Abstract
Krüppel-like factor 9 (KLF9) is a thyroid hormone-induced, immediate early gene implicated in neural development in vertebrates. We analyzed stressor and glucocorticoid (GC)-dependent regulation of KLF9 expression in the brain of the frog Xenopus laevis, and investigated a possible role for KLF9 in neuronal differentiation. Exposure to shaking/confinement stressor increased plasma corticosterone (CORT) concentration, and KLF9 immunoreactivity in several brain regions, which included the medial amygdala and bed nucleus of the stria terminalis, anterior preoptic area (homologous to the mammalian paraventricular nucleus), and optic tectum (homologous to the mammalian superior colliculus). The stressor-induced KLF9 mRNA expression in the brain was blocked by pretreatment with the GC receptor antagonist RU486, or mimicked by injection of CORT. Treatment with CORT also caused a rapid and dose-dependent increase in KLF9 mRNA in X. laevis XTC-2 cells that was resistant to inhibition of protein synthesis. The action of CORT on KLF9 expression in XTC-2 cells was blocked by RU486, but not by the mineralocorticoid receptor antagonist spironolactone. To test for functional consequences of up-regulation of KLF9, we introduced a KLF9 expression plasmid into living tadpole brain by electroporation-mediated gene transfer. Forced expression of KLF9 in tadpole brain caused an increase in Golgi-stained cells, reflective of neuronal differentiation/maturation. Our results support that KLF9 is a direct, GC receptor target gene that is induced by stress, and functions as an intermediary in the actions of GCs on brain gene expression and neuronal structure.
Krüppel-like factor 9 expression is induced by stress and may mediate activity and glucocorticoid-dependent actions on brain development and plasticity.
Stress can have profound and complex effects on brain function and morphology, and consequently on learning, memory, and behavior. The effects of stress can be facilitatory or inhibitory, the outcome dependent on the duration and type of stressor, and the behavioral context within which the stress is experienced. Chronic stress leads to neurodegeneration and impairment of learning and memory (1,2,3). By contrast, short-term stress may enhance neurotransmission, promote long-term potentiation (LTP), increase dendritic spine density in the hippocampus, and facilitate memory consolidation and reconsolidation (1,2,3,4,5). Stress hormone actions on the brain lead to changes in neuronal structure, but little is known about the transcriptional mechanisms that underlie cellular changes after exposure to a stressor (3).
The primary vertebrate stress hormones, the glucocorticoids (GCs), are responsible for many effects of stress on the brain, and their actions are mediated by two nuclear receptors, the mineralocorticoid receptor (MR) and the GC receptor (GR) (6,7). Receptors located in the plasma membrane transduce rapid, nongenomic actions of GCs on neural function (8,9,10). The initial, facilitatory effects of GCs on neurotransmission and induction of LTP may be mediated by membrane GRs, whereas subsequent genomic actions, dependent on the nuclear GR, may reverse and normalize the enhanced excitability (2) and generate structural changes in neurons (3). This is considered to be an adaptive mechanism for limiting the stress response (i.e. through negative feedback) and facilitating information storage.
Earlier, we showed that a member of the Sp/Krüppel-like family of zinc-finger domain transcription factors (11,12), the Krüppel- like factor 9 (KLF9) [also basic transcription element binding protein 1 (13)], plays a key role in thyroid hormone-dependent actions on neurite extension and branching (14,15,16,17,18). Forced expression of KLF9 in Neuro-2a cells caused a dose-dependent increase in neurite extension and branching (15). Suppression of KLF9 with antisense oligodeoxynucleotides in primary embryonic rat neuronal cultures inhibited thyroid hormone-induced neurite branching (17). Furthermore, KLF9 deficient mice showed reduced dendritic arborization in cerebellar Purkinje cells and behavioral deficits consistent with abnormal functions of the amygdala, hippocampus, and cerebellum (18).
In a pilot experiment, we discovered that KLF9 immunoreactivity (ir) was strongly up-regulated in the brain of juvenile Xenopus laevis frogs after exposure to a physical stressor. In the current study, we investigated the mechanisms by which a physical stressor influences KLF9 expression, and the consequences of KLF9 up-regulation for neuronal development. Frogs were exposed to shaking/handling stressor, or injected with corticosterone (CORT) or the GR antagonist RU486, and changes in brain KLF9 expression were measured by immunohistochemistry (IHC) and real-time quantitative RT-PCR. The regulation of KLF9 gene expression by GCs was investigated using the X. laevis cell line XTC-2. Finally, the effects on neuronal differentiation and morphology of increased KLF9 expression in the brain were studied using electroporation-mediated (EM) gene transfer, followed by Golgi staining. Our results support the hypothesis that KLF9 is a direct GR target gene, and that up-regulation of KLF9 promotes neuronal differentiation.
Materials and Methods
Animal care
Juvenile X. laevis frogs (age 3–4 months) were purchased from Xenopus I, Inc. (Dexter, MI), and maintained in the laboratory in well water (20–22 C) under a 12-h light, 12-h dark photoperiod and fed beef liver. Forty-eight hours before experiments, seven animals per treatment were distributed into 10-liter aquaria that were kept behind opaque barriers to minimize stress to the animals. Animals were not fed during the 48-h period before experimentation; fasting for this period does not change plasma CORT concentration (19). Tadpoles were obtained by in-house breeding and maintained as described by Yao et al. (20). All procedures involving animals were conducted in accordance with the guidelines of the University Committee on the Care and Use of Animals of the University of Michigan.
Shaking/confinement stressor, hormone and drug treatments in vivo
To determine whether exposure to a stressor alters KLF9 expression in the frog brain, we subjected juvenile X. laevis to shaking/confinement stressor for 4 h before being killed (21). This stressor paradigm causes rapid and robust increases in plasma CORT concentration, phosphorylation of cAMP response element binding protein, and activation of corticotropin-releasing factor (CRF) neurons (20,21). Briefly, two or three frogs were placed into square 32-oz white polypropylene containers containing 100 ml water and shaken on an orbital shaker (Lab-Line Instruments, Inc., Melrose Park, IL) at 100 revolutions per minute for 4 h. Controls were left undisturbed. At the end of the treatment, frogs were rapidly killed by decapitation. Blood was collected into heparinized capillary tubes for CORT RIA, and heads were fixed in 4% paraformaldehyde at 4 C overnight before the preparation of cryosections for IHC.
We next tested whether exogenous CORT could induce KLF9 expression, and if the effects of shaking/confinement stress on KLF9 could be blocked by the GR antagonist RU486. In this experiment there were six treatments: 1) uninjected control, unstressed; 2) oil-injected control, unstressed; 3) RU486 injected, unstressed; 4) oil injected, stressed; 5) RU486 injected, stressed; and 6) CORT injected, unstressed. The body weight of the juvenile frogs averaged 15 g, the dose of CORT used was 500 ng, and RU486 was 5 μg, both administered in a 50-μl injection volume. CORT or RU486 was first dissolved in 100% ethyl alcohol (EtOH), then added dropwise to vegetable oil with stirring. Controls received vegetable oil with a comparable concentration of EtOH (0.28%) to that of the CORT and RU486 treatments. Frogs received injections of oil, CORT, or RU486 1 h before exposure to shaking stressor, and the stressor was continued for 4 h. At the end of the experiment, frogs were rapidly killed by decapitation, and the brain was microdissected into the preoptic area/diencephalon and the thalamus/optic tectum regions. Tissues were snap frozen and stored at −80 C for subsequent RNA extraction and gene expression analysis.
Plasma CORT and T4 RIAs
Plasma CORT concentration was measured by RIA (22,23). Briefly, plasma was extracted once with 5 ml diethyl ether, and the extract was dried under nitrogen and reconstituted with 500 μl PBS-gelatin buffer [0.02 m sodium phosphate (pH 7.4) and 1% gelatin]. Plasma T4 was determined following the methods of Denver (23). For both CORT and T4, all samples were measured in one assay, and the intraassay coefficient of variation was less than 10%.
Immunohistochemical analysis of KLF9
We conducted IHC for KLF9 in frog brain using an affinity purified rabbit polyclonal antiserum to X. laevis KLF9 (16,24). The antiserum was purified by affinity column chromatography such that the purified IgG recognized only the N-terminal region of the protein. A Basic Alignment Search Tool (BLAST) search of the National Center for Biotechnology Information database showed no sequence similarity of the N-terminal region of frog KLF9 to known proteins other than KLF9s. A partial cDNA for X. laevis KLF9 was isolated that corresponds to amino acids 26–140, which is N terminal to the highly conserved zinc finger region (16). This cDNA was subcloned into the TOPO-PET151/D vector (Invitrogen Corp., Carlsbad, CA) to generate TOPO-PET151-xKLF9Nterm. BL21 cells (Invitrogen) were transformed with TOPO-PET151-xKLF9Nterm, and expression was induced with 0.5 mm isopropyl-β-d-thiogalactopyranoside (Roche Applied Science, Indianapolis, IN) for 4 h. The hexahistidine-tagged recombinant protein was purified using a nickel-conjugated resin (ProBond Purification System; Invitrogen) and conjugated to Affi-gel 15 (Bio-Rad Laboratories, Inc., Richmond, CA) following the manufacturer's instructions. The antiserum was first purified over a protein A column (Pierce, Rockford, IL), followed by affinity purification on the Affi-gel 15 column. The concentration of the purified IgG was determined using the BCA Protein Assay (Pierce). The specificity of this antiserum for KLF9 has been verified and described by Bagamasbad et al. (24).
We dissected frog brains from the fixed heads, postfixed, and submerged them in 30% sucrose before snap freezing and transverse cryosectioning at 10 μm. The slides were immersed in heated citric acid for 3 min for antigen retrieval, and then blocked in Tris SuperBlock (Pierce) containing 5% normal goat serum. Tissue sections were incubated in the affinity purified anti-N-terminal KLF9 IgG (0.2 μg/ml) overnight at 4 C before detection of immune complexes using the Vectastain elite ABC (rabbit) and Vector VIP kits (Vector Laboratories, Burlingame, CA) following the manufacturer's instructions.
We captured micrographic images using an Olympus IX81 inverted microscope (Olympus, Tokyo, Japan) and a Retiga 1300R Fast digital video camera (QI Imaging, Tuscon, AZ). Brightness, contrast, and evenness of illumination were adjusted uniformly for images shown in the figures using Adobe Photoshop CS2 (Adobe Systems, Inc., San Jose, CA); images used for morphometric analysis were not adjusted. KLF9-ir was quantified in discrete brain regions using MetaMorph software (version 6. 2r4; Universal Imaging, Downingtown, PA) following methods described by Yao et al. (21,25). All samples were processed simultaneously under identical conditions. Following the anatomical definitions of Tuinhof et al. (26), three to five coronal sections from each animal that contained the anterior preoptic area [homologous to the mammalian paraventricular nucleus (PVN)], medial pallium (homologous to the mammalian hippocampus), and optic tectum (homologous to the mammalian superior colliculus) were selected for analysis. These brain regions were selected because of their strong KLF9-ir, and/or because of their known roles in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis. All sections were carefully matched for anatomical level, and digital images were captured at ×100 magnification for morphometric analysis. Image analysis was conducted in a blinded manner. Brain regions on the captured images were isolated using a handmade frame that covered the area of interest. Each selected brain region analyzed on adjacent sections and from different brains was roughly equivalent in total area, but because each sample differed slightly in shape, we hand-drew boxes around each region of interest. Using the “Auto threshold for dark objects” tool in the MetaMorph software, the threshold was adjusted to eliminate background staining. This was repeated on five to eight sections, and a mean threshold was established that was then set for analysis of all sections. The signal density within a selected area was then counted automatically. The signal density was divided by the total area of the selected brain region to obtain a mean signal density, which allowed for correction for size differences between brains and between adjacent sections. The average of the mean densities in a given brain region on replicate brain sections was calculated, summed for all animals in the treatment, then divided by the number of animals in the treatment to obtain the average signal density for each brain region (see Refs. 21 and 25). This method allows for the analysis of changes in the immunoreactive signal density within a given area, which reflects changes in the expression level per cell and the number of cells reaching the threshold for detection. It does not directly measure the number of immunoreactive cells.
XTC-2 cell culture conditions and experiments
The X. laevis embryonic fibroblast-derived cell line XTC-2 was cultured in Leibovitz L-15 media (pH 7.4), with 10% fetal bovine serum that was stripped of thyroid hormone and steroids (25,27). Cells were cultured at 25 C in an atmosphere of 5% CO2. All experiments were conducted in six-well plates (BD Falcon; BD Biosciences, San Jose, CA), and cells were plated in growth medium at a density of 350,000 cells per well. When cells had reached approximately 75% confluency, and 24 h before hormone treatments, the medium was replaced with serum-free L-15.
We first tested for dose and time-dependent effects of CORT on KLF9 mRNA expression, and then if CORT action on KLF9 mRNA is mediated by the GR or MR using the GR antagonist RU486, or the MR antagonist spironolactone. The receptor antagonists were used at a 20-fold higher concentration than CORT to block their respective receptors, and were applied 1 h before CORT treatment. CORT, RU486 (mifepristone), and spironolactone (Sigma-Aldrich Corp., St. Louis, MO) were dissolved in 100% EtOH before addition to the culture media, and an equivalent dose of ETOH was added to controls (final concentration 0.001%).
To determine whether CORT induction of KLF9 mRNA requires ongoing protein synthesis, we treated cells with 10 μg/ml cycloheximide (Sigma-Aldrich) simultaneously with CORT. This dose was shown previously to block protein synthesis in XTC-2 cells (28). To verify the efficacy of cycloheximide in blocking protein synthesis, XTC-2 cells were incubated with 10 μCi EasyTag EXPRESS 35S Protein Labeling Mix (PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA) in methionine and cysteine-deficient medium for 4 h before harvest. Cells were washed three times with ice-cold amphibian-strength Dulbecco's Phosphate Buffered Saline [diluted 1:1.5 (pH 7.4); Invitrogen], labeled proteins were precipitated with 10% ice-cold trichloroacetic acid, and radioactivity was determined by liquid scintillation counting.
RNA isolation, cDNA synthesis, and quantitative real-time PCR
We extracted RNA from frog brain sections and XTC-2 cells using the TRIzol reagent (Invitrogen) following the manufacturer's instructions. The RNA was treated with deoxyribonuclease I and ribonuclease inhibitor before cDNA synthesis with Superscript II (Invitrogen) using random hexamers (Applied Biosystems, Foster City, CA). TaqMan assays (Applied Biosystems) were used to quantify transcripts for KLF9, GR, MR, and the housekeeping gene ribosomal protein L8 (rpL8). The assays were designed to span an exon/intron boundary in each gene; the KLF9 and rpL8 assays were the same as those described by Bagamasbad et al. (24). The GR TaqMan assay was as described by Yao et al. (25). For the MR TaqMan assay, the probe used was 6FAM-CAAAAGTGATTCCAGGATT-MGQ; the forward primer was 5′-ATGGTTCAGGTGGTGAAATGG-3′, and the reverse primer was 5′-GGTCCTCCAGAGGCAAATTTCT-3′. ABsolute QPCR Low ROX Mix (Abgene Ltd., Surrey, UK) was used, and real-time PCR was conducted using an ABI 3500 real-time quantitative PCR machine (Applied Biosystems). A relative quantification method (see Refs. 20 and 29) was used to compare expression levels among the different treatments by generating standard curves for each gene with pooled cDNAs. KLF9 mRNA expression was normalized to rpL8 mRNA.
EM gene transfer
To force expression of KLF9 in the amphibian brain, we used bulk EM gene transfer as described by Haas (30) and Yao (20) et al. with minor modifications. X. laevis tadpoles at Nieuwkoop and Faber (NF) (31) stage 49–51 were anesthetized by immersion in 0.002% benzocaine before intracerebroventricular microinjection of 184 nl DNA solution. Each DNA solution contained 2 μg/μl of the pCS2-KLF9 expression plasmid or the pCS2 empty vector (control), 600 ng/μl pEGFP-N1 plasmid (Clontech Laboratories, Inc., Mountain View, CA) to monitor transfection efficiency, and 0.02% fast green dye to monitor success of the microinjection. Immediately after microinjection a pair of platinum electrodes was placed over the skull and delivered five pulses of 30 V each, then the polarity was reversed and the current delivery repeated. Animals were allowed to recover and screened for high-enhanced green fluorescent protein expression 24 h after the procedure using a MZFLIII fluorescent stereomicroscope (Leica, Bannockburn, IL). Tadpoles with green fluorescent protein expression in the brain were reared in aerated, dechlorinated tap water (20–22 C, 12 h light, 12 h dark) and fed Frog Brittle powder daily (Nasco, Fort Atkinson, WI) for 5 d before being killed for Golgi staining and histological analysis. As a positive control, tadpoles that had been electroporated with pCS2 empty vector were treated with T3 (sodium salt; Sigma-Aldrich) for 3 d by adding it to the aquarium water to a final concentration of 5 nm.
Golgi staining and morphometric analysis
We used the FD Rapid GolgiStain kit (FD NeuroTechnologies, Inc., Ellicott City, MD) to stain tadpole brain sections. Briefly, tadpole heads (∼5 × 8 × 1 mm) were immersed in the impregnation solution supplied with the kit and stored at room temperature for 2 wk in the dark. Tissues were transferred into Solution C of the Golgi stain kit and stored at 4 C for 48 h before cryosectioning. The tissues were sectioned at 100 μm and stained following the manufacturer's instructions. The number of Golgi-stained cells was counted throughout the whole brain using a Zeiss Axioskop light microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Statistical analyses
Data were analyzed using SYSTAT version 10 statistical software (SPSS, Inc., Chicago, IL). Data were log10 transformed before statistical analysis when the variances were found to be heterogeneous (Bartlett's test). Data from the CORT RIA and KLF9 IHC experiments were analyzed by the Student's unpaired t test. Experiments with more than two factors were analyzed by one-way ANOVA, followed by Fisher's least significant difference (LSD) multiple comparisons test. A P value less than 0.05 was accepted as statistically significant. Data are reported as mean ± sem.
Results
Shaking/confinement stressor increased plasma CORT and KLF9-ir in discrete regions of the frog brain
Exposure to shaking stressor for 4 h caused a 10-fold increase in plasma CORT concentration in juvenile frogs (control: 3.0 ± 0.8 ng/ml; stressed: 29.9 ± 5.4 ng/ml; P < 0.001, Student's t test; n = 6 per treatment), which is comparable to what we reported previously (21,25). Because thyroid hormone is elevated by stressors in amphibian tadpoles (23,32) and KLF9 is a known thyroid hormone-induced gene (16,33), we also tested whether the shaking stressor affected plasma T4 concentration in juvenile frogs. There was no difference in plasma T4 between unstressed and stressed juvenile frogs (control: 1.96 ± 0.26 ng/ml vs. stressed: 1.50 ± 0.28 ng/ml; n = 6 per treatment).
Shaking stressor increased the mean signal density of KLF9-ir in the lateral and medial amygdala (P < 0.007, Student's unpaired t test; Fig. 1), the anterior preoptic area (P < 0.016), and the optic tectum (P < 0.014). The mean signal density of KLF9-ir was higher in the medial pallium of stressed vs. unstressed frogs, but this did not reach statistical significance (P = 0.053). KLF9-ir was observed in regions of the telencephalon and the hindbrain/spinal cord, but it was not altered by exposure to the stressor (data not shown).
Figure 1.
Shaking/confinement stressor increases KLF9-ir in the medial pallium (mp), lateral amygdala (LA) and medial amygdala (MeA), anterior preoptic area (POa), and optic tectum (tect) of juvenile X. laevis. Frogs were subjected to shaking/confinement stressor for 4 h, and their brains analyzed for KLF9-ir by IHC as described in Materials and Methods. A, Dorsal view of the frog brain and transverse sections through two planes showing anatomical locations of major brain nuclei analyzed for KLF9-ir. The anatomical drawings are from Tuinhof et al. (26). BNST, Bed nucleus of the stria terminalis; CeA, central amygdala; dp, dorsal pallium; lp, lateral pallium; nII, cranial nerve II; pd, pars distalis; pi, pars intermedia; pn, pars nervosa; tegm, mesencephalic tectum; TP, posterior tuberculum. B, Shown are representative micrographic images of KLF9-ir in four brain areas in unstressed and stressed frogs. To the right are graphs depicting the mean signal density of KLF9-ir for unstressed (U) or stressed (S) frogs. Asterisks indicate statistically significant differences (P < 0.05, Student's unpaired t test; n = 5 per treatment). Black bar, 200 μm. [Reproduced with permission from R. Tuinhof et al.: Cell Tissue Res 292:251–265, 1998 (26). © Springer Verlag].
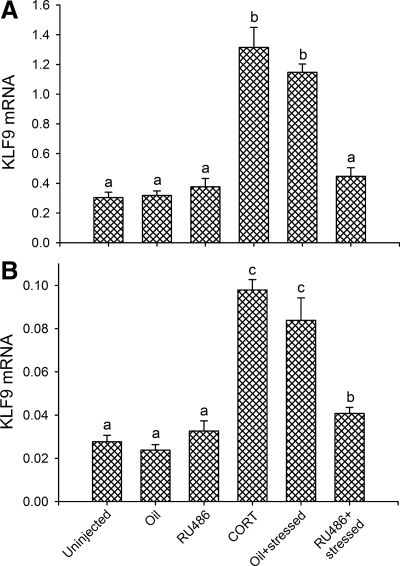
Shaking/confinement stressor or CORT injection increased KLF9 mRNA in frog brain, whereas the GR antagonist RU486 blocked the response to the stressor
KLF9 mRNA in the anterior preoptic area/diencephalon was increased by shaking/confinement stressor (∼4.5-fold) or by injection of CORT (∼4-fold) compared with uninjected, oil-injected, or RU486 injected controls, which were not different from each other [F (5,30) = 12.29; P < 0.001, ANOVA; Fig. 2A]. Pretreatment of frogs with RU486 before exposure to 4 h shaking/confinement stressor blocked the increase in KLF9 mRNA caused by the stressor (P < 0.001, Fisher's LSD test). We observed similar changes in KLF9 mRNA expression in the thalamus/optic tectum [F (5,30) = 33.24; P < 0.001; Fig. 2B].
Figure 2.
Shaking/confinement stressor and CORT act via the GR to increase KLF9 mRNA in the brain of X. laevis. Frogs received ip injections (50 μl) of oil vehicle, RU486 (5 μg), or CORT (0.5 μg) 1 h before initiation of the stressor. Juvenile X. laevis were subjected to shaking/confinement stressor for 4 h as described in Materials and Methods. Animals were rapidly euthanized, blood collected, and brains harvested, microdissected, RNA isolated and analyzed for KLF9 mRNA expression by real-time quantitative RT-PCR. A, Gene expression changes in the anterior preoptic area/diencephalon. B, Gene expression changes in the thalamus/optic tectum. Levels of KLF9 mRNA were normalized to mRNA levels of the housekeeping gene rpL8. Data were analyzed by one-way ANOVA, and letters indicate significant differences between the group means based on Fisher's LSD (P < 0.05; n = 6 per treatment).
CORT causes a time and dose-dependent increase in KLF9 mRNA in XTC-2 cells
Treatment of XTC-2 cells with CORT for 4 h caused a robust, dose-dependent increase in KLF9 mRNA [F (3,20) = 827.74; P < 0.0001, ANOVA; Fig. 3A]. CORT doses of 0.1, 1, and 100 nm resulted in KLF9 mRNA levels that were on average two, 50, and 116 times greater than untreated cells, respectively. We next conducted a time course experiment using 100 nm CORT and observed rapid (by 0.5 h), time-dependent elevation of KLF9 mRNA that reached a plateau by 2 h [F (4,25) = 700.39; P < 0.001; Fig. 3B].
Figure 3.
Treatment with CORT causes a time and dose-dependent increase in KLF9 mRNA expression in XTC-2 cells. A, Dose-dependent increase in KLF9 mRNA after 4 h treatment. B, Time-dependent increase in KLF9 mRNA of cells treated with 100 nm CORT. KLF9 gene expression was analyzed by real-time quantitative RT-PCR as described in Materials and Methods. Levels of KLF9 mRNA were normalized to mRNA levels of the housekeeping gene rpL8. Data were analyzed by one-way ANOVA, and letters indicate significant differences between the group means based on Fisher's LSD (P < 0.05; n = 6 per treatment). Each experiment was repeated three times.
CORT induction of KLF9 mRNA expression in XTC-2 cells is mediated by the GR and is resistant to protein synthesis inhibition
Treatment of XTC-2 cells with the GR antagonist RU486 (200 nm) reduced basal KLF9 mRNA and completely blocked the increase in expression caused by 10 nm CORT for 4 h [F (6,35) = 785.77; P < 0.001, ANOVA; Fig. 4A]. By contrast, the MR antagonist spironolactone (200 nm) caused only a slight reduction in basal and CORT stimulated KLF9 mRNA expression. Real-time quantitative RT-PCR analysis showed that XTC-2 cells express both GR and MR mRNAs, although a strict comparison of their expression levels cannot be made because the efficiencies of the individual TaqMan assays could differ. For GR the mean critical threshold (Ct) value was 28.40 ± 1.12, whereas for MR the mean Ct value was 33.14 ± 1.08 (n = 3).
Figure 4.
CORT induction of KLF9 mRNA in XTC-2 cells is mediated by the GR and is resistant to inhibition of protein synthesis. A, XTC-2 were cells treated with CORT (10 nm), the GR antagonist RU486 (200 nm), the MR antagonist spironolactone (SPR) (200 nm), or CORT plus the antagonists for 4 h before harvest. B, CORT induction of KLF9 mRNA in XTC-2 cells is resistant to protein synthesis inhibition. Cells were treated with or without cycloheximide (Chx) (10 μg/ml) and CORT (10 nm) for 4 h before harvest. RNA was isolated from cells, and gene expression was analyzed by real-time quantitative RT-PCR as described in Materials and Methods. Levels of KLF9 mRNA were normalized to mRNA levels of the housekeeping gene rpL8. Data were analyzed by one-way ANOVA, and letters indicate significant differences between the group means based on Fisher's LSD (P < 0.05; n = 6 per treatment). Each experiment was repeated two to three times.
Exposure to cycloheximide (10 μg/ml) for 4 h reduced protein synthesis in XTC-2 cells by 96.4% compared with control (data not shown). Treatment with CORT caused a 4-fold increase in KLF9 mRNA [F (3,20) = 244.01; P < 0.001; Fig. 4B]. Treatment with cycloheximide caused a small (∼25%) reduction in basal KLF9 mRNA expression and reduced (∼35%), but did not block, the up-regulation of KLF9 mRNA caused by CORT.
Forced expression of KLF9 in tadpole brain increases the number of Golgi-stained cells
We used EM gene transfer to force expression of KLF9 in the tadpole brain, and then analyzed effects on neuronal differentiation and morphology using Golgi staining. Premetamorphic tadpoles (NF stage 49–51) were chosen rather than later stage tadpoles or juvenile frogs because the efficiency of the electroporation technique declines dramatically after NF stage 54 (30). Few Golgi-stained cells were observed in empty vector transfected, untreated control tadpole brain. By contrast, treatment with T3 (for 3 d) or forced expression of KLF9 (for 5 d) caused dramatic increases in the number of Golgi-stained cells throughout the tadpole brain [F (2,19) = 29.5; P < 0.0001, ANOVA; n = 5–9 per treatment; Fig. 5]. The effect of T3 was greater than for forced expression of KLF9, which may reflect limits to the efficiency of the EM gene transfer. We noted that Golgi-stained cells in brains from the T3 treatment radiated out from the ventricles, with many cells closer to the ventricles than those from KLF9-transfected animals (compare panels 2 and 3 in Fig. 5A). This may indicate that the KLF9-transfected cells migrated out of the subventricular zone and differentiated over the course of the 5 d from EM gene transfer to being killed. By contrast, over the 3 d T3 treatment, some cells moved out of the subventricular zone, whereas other, newly differentiated cells were in the process of migrating. T3 treatment over this time course causes a burst of proliferation in periventricular neurogenic zones and lateral cell migration (34).
Figure 5.
Forced expression of KLF9 in tadpole brain increases the number of Golgi-stained cells. Brains of living tadpoles (NF stage 49–51) were transfected with a KLF9 expression vector pCS2-KLF9 or empty vector pCS2 by EM gene transfer and reared for 5 d before being killed as described in Materials and Methods. The empty vector transfected animals were treated with or without 5 nm T3 added to the aquarium water for 3 d before being killed. A, Representative micrographic images showing Golgi-stained neurons in the anterior preoptic area (panels 1–3) or the optic tectum (panels 4–6). pCS2 (empty), no T3 − 1,4; pCS2 (empty), +T3 − 2,5; pCS2-KLF9, no T3 – 3,6. Black bar, 100 μm. B, Effects of forced expression of KLF9 or T3 on the mean number of Golgi-stained cells throughout the tadpole brain. Data were analyzed by one-way ANOVA, and letters indicate significant differences between the group means based on Fisher's LSD (P < 0.05; n = 8 for pCS2 (empty), n = 8 for pCS2 (empty) +T3, and n = 5 for pCS2-KLF9).
Discussion
Stress hormones have diverse, complex, and even paradoxical effects on the brain, which underlie profound alterations in learning and memory, and behavior. Exposure to chronic stress causes neurodegeneration (2,3), whereas the effects of acute, short-term stress may be facilitatory depending on the type and intensity of the stressor, and the stage of development when stress is experienced (2,3,4). Stress hormones modulate brain function in part by altering the structure of neurons, and the maintenance of these changes depends on de novo gene transcription and protein synthesis. Here, we show that KLF9, a zinc finger transcription factor previously shown to mediate thyroid hormone action on neurite extension and branching (15,17), is strongly induced in the brain after exposure to a physical stressor. Shaking/confinement stressor caused a 10-fold increase in plasma CORT concentration, and increased KLF9 protein and mRNA in discrete regions of the frog brain. The increase in KLF9 mRNA caused by the stressor was mimicked by CORT injection, and stressor-dependent expression of KLF9 mRNA was completely blocked by injection of the GR antagonist RU486, thus supporting that KLF9 gene activation is mediated by the GR. We also show that forced expression of KLF9 in the amphibian brain increased the number of Golgi-stained cells, which supports previous findings in mammals that this transcription factor mediates hormone action on neuronal differentiation and structure.
We observed significant, stressor-induced increases in KLF9-ir in several brain areas that are known to play roles in the regulation of the HPA axis and in behavioral stress responses. For example, KLF9-ir and mRNA were strongly increased in the anterior preoptic area, a region of the frog brain that houses hypophysiotropic CRF neurons and a high density of GR-ir that colocalizes with CRF (35). Exposure to stressors or GCs causes functional and morphological changes in mammalian PVN neurons (2,3). There were also significant increases in KLF9-ir in limbic structures that express GR (25) such as the lateral and medial amygdala, but lesser effects in the medial pallium, a region of the frog brain that is homologous to the mammalian hippocampus (25,35). The amygdala is strongly influenced by stress hormones, and plays critical roles in stress-related behaviors and the activity of the HPA axis (36,37). Chronic stress enhances amygdala-dependent unlearned fear and fear conditioning (38), and causes dendritic hypertrophy in the amygdala (36,39,40), an effect opposite to what is seen in the hippocampus (3). The mammalian hippocampus plays a central role in negative feedback by GCs on the HPA axis via inhibitory projections to PVN neurons [reviewed by Yao and Denver (41)]. Adaptive structural plasticity occurs in CA3 pyramidal cells of the hippocampus that undergo rapid and reversible remodeling of their dendrites, in part modulated by GCs (42,43). Our finding of GC-induced KLF9 expression suggests the hypothesis that this transcription factor, which is known to influence neuronal structure, could play a key role in mediating GC actions on the development and plasticity of the limbic system and the neuroendocrine brain.
We also observed strong stressor and GC-induced increases in KLF9 expression in the optic tectum, which in the frog serves as the interface for visuomotor processing and is considered to be homologous to the mammalian superior colliculus. The optic tectum receives input from the retina, and also houses “command” neurons that initiate motor responses (see Ref. 44). These command neurons play a central role in initiating prey catching behavior, and their activity can be negatively (short term) or positively (longer term) modulated by stress. The GR is expressed in the optic tectum (25), and this region of the amphibian brain shows robust activity dependent plasticity, and has, therefore, been used as a model system to study activity dependent dendritic remodeling, axonal and synaptic development (45,46,47).
To investigate the mechanisms by which GCs influence KLF9 mRNA expression, we turned to the Xenopus tissue culture cell line XTC-2, which is known to up-regulate KLF9 mRNA in response to thyroid hormone (24). In XTC-2 cells, as in the brain in vivo, CORT induced KLF9 mRNA in a dose and time-dependent manner, and this gene induction could be blocked by RU486, but not by the MR antagonist spironolactone. KLF9 up-regulation exhibited rapid kinetics, occurring within 30 min exposure to the hormone. The action of CORT on KLF9 expression was largely, but not entirely, independent of new protein synthesis. We recently found that KLF9 is up-regulated by CORT via a GR-dependent pathway in the mouse hippocampus-derived cell line HT-22 (P.B. and R.J.D., unpublished data). Furthermore, Irlbacher and colleagues (48) identified functional corticosteroid receptor binding sites in the 5′ proximal region of the human KLF9 gene. Together, the evidence supports that KLF9 is a direct GR target gene that is rapidly induced upon exposure to stressors that elevate plasma GCs, and that this mode of gene regulation is evolutionarily conserved.
Previously, we showed that KLF9 promotes differentiation of mammalian neuronal cells, and mediates thyroid hormone actions on neurite extension and branching (15,17). The number of dendritic spines influences synaptic efficacy and network excitability, and is thus implicated in learning and memory (49). Disruption of the KLF9 gene in the mouse led to behavioral abnormalities characteristic of defects in the hippocampus, cerebellum, and amygdala, and reduced dendritic arborization of cerebellar Purkinje cells (18). The expression of KLF9 in mouse hippocampus increases dramatically at postnatal d 7, which is a time of major synaptic development in this brain region. KLF9 was recently discovered to be an activity regulated gene in cortical neurons and is implicated in the development of inhibitory synapses (50).
We found that forced expression of KLF9 in the amphibian brain led to a dramatic increase in the number of Golgi-stained cells. For comparison, tadpoles were treated with T3, which is known to promote neuronal differentiation (see Ref. 51). We interpret the increase in Golgi-stained cells in T3-treated and KLF9-transfected animals to reflect increased neuronal differentiation and/or maturation in the tadpole brain. The Golgi technique impregnates cells with silver chromate and stains a limited number of cells in their entirety, but the mechanism by which this occurs and the selectivity for some cells over others are not understood (52). We anticipated that this technique would allow us to examine differences in the number, length, and number of branch points of neuronal processes. However, the main effect of the treatments was to increase the number of Golgi-stained cells. A rough analysis of the number of branches showed no significant differences between groups, but the number of Golgi-stained cells in the controls was too few to conduct an accurate analysis. Further study is required to identify specific changes in neuronal differentiation and morphology in the amphibian brain induced by KLF9.
Structural plasticity in the brain depends on de novo transcription and local dendritic protein synthesis (53). The maintenance of LTP depends in part on structural remodeling of potentiated synapses, which involves the growth and remodeling of dendritic spines and increases in the size of the postsynaptic density. Dendritic spines undergo rapid shape changes under the influence of neuronal activity, and as discussed previously, by stress hormones. Very little is known about the transcriptional mechanisms that underlie synaptic plasticity. We propose that KLF9 is an immediate early transcription factor induced by stress that integrates activity and hormone-dependent pathways in the maintenance of structural plasticity in the brain.
Acknowledgments
We thank Meng Yao for providing technical assistance.
Footnotes
This research was supported by National Institutes of Health Grant 1 R01 NS046690 and National Science Foundation Grant IBN 0235401 (to R.J.D.).
Present address for R.M.B.: Department of Biological Sciences, University of Tulsa, 800 South Tucker Drive, Tulsa, Oklahoma 74104.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 26, 2008
Abbreviations: CORT, Corticosterone; CRF, corticotropin-releasing factor; EM, electroporation-mediated; EtOH, ethyl alcohol; GC, glucocorticoid; GR, glucocorticoid receptor; HPA, hypothalamic-pituitary-adrenal; IHC, immunohistochemistry; ir, immunoreactivity; KLF9, Krüppel-like factor 9; LSD, least significant difference; LTP, long-term potentiation; MR, mineralocorticoid receptor; NF, Nieuwkoop and Faber; PVN, paraventricular nucleus; rpL8, ribosomal protein L8.
References
- Radley JJ, Morrison JH 2005 Repeated stress and structural plasticity in the brain. Ageing Res Rev 4:271–287 [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ 2007 Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol 28:72–96 [DOI] [PubMed] [Google Scholar]
- McEwen BS 2007 Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904 [DOI] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S 2006 Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141:907–915 [DOI] [PubMed] [Google Scholar]
- Shors TJ 2006 Stressful experience and learning across the lifespan. Annu Rev Psychol 57:55–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M 2006 Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 27:244–250 [DOI] [PubMed] [Google Scholar]
- Denver RJ, Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann NY Acad Sci, in press [DOI] [PubMed] [Google Scholar]
- Borski RJ 2000 Nongenomic membrane actions of glucocorticoids in vertebrates. Trends Endocrinol Metab 11:427–436 [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP 2004 Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R 2006 Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147:5549–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R 2003 Sp1- and Krüppel-like transcription factors. Genome Biol 4:206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntram S, Bruford E, Philipsen S 2005 Mammalian SP/KLF transcription factors: bring in the family. Genomics 85:551–556 [DOI] [PubMed] [Google Scholar]
- Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y 1992 Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J 11:3663–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver RJ, Pavgi S, Shi YB 1997 Thyroid hormone-dependent gene expression program for Xenopus neural development. J Biol Chem 272:8179–8188 [DOI] [PubMed] [Google Scholar]
- Denver RJ, Ouellet L, Furling D, Kobayashi A, Fujii-Kuriyama Y, Puymirat J 1999 Basic transcription element binding protein (BTEB) is a thyroid hormone-regulated gene in the developing central nervous system: evidence for a role in neurite outgrowth. J Biol Chem 274:23128–23134 [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, Huang LY, Denver RJ 2002 Basic transcription element binding protein is a thyroid hormone-regulated transcription factor expressed during metamorphosis in Xenopus laevis. Dev Growth Differ 44:365–381 [DOI] [PubMed] [Google Scholar]
- Cayrou C, Denver RJ, Puymirat J 2002 Suppression of the basic transcription element-binding protein in brain neuronal cultures inhibits thyroid hormone-induced neurite branching. Endocrinology 143:2242–2249 [DOI] [PubMed] [Google Scholar]
- Morita M, Kobayashi A, Yamashita T, Shimanuki T, Nakajima O, Takahashi S, Ikegami S, Inokuchi K, Yamashita K, Yamamoto M, Fujii-Kuriyama Y 2003 Functional analysis of basic transcription element binding protein by gene targeting technology. Mol Cell Biol 23:2489–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Vaudry H, Denver RJ 2004 Roles of corticotropin-releasing factor, neuropeptide Y and corticosterone in the regulation of food intake in Xenopus laevis. J Neuroendocrinol 16:279–288 [DOI] [PubMed] [Google Scholar]
- Yao M, Stenzel-Poore M, Denver RJ 2007 Structural and functional conservation of vertebrate corticotropin-releasing factor genes: evidence for a critical role for a conserved cyclic AMP response element. Endocrinology 148:2518–2531 [DOI] [PubMed] [Google Scholar]
- Yao M, Westphal NJ, Denver RJ 2004 Distribution and acute stressor-induced activation of corticotrophin-releasing hormone neurones in the central nervous system of Xenopus laevis. J Neuroendocrinol 16:880–893 [DOI] [PubMed] [Google Scholar]
- Licht P, McCreery BR, Barnes R, Pang R 1983 Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol 50:124–145 [DOI] [PubMed] [Google Scholar]
- Denver RJ 1998 Hormonal correlates of environmentally induced metamorphosis in the Western spadefoot toad, Scaphiopus hammondii. Gen Comp Endocrinol 110:326–336 [DOI] [PubMed] [Google Scholar]
- Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ 2008 A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor β. J Biol Chem 283:2275–2285 [DOI] [PubMed] [Google Scholar]
- Yao M, Hu F, Denver RJ 2008 Distribution and corticosteroid regulation of glucocorticoid receptor in the brain of Xenopus laevis. J Comp Neurol 508:967–982 [DOI] [PubMed] [Google Scholar]
- Tuinhof R, Ubink R, Tanaka S, Atzori C, van Strien FJ, Roubos EW 1998 Distribution of pro-opiomelanocortin and its peptide end products in the brain and hypophysis of the aquatic toad, Xenopus laevis. Cell Tissue Res 292:251–265 [DOI] [PubMed] [Google Scholar]
- Samuels HH, Stanley F, Casanova J 1979 Depletion of l-3,5,3′-triiodothyronine and l-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80–85 [DOI] [PubMed] [Google Scholar]
- Kanamori A, Brown DD 1993 Cultured cells as a model for amphibian metamorphosis. Proc Natl Acad Sci USA 90:6013–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ 2006 Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proc Natl Acad Sci USA 103:10092–10097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT 2002 Targeted electroporation in Xenopus tadpoles in vivo—from single cells to the entire brain. Differentiation 70:148–154 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J 1994 In: Normal table of Xenopus laevis (Daudin). New York: Garland Publishing Inc. [Google Scholar]
- Denver RJ 1997 Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav 31:169–179 [DOI] [PubMed] [Google Scholar]
- Furlow JD, Kanamori A 2002 The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology 143:3295–3305 [DOI] [PubMed] [Google Scholar]
- Denver RJ, Hu F, Scanlan TS, Furlow JD 2009 Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol 326:155–168 [DOI] [PubMed] [Google Scholar]
- Yao M, Schulkin J, Denver RJ 2008 Evolutionarily conserved glucocorticoid regulation of corticotropin-releasing factor expression. Endocrinology 149:2352–2360 [DOI] [PubMed] [Google Scholar]
- Samson RD, Duvarci S, Pare D 2005 Synaptic plasticity in the central nucleus of the amygdala. Rev Neurosci 16:287–302 [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE 2007 Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52:215–227 [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magarinos AM, LeDoux JE, McEwen BS 1999 Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113:902–913 [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BS, Chattarji S 2002 Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM 2008 Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 105:5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Denver RJ 2007 Regulation of vertebrate corticotropin-releasing factor genes. Gen Comp Endocrinol 153:200–216 [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS 1999 Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 371:113–122 [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM 2000 Erratum to “Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement.” Neuroscience 101:483 [DOI] [PubMed] [Google Scholar]
- Carr JA 2006 Novel effects of CRF on visuomotor behavior and autonomic function in anuran amphibians. Gen Comp Endocrinol 146:28–35 [DOI] [PubMed] [Google Scholar]
- Cline HT 2001 Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11:118–126 [DOI] [PubMed] [Google Scholar]
- Debski EA, Cline HT 2002 Activity-dependent mapping in the retinotectal projection. Curr Opin Neurobiol 12:93–99 [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT 2003 Control of axon branch dynamics by correlated activity in vivo. Science 301:66–70 [DOI] [PubMed] [Google Scholar]
- Ziera T, Irlbacher H, Borden S, Specific gene regulation by the mineralocorticoid receptor. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P1-629) [Google Scholar]
- Leuner B, Shors TJ 2004 New spines, new memories. Mol Neurobiol 29:117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME 2008 Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455:1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel JM, Dussault JH, Puymirat J 1994 Overexpression of the β-1 thyroid receptor induces differentiation in Neuro-2a cells. Proc Natl Acad Sci USA 91:2644–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ 1980 The Golgi method: its application to the insect nervous system and the phenomenon of stochastic impregnation. In: Strausfeld NJ, Miller, TA, eds. Neuroanatomical techniques: insect nervous system. New York: Springer-Verlag; 132–205 [Google Scholar]
- Citri A, Malenka RC 2008 Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41 [DOI] [PubMed] [Google Scholar]