA. The data indicate that decorin is present at an average of 1.37 μg/cornea (±0.26 S.E.), whereas biglycan is present at an average of 0.13 μg/cornea (±0.06 S.E.), making decorin 10 times more abundant than biglycan in the wild-type cornea (p = 0.034) during the period of peak biglycan expression.
FIGURE 1.
Differential temporal expression patterns of decorin and biglycan. A, decorin and biglycan protein core expression was analyzed using semi-quantitative immunoblotting. The expression of decorin was relatively constant throughout the developmental stages studied, P10 to P90. Biglycan expression was high early and decreased with development to barely detectable levels at P90. Representative immunoblots are presented. Actin and type I collagen were used as loading controls. B, the graphs present data from 4 independent immunoblots with the mean density of decorin and biglycan presented relative to P10 band density.
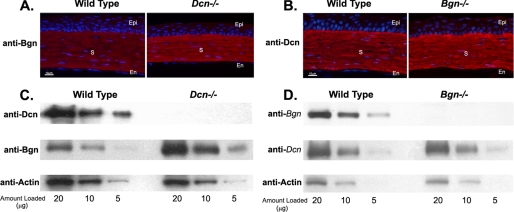
Spatially, both decorin and biglycan were homogeneously expressed throughout the corneal stroma at P10 as determined by immunochemical localization analyses. The localization was comparable with that observed for type I collagen. However, as the corneal stroma matured, decorin retained its homogeneous distribution throughout the stroma, whereas biglycan reactivity decreased (Fig. 2), consistent with the biochemical analyses of protein core expression. The overlapping spatial localization coupled with a differential temporal expression pattern support coordinate regulatory functions for decorin and biglycan in regulation of fibrillogenesis.
FIGURE 2.
Spatial expression of decorin and biglycan. A, light micrographs showing the organization of the cornea from mice at P10 and P60. The sections are comparable with those shown in B. Thin plastic section (1 μm) stained with methylene blue/azur blue. B, immunofluorescence localization demonstrated that both decorin and biglycan were localized homogeneously throughout the stroma. Decorin expression was relatively constant with development, biglycan expression decreased significantly from P10 to P60. An antibody against type I collagen was used as a positive control and no primary antibody was used as a negative control. Nuclei were localized with 4′,6-diamidino-2-phenylindole (blue). P10, post-natal day 10; P60, post-natal day 60. Epi, epithelium; En, endothelium; and S, stroma.
Regulation of Corneal Fibrillogenesis in Decorin- and Biglycan-deficient Mice—To investigate the regulatory role(s) of each member of this pair independently, corneal stromal fibril structure from decorin-null or biglycan-null mice was compared with wild-type controls from the stroma of mature mice (P60) using transmission electron microscopy. In wild-type corneas, collagen fibrils were cylindrical and stromal architecture was highly organized, as expected. Both the collagen fibrils and stromal structures from decorin-deficient and biglycan-deficient mice were comparable with the wild-type controls (Fig. 3). The decorin-null corneas contained occasional abnormal fibrils with larger diameters and irregular shapes. The stroma was examined in both the anterior and posterior regions because developmental anterior-posterior differences in fibril structure have been described (9). The ultrastructure of both regions as well as the diameter distributions were comparable in all 3 genotypes (Fig. 3). These results were unexpected based on the altered fibril structures seen in other connective tissues, e.g. tendon, skin, bone, in the null mice (27, 31). Therefore, we hypothesize that the regulatory function of these closely related class I SLRPs involves the interaction of both decorin and biglycan.
FIGURE 3.
Collagen fibril structure in decorin and biglycan-null corneal stromas. Both fibril and stromal architecture in decorin-deficient (Dcn-/-) and biglycan-deficient (Bgn-/-) corneas were comparable with wild-type controls. A, transmission electron micrographs of the anterior and posterior stroma from wild-type (WT), Dcn-/-, and Bgn-/- mice. Both the Dcn-/- and Bgn-/- mice had fibril and stromal structures comparable with that seen in the wild-type cornea. There were occasional abnormal fibrils observed in the Dcn-/- stroma (arrow). All micrographs are from P60 mice, other developmental stages were comparable. B, fibril diameter distributions in decorin- and biglycan-deficient corneas and wild-type controls were comparable.
Biglycan Compensates for the Loss of Decorin—Because decorin and biglycan interact with a similar region on the type I collagen molecule with different affinities (36, 37), the possibility of a functional compensation in the single null genotypes was examined. In the decorin-null corneas, stronger biglycan reactivity compared with wild-type controls was observed by immunofluorescence microscopy. However, no reciprocal change in decorin expression was observed in biglycan-null corneas (Fig. 4, A and B). Further analyses demonstrated that biglycan protein core expression was significantly stronger in decorin-null corneas than wild-type controls as determined from semi-quantitative immunoblots (Fig. 4C). In contrast, no significant change in decorin expression was observed in biglycan-null corneas (Fig. 4D). The data illustrate that in the absence of decorin, biglycan expression is up-regulated, but not the reverse. This suggests that the lack of a phenotype in the decorin-null cornea may be due to a compensatory induction of biglycan gene expression and a resulting replacement of function.
FIGURE 4.
Biglycan expression is up-regulated in the absence of decorin. A, biglycan reactivity was increased in Dcn-/- corneas compared with wild-type (WT) controls by immunolocalization. B, in contrast decorin reactivity was comparable in Bgn-/- and wild-type mice. C, biglycan protein core expression was up-regulated in Dcn-/- corneas. Semi-quantitative immunoblots demonstrated that biglycan reactivity was significantly stronger in Dcn-/- mice than in wild-type controls. D, decorin protein core expression was comparable in Bgn-/- and wild-type corneas. Anti-actin reactivity showed comparable sample loadings between the groups.
Dysfunctional Regulation of Mature Fibril Assembly in the Absence of Decorin and Biglycan—To prevent the compensation mechanism and to unmask the roles of decorin and biglycan in the regulation of fibrillogenesis, a compound decorin/biglycan-null mouse model was produced by crossing decorin- and biglycan-null mice. In the compound decorin/biglycan-null mice, a striking disruption in fibril structure and organization was observed throughout the stroma at P60. Fibril structure was abnormal in the compound mutant cornea. Large, irregular fibrils were present in both anterior and posterior stroma (Fig. 5). However, the posterior region had a more severe fibril phenotype. The aberrant fibrils in the posterior stroma were larger and more irregular than in the anterior region. Fibril diameter analyses demonstrated that there was a fibril subpopulation with very large diameters in both anterior and posterior stroma in compound decorin/biglycan-null mice that was absent in wild-type controls (Fig. 6). This distinct fibril subpopulation with very large diameters and irregular contours in the posterior stroma indicated an abnormal lateral association and fusion of fibrils indicating a dysfunctional regulation of the fibril growth stages. However, during early developing stages (P4), fibril structure is unaltered in the compound decorin/biglycan-null corneas (Fig. 7). The fibril contours and diameters were comparable in the compound mutant and wild-type mice. P4 is an early developing stage of stromal development where initiation of fibril intermediate assembly predominates. The data suggest that class I SLRPs, decorin and biglycan, are involved in the regulation of fibril linear growth and lateral association to form mature fibrils at later developing stages, i.e. P60. Lateral growth does not occur during normal corneal development and the abnormal growth seen in the compound mutant mice indicated a loss of this key regulatory step in corneal fibrillogenesis. The disruption of decorin and biglycan does not affect the initial formation of fibril intermediates. In addition, fibril packing, lamellar organization, and stromal architecture were severely disrupted in the compound decorin/biglycan-null cornea. This disruption was present throughout the stroma, but was more severe in the posterior stroma.
FIGURE 5.
Aberrant fibril structure in the compound decorin/biglycan-null corneas. Transmission electron micrographs of the posterior and anterior stroma from: wild type (WT) and double-null (Dcn-/-,Bgn-/-) mice at P60. The compound mutants demonstrated an aberrant fibril phenotype (arrows). Large, irregular fibrils were present in both anterior and posterior stroma, but the posterior region had the more severe phenotype. In addition, there were disruptions in fibril packing and organization in the double-null mice.
FIGURE 6.
The compound decorin/biglycan-null cornea demonstrated a fibril subpopulation with abnormally large diameters. The fibril diameter distribution is abnormal in the compound Dcn-/-/Bgn-/- corneas containing a fibril subpopulation with abnormally large diameters in both the anterior and posterior stroma compared with wild-type controls. The compound mutant corneas showed a broader fibril diameter range indicating a disrupted regulation in fibril growth.
FIGURE 7.
Fibril structure is unaltered in the compound decorin/biglycan-deficient corneas during early stages of fibrillogenesis. Fibril structure was comparable in compound decorin/biglycan-deficient (Dcn-/-,Bgn-/-) and wild-type (WT) corneas at P4, a developmental stage dominated by the initiation of fibril intermediate assembly. The absence of a mutant phenotype suggests that decorin and biglycan function in regulation of linear and lateral fibril association to form mature fibrils at later developing stages, i.e. P60. The disruption of decorin and biglycan has little or no effect on the initial fibril precursor assembly.
The cornea contains high concentrations of class II SLRPs, particularly lumican and keratocan. To address the possibility that the observed alterations in stromal fibrillogenesis were secondary effects due to a compensatory up-regulation of other regulatory SLRPs, keratocan, lumican, and fibromodulin expression in the decorin- and biglycan-null corneas were analyzed (Fig. 8). Expression was analyzed at P30, a period when both decorin and biglycan are normally expressed. A semi-quantitative immunoblot analysis demonstrated no changes in the expression of the major corneal class II SLRPs keratocan and lumican in either the decorin-deficient (Fig. 8A) or biglycan-deficient mouse (Fig. 8B). In contrast, there was a consistent decrease in fibromodulin expression in the decorin- and biglycan-deficient corneas. However, our data and others indicate very low levels of fibromodulin expression in the corneal stroma (24). In addition, a corneal phenotype has not been reported for the fibromodulin-deficient mouse. Overall, the data support a direct relationship between the absence of decorin and dysfunctional regulation of corneal fibrillogenesis and stromal organization.
FIGURE 8.
Class II leucine-rich proteoglycan expression in decorin- and biglycan-null mice. Semiquantitative immunoblots of representative experiments. A, no change in lumican or keratocan protein core expression was detected in Dcn-/- corneas compared with wild-type controls. Fibromodulin expression decreased slightly in Dcn-/- corneas compared with controls, however, fibromodulin expression was very low compared with both lumican and keratocan. B, the results in the Bgn-/- corneas were comparable with those in the Dcn-/- corneas. There was no obvious change in lumican or keratocan with a slight, consistent decrease in fibromodulin protein core expression compared with wild-type controls.
Biglycan Substitutes for Decorin in in Vitro Fibrillogenesis—To further test our hypothesis that higher concentrations of biglycan could substitute for the regulatory function of decorin; in vitro fibrillogenesis turbidity assays were performed with native type I collagen. Recombinant decorin and biglycan protein cores and intact proteoglycans were used. Turbidity assays demonstrated that decorin and biglycan have the same effect on in vitro fibrillogenesis and reduce the maximum A313 nm value, compared with the control, in a dose-dependent manner (Fig. 9). Comparable effects were observed for core proteins and intact proteoglycans, for both decorin and biglycan. At high proteoglycan to collagen ratios, decreased rates of fibrillogenesis and the decrease in the maximum A313 nm is similar for both decorin and biglycan. However, to obtain similar effects on fibrillogenesis at lower proteoglycan to collagen ratios, more biglycan was required than decorin. The data indicate that decorin and biglycan coordinately regulate in vitro fibrillogenesis. Decorin is the higher affinity, major component and biglycan is the lower affinity, minor component. Therefore, in the absence of decorin; biglycan at higher concentrations can compensate for and rescue the loss of the regulatory roles of decorin.
FIGURE 9.
Biglycan can substitute for decorin in regulation of fibrillogenesis. Representative in vitro fibrillogenesis assays where increased turbidity is followed as changes in absorbance as a function of time. A, at a stoichiometry (protein core/proteoglycan:collagen) of 1:6, there were alterations in the fibrillogenesis assays with decorin and biglycan, but the magnitude of the biglycan effect was markedly reduced compared with decorin. B, the differences were mitigated at a molar ratio of 1:1 for both protein cores and intact proteoglycans, but decorin was consistently more efficient than biglycan. C, at very high molar ratios of either protein core, 10:1, both decorin and biglycan demonstrated comparable effects on fibrillogenesis. D, normal, striated fibrils were assembled under all experimental conditions. Transmission electron micrographs of the negatively stained preparation of type I collagen alone, with decorin (6:1), and with biglycan (6:1) are shown. Stoichiometry was based on the molar ratio of proteoglycans or core protein to type I collagen (300 kDa). Proteoglycan and protein core concentrations were determined from protein concentrations using a molecular mass of 38.7 kDa for the biglycan core and 38.4 kDa for the decorin core.
DISCUSSION
In this study, we investigated the regulatory roles of decorin and biglycan in corneal fibrillogenesis using decorin-null, biglycan-null, and compound null mouse models. In the normal developing cornea, there was a differential temporal expression of decorin and biglycan. However, spatial expression was comparable and overlapped that of type I collagen consistent with a regulatory role in fibrillogenesis. Our data and previously published work utilizing the decorin-null mouse (27) demonstrated a comparable fibril structure and diameter distribution in all three genotypes. Similarly, decorin- and biglycan-null mice had a comparable and mild cutaneous phenotype, however, a more severe phenotype was observed in the compound mutant mice, indicating an interaction between decorin and biglycan in skin and bone (38). This could be the result of cooperative regulatory role(s) in fibrillogenesis or/and a compensation or rescue involving decorin and biglycan. These data suggest that the regulatory functions may require the interaction of both decorin and biglycan. This is consistent with our data demonstrating a severe corneal stromal fibril phenotype in compound decorin/biglycan-null corneas.
Six SLRPs; class I, decorin and biglycan; and class II, lumican, keratocan, fibromodulin, and osteoglycin are known to be expressed in cornea (9, 20-24, 39). All of the corneal SLRPs have been “knocked out” in the mouse (25-27, 31, 40-42) and all demonstrate abnormal connective tissue phenotypes. To date, only mice deficient in lumican were shown to have a significant disruption in corneal fibril architecture. The fibrils in the posterior stroma of adult lumican-null corneas showed a wide range in fibril diameter, with a population of abnormally large fibrils having irregular contours (8, 9, 25). Alterations in fibril packing also were suggested by x-ray diffraction (43). However, the anterior stroma was virtually normal. No significant corneal fibril phenotype was observed in mice deficient in other SLRPs. The phenotype in compound decorin/biglycan-deficient mice, described in this study, is the most severe corneal stromal phenotype reported. Fibril structure and organization were disrupted throughout the stroma. This is the only SLRP knock-out model demonstrating an anterior stromal phenotype.
Decorin and biglycan are two closely related class I SLRP members that have striking homology in their primary structure (11). They are widely co-distributed in type I and II collagen fibril-rich connective tissues, e.g. corneas, tendons, skin, bone, and cartilage (44). Decorin and biglycan compete for the same site on type I collagen with decorin demonstrating greater affinity than biglycan. This locus is distinct from the fibromodulin/lumican binding site (36, 37, 45). In this study, we demonstrated a cooperative relationship between decorin and biglycan in the regulation of corneal fibrillogenesis. The differential temporal and comparable spatial expression patterns in the developing cornea; the significantly higher content of decorin than biglycan in cornea; the up-regulation of biglycan in the absence of decorin, but not the reverse; and the synergistic fibril phenotype in compound null mice all indicate a coordinate regulation of corneal fibrillogenesis by decorin and biglycan. Our data suggest that during normal cornea development, decorin is the dominant regulator, whereas biglycan, expressed early in development, modulates the role of decorin during this period.
Our data indicate that biglycan can compensate for the loss of decorin. This is supported by the data indicating a significant increase in biglycan expression associated with the lack of a significant phenotype in decorin-null corneas. In vitro fibrillogenesis assays confirmed that biglycan can replace the regulatory function of decorin, but not as efficiently. Biglycan required higher concentrations for comparable effects. Both intact proteoglycans and core proteins had comparable effects. In contrast, decorin expression in the biglycan-null cornea remained unchanged suggesting that decorin is the dominant class I SLRP; therefore biglycan-deficient corneas do not have a phenotype. When compensation is blocked in the compound null mice, a significant phenotype was observed in mature corneas.
Human corneal congenital stromal dystrophy is an autosomal dominant disease where a point mutation results in a truncation of the C-terminal region of the decorin core protein (28, 29). Clinically the cornea is cloudy shortly after birth and this is associated with thin filamentous collagen that is poorly arranged. This defective matrix exists adjacent to normal appearing lamellae (28). Like the phenotype described here, abnormal fibrils are seen throughout the stroma with greater severity in the posterior region. The affected individuals are heterozygous for the mutant allele and the mutant core presumably alters the normal decorin-collagen regulatory interactions in a dominant-negative fashion. However, the dysfunctional regulatory step(s) remain to be elucidated.
During tendon development, fibromodulin and lumican are involved in the regulation of fibrillogenesis (12). As was the case for decorin and biglycan, with development, fibromodulin expression increases and lumican decreases. In the absence of fibromodulin, lumican deposition in tendon is increased to compensate for the loss of regulatory roles of fibromodulin (42). Lumican and fibromodulin deficiency results in a comparable phenotype with irregular contour and larger fibril diameters in tendon at early developing stages. At maturation, tendon fibrils are virtually normal in lumican-null mice, but show a severe disruption in fibrillogenesis in fibromodulin-deficient mouse. The phenotype in lumican and fibromodulin double deficient tendons is additive (12). Functional studies demonstrated that fibromodulin was required for development of normal biomechanical properties and fibromodulin-null mice had decreased mechanical properties. The absence of lumican had no effect. However, in compound mutant tendons decreased lumican (+/+, +/-, -/-) modified the effect in a dose-dependent manner (13). These data indicate a coordinate relationship between lumican and fibromodulin in the regulation of tendon fibrillogenesis. We suggest that biglycan has a similar modulating role in cornea. In contrast to the tendon, in the cornea lumican is a quantitatively major proteoglycan. At early developing stages lumican is expressed throughout the stroma, but restricted to the posterior region in mature stroma. Consequently, in lumican-null mice, a phenotype with altered fibril structure and fibril diameter was observed more markedly in posterior stroma than the anterior region (8, 9, 25). The expression of lumican and keratocan is coupled (16). Decreased keratocan expression was observed in the lumican-null cornea and overexpression of lumican resulted in an increased keratocan expression. Different SLRP classes also show coordinate regulatory roles in fibrillogenesis (46). Studies with biglycan/fibromodulin-deficient mice demonstrate that biglycan interacts with fibromodulin (10, 14). The phenotype of the biglycan/fibromodulin double mutant mice is synergistic, indicating the cooperative relationship between the two SLRPs. The regulation of fibrillogenesis by SLRPs requires an integrated interaction of several fibril-associated molecules to establish the functional architecture and mechanical properties of connective tissues.
Given the possibility of redundant and/or complementary effects involving the class II SLRPs, we examined the expression of keratocan, lumican, and fibromodulin in the decorin- and biglycan-null mice. There was no effect on expression of keratocan or lumican in either null cornea. Quantitatively, these are the major corneal class II SLRPs and therefore, this supports the direct role for decorin in regulation of corneal fibrillogenesis. However, we observed a consistent decrease in fibromodulin in the null corneas. Fibromodulin is not generally considered a component of the stromal matrix, but it is expressed at low levels (24, 39). The low level expression is confirmed by our analyses; however, the demonstration of synergistic interaction in the compound decorin-fibromodulin and biglycan-fibromodulin null mice indicated further analyses are required.
Collagen fibrillogenesis is a multistep process and each step is distinct and independently regulated (2, 4, 5, 47, 48). The first step involves the collagen assembly to form fibril intermediates. The immature fibril intermediates assemble to form mature fibrils. Mature fibrils are formed by regulated end-to-end assembly and lateral assembly from the immature preformed intermediates. The characteristic feature of cornea stromal architecture is the fibrils with universal small diameters, a requisite for transparency and a functional cornea. Therefore, there is no significant lateral growth in corneal fibrillogenesis. The aberrant fibrils with very large diameters and irregular contours in mature (P60) corneas from compound decorin/biglycan-deficient mice indicate that the lack of class I SLRPs results in dysfunctional regulation of fibrillogenesis in cornea development. However, our data show that fibril structure and diameter at P4, an early stage in corneal development, dominated by fibril intermediate assembly rather than fibril growth, is comparable with the wild-type controls. Regulatory role(s) for class I SLRPs, decorin, and biglycan, were indicated in fibril growth during the later stages of fibrillogenesis, but not the initial formation of fibril intermediates.
In conclusion, our data indicate that decorin has a key role in the regulation of stromal fibril assembly, primarily regulating lateral fibril growth. Decorin and biglycan have a coordinate and cooperative relationship in the regulation of stromal fibrillogenesis. In the absence of decorin, biglycan expression and deposition in the cornea is up-regulated to compensate for the loss of the regulatory roles of decorin, resulting in relatively normal fibrillogenesis. However, in double-deficient mice when compensation was prevented, the dominant decorin-deficient phenotype prevailed.
Acknowledgments
We gratefully acknowledge Diana Menezes, Kaitlin Petrella, and Kate Buchler for expert technical assistance.
This work was supported, in whole or in part, by National Institutes of Health NEI Grant EY005129 (to D. E. B.) and support from the Intramural Research Program, NIDCR (to M. F. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SLRP, small leucine-rich proteoglycan; PBS, phosphate-buffered saline; WT, wild type; P, postnatal; ELISA, enzyme-linked immunosorbent assay.
References
- 1.Wenstrup, R. J., Florer, J. B., Brunskill, E. W., Bell, S. M., Chervoneva, I., and Birk, D. E. (2004) J. Biol. Chem. 279 53331-53337 [DOI] [PubMed] [Google Scholar]
- 2.Birk, D. E., Zycband, E. I., Winkelmann, D. A., and Trelstad, R. L. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 4549-4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birk, D. E., Nurminskaya, M. V., and Zycband, E. I. (1995) Dev. Dyn. 202 229-243 [DOI] [PubMed] [Google Scholar]
- 4.Birk, D. E., Zycband, E. I., Woodruff, S., Winkelmann, D. A., and Trelstad, R. L. (1997) Dev. Dyn. 208 291-298 [DOI] [PubMed] [Google Scholar]
- 5.Zhang, G., Young, B. B., Ezura, Y., Favata, M., Soslowsky, L. J., Chakravarti, S., and Birk, D. E. (2005) J. Musculoskelet. Neuronal. Interact. 5 5-21 [PubMed] [Google Scholar]
- 6.Blaschke, U. K., Eikenberry, E. F., Hulmes, D. J., Galla, H. J., and Bruckner, P. (2000) J. Biol. Chem. 275 10370-10378 [DOI] [PubMed] [Google Scholar]
- 7.Iozzo, R. V. (1999) J. Biol. Chem. 274 18843-18846 [DOI] [PubMed] [Google Scholar]
- 8.Chakravarti, S., Petroll, W. M., Hassell, J. R., Jester, J. V., Lass, J. H., Paul, J., and Birk, D. E. (2000) Investig. Ophthalmol. Vis. Sci. 41 3365-3373 [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti, S., Zhang, G., Chervoneva, I., Roberts, L., and Birk, D. E. (2006) Dev. Dyn. 235 2493-2506 [DOI] [PubMed] [Google Scholar]
- 10.Ameye, L., and Young, M. F. (2002) Glycobiology 12 107R-116R [DOI] [PubMed] [Google Scholar]
- 11.Schaefer, L., and Iozzo, R. V. (2008) J. Biol. Chem. 283 21305-21309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezura, Y., Chakravarti, S., Oldberg, A., Chervoneva, I., and Birk, D. E. (2000) J. Cell Biol. 151 779-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jepsen, K. J., Wu, F., Peragallo, J. H., Paul, J., Roberts, L., Ezura, Y., Oldberg, A., Birk, D. E., and Chakravarti, S. (2002) J. Biol. Chem. 277 35532-35540 [DOI] [PubMed] [Google Scholar]
- 14.Ameye, L., Aria, D., Jepsen, K., Oldberg, A., Xu, T., and Young, M. F. (2002) FASEB J. 16 673-680 [DOI] [PubMed] [Google Scholar]
- 15.Zhang, G., Ezura, Y., Chervoneva, I., Robinson, P. S., Beason, D. P., Carine, E. T., Soslowsky, L. J., Iozzo, R. V., and Birk, D. E. (2006) J. Cell Biochem. 98 1436-1449 [DOI] [PubMed] [Google Scholar]
- 16.Carlson, E. C., Liu, C. Y., Chikama, T., Hayashi, Y., Kao, C. W., Birk, D. E., Funderburgh, J. L., Jester, J. V., and Kao, W. W. (2005) J. Biol. Chem. 280 25541-25547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birk, D. E., Silver, F. H., and Trelstad, R. L. (1991) in Cell Biology of the Extracellular Matrix (Hay, E. D., ed) pp. 221-254, Plenum Press, New York
- 18.Birk, D. E., Hahn, R. A., Linsenmayer, C. Y., and Zycband, Z. I. (1996) Matrix Biol. 15 111-118 [DOI] [PubMed] [Google Scholar]
- 19.Birk, D. E., and Bruckner, P. (2005) in Topics in Current Chemistry: Collagen (Brinckmann, J., Müller, P. K., and Notbohm, H., eds) Vol. 247, pp. 185-205, Springer-Verlag, Berlin [Google Scholar]
- 20.Blochberger, T. C., Cornuet, P. K., and Hassell, J. R. (1992) J. Biol. Chem. 267 20613-20619 [PubMed] [Google Scholar]
- 21.Funderburgh, J. L., Corpuz, L. M., Roth, M. R., Funderburgh, M. L., Tasheva, E. S., and Conrad, G. W. (1997) J. Biol. Chem. 272 28089-28095 [DOI] [PubMed] [Google Scholar]
- 22.Beales, M. P., Funderburgh, J. L., Jester, J. V., and Hassell, J. R. (1999) Investig. Ophthalmol. Vis. Sci. 40 1658-1663 [PubMed] [Google Scholar]
- 23.Dunlevy, J. R., Beales, M. P., Berryhill, B. L., Cornuet, P. K., and Hassell, J. R. (2000) Exp. Eye Res. 70 349-362 [DOI] [PubMed] [Google Scholar]
- 24.Schonherr, E., Sunderkotter, C., Schaefer, L., Thanos, S., Grassel, S., Oldberg, A., Iozzo, R. V., Young, M. F., and Kresse, H. (2004) J. Vasc. Res. 41 499-508 [DOI] [PubMed] [Google Scholar]
- 25.Chakravarti, S., Magnuson, T., Lass, J. H., Jepsen, K. J., LaMantia, C., and Carroll, H. (1998) J. Cell Biol. 141 1277-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saika, S., Shiraishi, A., Liu, C. Y., Funderburgh, J. L., Kao, C. W., Converse, R. L., and Kao, W. W. (2000) J. Biol. Chem. 275 2607-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danielson, K. G., Baribault, H., Holmes, D. F., Graham, H., Kadler, K. E., and Iozzo, R. V. (1997) J. Cell Biol. 136 729-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredrup, C., Knappskog, P. M., Majewski, J., Rodahl, E., and Boman, H. (2005) Investig. Ophthalmol. Vis. Sci. 46 420-426 [DOI] [PubMed] [Google Scholar]
- 29.Rodahl, E., Van Ginderdeuren, R., Knappskog, P. M., Bredrup, C., and Boman, H. (2006) Am. J. Ophthalmol. 142 520-521 [DOI] [PubMed] [Google Scholar]
- 30.McEwan, P. A., Scott, P. G., Bishop, P. N., and Bella, J. (2006) J. Struct. Biol. 155 294-305 [DOI] [PubMed] [Google Scholar]
- 31.Xu, T., Bianco, P., Fisher, L. W., Longenecker, G., Smith, E., Goldstein, S., Bonadio, J., Boskey, A., Heegaard, A. M., Sommer, B., Satomura, K., Dominguez, P., Zhao, C., Kulkarni, A. B., Robey, P. G., and Young, M. F. (1998) Nat. Genet. 20 78-82 [DOI] [PubMed] [Google Scholar]
- 32.Fisher, L. W., Stubbs, J. T., III, and Young, M. F. (1995) Acta Orthop. Scand. Suppl. 266 61-65 [PubMed] [Google Scholar]
- 33.Birk, D. E., and Trelstad, R. L. (1984) J. Cell Biol. 99 2024-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birk, D. E., and Silver, F. H. (1984) Arch. Biochem. Biophys. 235 178-185 [DOI] [PubMed] [Google Scholar]
- 35.Goldoni, S., Owens, R. T., McQuillan, D. J., Shriver, Z., Sasisekharan, R., Birk, D. E., Campbell, S., and Iozzo, R. V. (2004) J. Biol. Chem. 279 6606-6612 [DOI] [PubMed] [Google Scholar]
- 36.Schonherr, E., Hausser, H., Beavan, L., and Kresse, H. (1995) J. Biol. Chem. 270 8877-8883 [DOI] [PubMed] [Google Scholar]
- 37.Schonherr, E., Witsch-Prehm, P., Harrach, B., Robenek, H., Rauterberg, J., and Kresse, H. (1995) J. Biol. Chem. 270 2776-2783 [DOI] [PubMed] [Google Scholar]
- 38.Corsi, A., Xu, T., Chen, X. D., Boyde, A., Liang, J., Mankani, M., Sommer, B., Iozzo, R. V., Eichstetter, I., Robey, P. G., Bianco, P., and Young, M. F. (2002) J. Bone Miner. Res. 17 1180-1189 [DOI] [PubMed] [Google Scholar]
- 39.Liu, C. Y., Birk, D. E., Hassell, J. R., Kane, B., and Kao, W. W. (2003) J. Biol. Chem. 278 21672-21677 [DOI] [PubMed] [Google Scholar]
- 40.Carlson, E. C., Mamiya, K., Liu, C. Y., Gendron, R. L., Birk, D. E., Funderburgh, J. L., and Kao, W. W. (2003) Biochem. J. 369 461-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasheva, E. S., Koester, A., Paulsen, A. Q., Garrett, A. S., Boyle, D. L., Davidson, H. J., Song, M., Fox, N., and Conrad, G. W. (2002) Mol. Vis. 8 407-415 [PubMed] [Google Scholar]
- 42.Svensson, L., Aszodi, A., Reinholt, F. P., Fassler, R., Heinegard, D., and Oldberg, A. (1999) J. Biol. Chem. 274 9636-9647 [DOI] [PubMed] [Google Scholar]
- 43.Quantock, A. J., Meek, K. M., and Chakravarti, S. (2001) Investig. Ophthalmol. Vis. Sci. 42 1750-1756 [PubMed] [Google Scholar]
- 44.Bianco, P., Fisher, L. W., Young, M. F., Termine, J. D., and Robey, P. G. (1990) J. Histochem. Cytochem. 38 1549-1563 [DOI] [PubMed] [Google Scholar]
- 45.Svensson, L., Narlid, I., and Oldberg, A. (2000) FEBS Lett. 470 178-182 [DOI] [PubMed] [Google Scholar]
- 46.Reed, C. C., and Iozzo, R. V. (2002) Glycoconj. J. 19 249-255 [DOI] [PubMed] [Google Scholar]
- 47.Birk, D. E., Zycband, E. I., Winkelmann, D. A., and Trelstad, R. L. (1990) Ann. N. Y. Acad. Sci. 580 176-194 [DOI] [PubMed] [Google Scholar]
- 48.Birk, D. E., and Zycband, E. (1994) J. Anat. 184 457-463 [PMC free article] [PubMed] [Google Scholar]