Abstract
As the primary mediators of estrogen signaling in vertebrates, estrogen receptors (ERs) play crucial roles in reproduction, development, and behavior. They are also the major mediators of endocrine disruption by xenobiotic pollutants that mimic or block estrogen action. ERs that are sensitive to estrogen and endocrine disrupters have long been thought to be restricted to vertebrates: although there is evidence for estrogen signaling in invertebrates, the only ERs studied to date, from mollusks and cephalochordates, have been insensitive to estrogen and therefore incapable of mediating estrogen signaling or disruption. To determine whether estrogen sensitivity is ancestral or a unique characteristic of vertebrate ERs, we isolated and characterized ERs from two annelids, Platynereis dumerilii and Capitella capitata, because annelids are the sister phylum to mollusks and have been shown to produce and respond to estrogens. Functional assays show that annelid ERs specifically activate transcription in response to low estrogen concentrations and bind estrogen with high affinity. Furthermore, numerous known endocrine-disrupting chemicals activate or antagonize the annelid ER. This is the first report of a hormone-activated invertebrate ER. Our results indicate that estrogen signaling via the ER is as ancient as the ancestral bilaterian animal and corroborate the estrogen sensitivity of the ancestral steroid receptor. They suggest that the taxonomic scope of endocrine disruption by xenoestrogens may be very broad and reveal how functional diversity evolved in a gene family central to animal endocrinology.
The estrogen receptors of annelid invertebrates are activated by estrogens and sensitive to endocrine disrupting chemicals, corroborating the estrogen sensitivity of the ancestral steroid receptor and indicating that the taxonomic scope of endocrine disruption is very broad.
Estrogen receptors (ERs) are major regulators of development, behavior, and reproduction in vertebrates (1). They function as hormone-activated transcription factors, increasing the expression of target genes when estrogens are present. ERs are members of the steroid receptor (SR) gene family, which also includes receptors for androgens, progestins, and corticosteroids. Like other SRs, ERs have a modular structure composed of several semiautonomous domains. The DNA-binding and ligand-binding domains (DBDs and LBDs) are well conserved among species and, to a lesser extent, among paralogs (2). The DBD recognizes and binds to specific response elements, short palindromic sequences in DNA near target genes (3,4). The LBD regulates hormone-dependent transcription by binding ligand and shifting to an active conformation, exposing a newly assembled surface [the activation function 2 (AF-2)], which recruits coactivators and thereby potentiates transcription of target genes (5,6). ERs also contain poorly conserved hinge and an N-terminal domain, which contains a ligand-independent transcriptional activation function. In addition to their classical functions as direct regulators of target gene expression, ERs also play roles in a number of other signaling pathways (7,8,9).
Disruption of hormone signaling by xenobiotic pollutants has emerged as an important issue in environmental health (10,11). Numerous natural and synthetic chemicals, including pharmaceuticals, pesticides, plastics feedstocks and additives, and phytochemicals, can act as estrogen agonists or antagonists, mimicking or blocking the natural effects of estrogens on the ER (12,13,14,15,16,17,18,19,20,21). Disruption of estrogen/ER signaling by such substances can have profound effects on the organism, including abnormal development of male and female reproductive tracts, feminization of males, lowered sperm counts, disrupted reproductive cycling and reduced fertility, carcinogenesis, and behavioral changes (10,22,23,24,25,26). Many endocrine-disrupting chemicals are now ubiquitous in the global environment (27). Health damage by endocrine disrupting chemicals that target the ER has been documented in a variety of vertebrates, including humans (27,28,29,30,31,32,33,34). Invertebrates can be affected by some endocrine-disrupting pollutants, with potentially significant ecological consequences, but the effects that have been documented have not been mediated by ERs or other steroid hormone receptors (35,36,37,38).
Ligand-sensitive ERs have not yet been identified in any invertebrate species. SRs were traditionally thought to be a vertebrate-specific novelty because they are absent from arthropod and nematode genomes—the first invertebrate phyla to be fully sequenced—and from those of urochordates and echinoderms as well (39,40,41,42,43). ER orthologs, however, have recently been isolated from several mollusk species (44,45,46,47,48) and the cephalochordate Branchiostoma floridae (49,50). These receptors descend from the same gene in an ancient bilaterian animal as the vertebrate ERs do, so they are appropriately classified as ERs (see Fig. 1). Unlike the vertebrate ERs, however, these invertebrate receptors are not activated by estrogen. Mollusk ERs activate transcription constitutively and are unaffected by the addition of estrogens or other ligands (44,45,46,47). The cephalochordate ER, in contrast, is incapable of activating transcription whether or not ligand is present; however, it does maintain estrogen response element (ERE) recognition and acts as a repressor of ERE-based transcriptional activation by other proteins (49,50). Because they are not ligand regulated, mollusk and cephalochordate ERs are unlikely to be subject to endocrine disruption. Cephalochordates do, however, have a second SR family member, Branchiostoma floridae SR (BfSR), an ortholog of the vertebrate androgen, progestin, and corticosteroid receptors (49,50). BfSR displays the classic properties of a vertebrate ER, including recognition of EREs and specific sensitivity to estrogens (49).
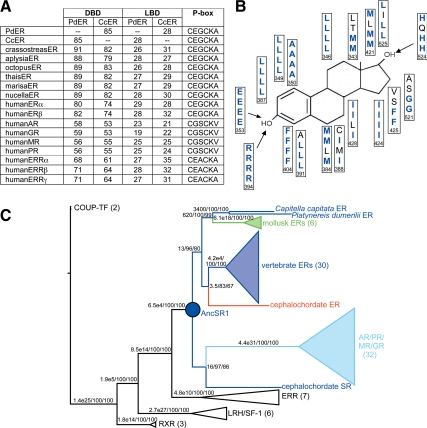
Figure 1.
PdER and CcER are ER orthologs. A, Percent sequence identity of the annelid ER sequences to mollusk and human steroid and related receptors. The characteristic P-box sequence in the DBD is also shown. B, Conservation of ligand-contacting residues among estrogen-sensitive receptors. Amino acid positions that contact estradiol in the human ERα structure (67) are shown, numbered by position in the human ERα. Amino acid states (from top to bottom) in PdER, CcER, human ERα, and the reconstructed ancestral gene AncSR1 are shown. States unchanged from AncSR1 are shown in blue. Arrows, Residues that discriminate between estrogens and other ligands. C, Phylogeny of 90 steroid and related receptors inferred by ML and Bayesian methods. Node labels show the approximate likelihood ratio, χ2 confidence estimate, and Bayesian posterior probability. Parentheses, Number of sequences in each clade; blue, ligand-activated receptors (dark blue, estrogens; light blue, 3-ketosteroids); green, constitutive receptors; red, transcriptionally inactive receptors. Blue dot shows AncSR1, the ancestral SR gene. For complete phylogenies and accessions, see supplemental Figs. 2–5 and supplemental Table 3.
Although ERs from mollusks and cephalochordates are insensitive to estrogens and their mimics, it remains unknown whether all invertebrate ERs have this property. The SR family descends from a single ancestral receptor gene (AncSR1) by duplication and divergence. AncSR1 existed before the last common ancestor of the two major clades of bilaterian animals, deuterosomes (including chordates and echinoderms) and protostomes (including arthropods, nematodes, mollusks, annelids, and a host of other invertebrates). All phyla in these groups are expected to have ERs unless they have been secondarily lost. The functional characteristics of ERs in protostome phyla other than mollusks, however, have not been studied.
A better understanding of invertebrate ERs would also illuminate the evolutionary history of SR function. The evidence to date suggests that the ER's hormone-dependence is very ancient. When the sequence of AncSR1 was computationally inferred, synthesized, and experimentally characterized, it had the functions of vertebrate ERs, including binding to ER-specific response elements and specific activation of transcription in response to estrogens (44,51). This finding implies that the constitutive activity of the mollusk ERs is due to a secondary loss of the ancestor's hormone-independence, but whether this loss is unique to the mollusks or was lost early in the protostome lineage is unresolved. Furthermore, it has been suggested that the existence of the disabled ER in cephalochordates implies that AncSR1 may in fact have been ligand independent; according to this hypothesis, hormone sensitivity would have been gained independently in the vertebrate ERs and other SRs, and the apparent hormone dependence of AncSR1 would be due to errors in the phylogenetic reconstruction (50,52).
To elucidate the functions and potential endocrine disruption of ERs in invertebrates and to elucidate the evolution of ERs from AncSR1, we sought to determine whether other protostomes contain estrogen-responsive ERs. We focused on the annelids, the anciently diverged sister phylum to mollusks. In the polychaete Nereis virens, estradiol has been detected in male and female coelomic fluid, and ER-like proteins have been detected in reproductive tissues using immunocytochemistry (53). Incubation of N. virens eleocytes with nanomolar concentrations of estradiol induces expression of the classical estrogen-responsive gene vitellogenin (54). In this paper, we report the isolation and characterization of an estrogen-sensitive ER ortholog from the closely related species Platynereis dumerilii (PdER). P. dumerilii is closely related to N. virens, and molecular resources are available for this species. We confirmed that estrogen sensitivity was not species specific by testing the estrogen responsiveness of the ER from another annelid, Capitella capitata (CcER). We also tested PdER with a range of endocrine disrupting chemicals to determine whether these could interfere with ER function and analyzed the evolutionary implications of the annelid ER.
Materials and Methods
Isolation of ERs
Degenerate primers were designed from the highly conserved DBDs of vertebrate ERs; flanking restriction sites were added to the primers to increase specificity in later rounds of amplification. Primer sequences are available on request. Nested degenerate PCR was used to amplify a DBD fragment of PdER from a P. dumerilii cDNA library (a gift of Stephan Schneider, University of Oregon, Eugene, OR) using the following cycling conditions: 94 C/3 min, five cycles (92 C/20 sec, 50 C/30 sec, 72 C/1 min), 30 cycles (92 C/20 sec, 62 C/30 sec, 72 C/1 min), and 72 C/5 min. Gene-specific primers were then designed against the DBD fragment, and rapid amplification of cDNA ends (RACE; SmartRace; CLONTECH, Mountain View, CA) was conducted using RNA extracted from whole adults (Rneasy; QIAGEN, Valencia, CA). Products were cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) and sequenced on both strands. A single transcript of the entire open reading frame was then amplified and sequenced by PCR using cDNA as a template. Site-directed mutagenesis was performed using the Stratagene QuikChange kit (La Jolla, CA). A sequence fragment of CcER was identified in the C. capitella genome sequence (Joint Genome Institute, Walnut Creek, CA; U.S. Department of Energy) using a BLAST search with human ERα as query. The sequence of the CcER hinge, LBD, and carboxy-terminal extension was determined by RACE and then amplified as a single PCR amplicon and resequenced. 5′-RACE to recover the full open reading frame was not successful.
Phylogenetic analyses
To analyze phylogenetic relationships, we aligned protein sequences of 90 steroid and related receptors. The N-terminal domain, hinge, and C-terminal extension cannot be aligned with confidence and were excluded. DBDs were aligned using ClustalX version 2.0 (61). LBDs with available crystal structures were aligned using 3DCoffee (55), and the remaining LBD sequences were then aligned to that profile using ClustalX and manually edited. DBD and LBD alignments were then concatenated and analyzed together. Bayesian phylogenetic analysis was conducted with parallel MrBayes version 3.1.2 (72,73), using two independent runs of four chains each (three heated) for 2 million generations, with the first 500,000 generations discarded as burn-in. Protein models were integrated over, with a γ+invariant model of among-site rate variation and uniform priors over trees and branch lengths (10). Maximum likelihood (ML) analysis was conducted using Phyml version 2.4.5 (74), using the JTT+G+I model (which had posterior probability 1.0 in the Bayesian analysis). Support for splits was calculated as the approximate likelihood ratio (the likelihood of the ML phylogeny divided by the likelihood of the best rearrangement lacking the split of interest) and as the χ2 support (1 minus the estimated probability that a likelihood ratio as great or greater than that observed for a split would occur if the split is not present in the true tree) (56).
Ancestral reconstruction was performed using an extended database of 210 steroid and related receptors. DBD and LBD amino acid sequences were aligned using Muscle (http://www.ebi.ac.uk/Tools/muscle/index. html). The ML phylogeny was inferred using Phyml as described above. The ML sequence of AncSR1 was calculated (57) assuming the JTT+G model and the ML phylogeny, using ART software (http://ix.cs. uoregon.edu/∼victorhs/website/computerscience/art/), a wrapper and parser for the codeml program of PAML version 3.15 (75,76). The ML reconstruction is defined as the ancestral sequence, which maximizes the probability that all extant receptor sequences would evolve, given their phylogenetic relationships.
Cell culture and reporter activation
For reporter assays, DBDs of PdER and human ERα (gift of B. Katzenellenbogen, University of Illinois at Urbana-Champaign) were cloned into pCMV-AD (Stratagene). LBDs (including hinge) were cloned into pSG5-Gal4-DBD (gift of D. Furlow, University of California, Davis). Full-length receptors were cloned into pcDNA3. CHO-K1 cells were maintained in phenol red-free αMEM (Invitrogen) with 10% dextran-charcoal stripped fetal bovine serum (Hyclone, Logan, UT). For DBD assays, 2 ng of receptor plasmid per well were transfected with 10 ng of 4-ERE-c38-luc (gift of C. Klinge, University of Louisville School of Medicine, Louisville, KY), 0.1 ng of pRL-TK (Promega, Madison, WI), and 90 ng of pUC19 as carrier, using Lipofectamine and Plus reagents (Invitrogen). For LBD reporter assays, 5 ng of receptor plasmid were transfected as above with 100 ng of pFR-luc reporter (Promega) and 0.1 ng of pRL-TK. For full-length receptor assays, HepG2 cells were maintained in MEM with 10% stripped serum. We transfected 10 ng of receptor plasmid, 20 ng of 4-ERE-c38-luc reporter plasmid, 60 ng of puc19 DNA, and 1 ng of normalization plasmid pRL-CMV. After 4 h, the transfection mixture was replaced with medium supplemented with stripped serum. The following day, cells were incubated with hormones (1 pm to 10 μm) diluted in serum+medium for 24 h and then assayed using Dual-Glo luciferase (Promega). Hormones tested included progesterone, corticosterone, aldosterone, estrone, estradiol, androstenedione (Steraloids, Newport, RI), testosterone, dihydrotestosterone, estriol, and dehydroepiandrosterone (Sigma-Aldrich, St. Louis, MO). Endocrine-disrupting chemicals tested included ICI 182,780 (Tocris Bioscience, Ellisville, MO) diethylstilbestrol, 4-hydroxytamoxifen (Sigma), 4-octylphenol, bisphenol A, and genistein (Sigma-Aldrich). All assays were conducted in triplicate for each treatment and repeated multiple times. Dose-response relationships were estimated using Prism4 software (GraphPad, San Diego, CA).
Ligand binding assays
For binding assays, CHO-K1 cells were grown to approximately 90% confluence on two 100-mm plates and transfected with empty pSG5-Gal4DBD or PdER LBD in pSG5-Gal4DBD. Cells were transfected with 4 μg DNA, 30 μl Lipofectamine, and 20 μl Plus reagent per plate and incubated for 4 h. The next day, cells were harvested, spun down, resuspended in 200 μl ice-cold TEGDK buffer [10 mm Tris-HCl, 1 mm EDTA, 0.4 m KCl, 10% (vol/vol) glycerol, 1 mm dithiothreitol] with 1% protease inhibitor (Sigma-Aldrich). Cells were lysed by four freeze-thaw cycles and spun down at 10,000 × g for 3 min at 4 C. Supernatant was diluted into 10 ml of ice-cold TEGDK, divided into 200-μl aliquots, and incubated overnight at 4 C in triplicate with 2,4,6,7-3H-estradiol (NEN Life Science Products/PerkinElmer, Boston, MA) alone for total binding or with a 200-fold molar excess of unlabeled diethylstilbestrol (DES) for nonspecific binding. Samples were incubated for 15 min at 4 C with 200 μl of a 50% slurry of hydroxyapatite (Bio-Rad, Hercules, CA) in TEGDK buffer with vortexing every 5 min, followed by three cycles of spinning (10,000 × g for 20 sec), resuspension, and washing in 1 ml cold TEGDK. After bound ligand was extracted overnight from the washed hydroxyapatite in 1 ml ethanol, 500 μl suspension was added to 5 ml scintillation fluid and counted. All experiments were conducted in triplicate for each treatment and repeated multiple times.
Ligand dependence of extant receptors was coded as a discrete unordered character. Ancestral states were inferred and changes of states identified on the phylogeny using Fitch parsimony reconstruction (58). The rescaled consistency index (59) was calculated as (m/s)[(g − s)/(g − m)], where m is the minimum number of character changes on the most parsimonious phylogeny possible, s is the actual number required on a given phylogeny, and g is the maximum number of steps required on the least parsimonious possible phylogeny. This index ranges from 0.0 (explaining the fewest possible number of extant character states as due to descent from a common ancestor and requiring all shared states to be due to convergence) to 1.0 (explaining all shared states as due to common descent and requiring no convergence/reversal).
Results
We isolated the PdER using degenerate PCR and RACE. We isolated the LBD of the CcER using a BLAST search of the C. capitella genome, followed by RACE on cDNA from adult animals.
We compared the protein sequences of the annelid ERs, human ERα, and the reconstructed ancestral steroid receptor AncSR1.
PdER and CcER are most similar to each other and to the mollusk ERs, followed by the vertebrate ERs, with lower similarity to other SRs and related proteins. In the DBD, both sequences contain the classic ER-like P-box, a signature motif that mediates response element recognition (60) and is conserved among all ERs but differs in the other steroid and related receptors (Fig. 1A). In the LBD, the amino acids that contact estradiol (Fig. 1B) are highly conserved between the annelid ERs, human ERα, and AncSR1. This result contrasts with the divergence of the mollusk receptors at the majority of these sites (45). The complete aligned sequences of PdER and CcER are presented in supplemental Fig. 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
To determine orthology relationships, we conducted phylogenetic analysis of an alignment of 90 SR and related sequences using maximum likelihood and Bayesian methods. As expected, PdER and CcER were both included in a clade with the mollusk estrogen receptors, and this clade is placed as sister to the chordate ERs, within the family of steroid receptors (Fig. 1B and supplemental Figs. 2–4). These relationships are well supported, with very high likelihood ratios and posterior probabilities, indicating that the annelid ERs are orthologs of the mollusk ERs and the vertebrate ERs. Support for the node that pairs the annelid+mollusk ERs with the chordate ERs was only moderate, but this softness appears to be due to ambiguity in the placement of the long branches leading to the SRs of cephalochordates; when the BfSRs were excluded from the analysis, support for the orthology of the protostome ERs and vertebrate ERs increased dramatically (supplemental Fig. 5). We conclude that PdER and CcER are certainly orthologs of the mollusk ERs and are likely to be specific orthologs of the chordate ERs, although we cannot rule out the possibility that the protostome ERs could be equally orthologous to the entire SR family.
To determine PdER's functions, we first characterized its DNA recognition capacity using an ERE-driven luciferase reporter assay. The PdER DBD mediated transcriptional activation from the ERE at a level comparable with that elicited by the human ERα DBD (Fig. 2). The full-length PdER protein also activated transcription from an ERE-driven reporter (supplemental Fig. 6A). This demonstrates that in PdER, as in the mollusk ERs (44,45,46), the DNA-binding function of vertebrate ERs is conserved. Because all known ERs, including those from both vertebrates and invertebrates, are capable of binding EREs, we conclude that ERE recognition is as old as the protostome-deuterosome ancestor. These results corroborate previous experimental findings that AncSR1 was ER-like in function (44).
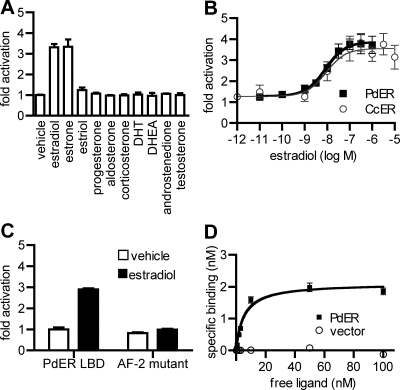
Figure 2.
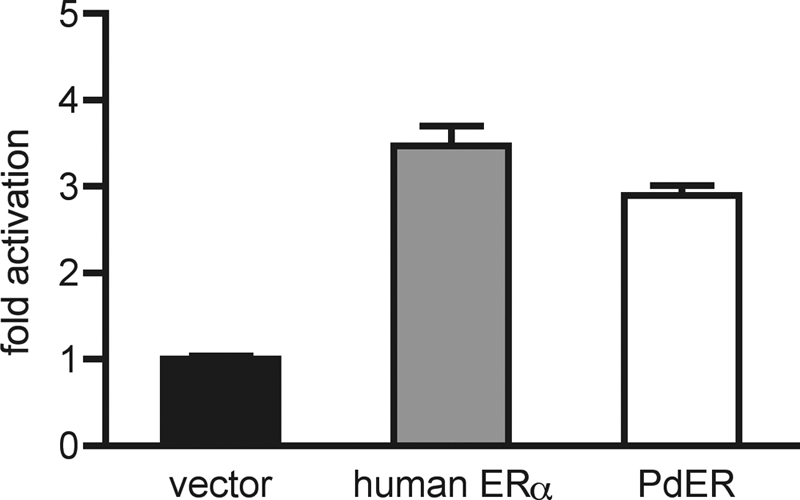
PdER activates transcription from EREs. Receptor DBDs were expressed as fusion proteins with the constitutive nuclear factor-κB activation domain in the presence of an ERE-driven luciferase reporter. Fold activation indicates normalized luciferase activity relative to empty vector. Mean ± sem of three replicates is shown.
To determine the hormone sensitivity of PdER, we measured luciferase reporter expression by the PdER LBD in the presence and absence of a variety of hormones. PdER showed virtually no constitutive activity. It was activated by the vertebrate estrogens estradiol and estrone but did not respond to androgens, progestins, or corticosteroids (Fig. 3A). Dose-response analysis showed that PdER is highly sensitive to estrogen, with an EC50 of 8.5 nm (Fig. 3B). The full-length PdER protein was also estrogen responsive, activating expression of an ERE-driven luciferase reporter in an estrogen-dependent manner with similar sensitivity (supplemental Fig. 6A). PdER's estrogen sensitivity is not species specific because CcER is also a sensitive transcriptional activator in the presence of several vertebrate estrogens (estradiol EC50 = 9.2 nm; Fig. 3B) but not other steroid hormones (supplemental Fig. 7).
Figure 3.
PdER is estrogen sensitive. A, Activation of a luciferase reporter gene by PdER-LBD in the presence of steroid hormones at 1 μm, relative to vehicle-only control. DHT, Dihydrotestosterone; DHEA, dehydroepiandrosterone. B, Dose-responsive activation of a luciferase reporter by PdER-LBD and CcER-LBD. C, Estrogen activation of PdER-LBD requires the classic AF-2 motif. When the AF-2 region is disabled by mutation E477Q, the LBD no longer activates luciferase expression. D, Estrogen binding assay of PdER-LBD. A PdER-LBD fusion protein was expressed and extracted from CHO-K1 cells. Binding of the cell extract to radiolabeled estradiol was tested at a range of concentrations, with or without a 200-fold excess of unlabeled competitor to distinguish specific from nonspecific binding. Circles show specific binding by cells without transfected ER LBD. Mean ± sem of three replicates is shown for all graphs.
To determine whether estrogen activation of the PdER LBD is mediated by the classic AF-2-dependent mechanism, we introduced a single amino acid change (E477Q) that disables the AF-2 core sequence of PdER; we found that transcriptional activity was completely abolished (Fig. 3C), as previously observed for human SRs (6) and AncSR1 (44). The magnitude of activation by PdER in all reporter experiments was lower than that induced by human ERα; this result may be due to intrinsically lower activity of PdER or reduced efficiency when the annelid LBD is assayed in a mammalian cell line. To confirm that PdER physically interacts with estrogen, we conducted a cell-free binding assay and found that the PdER LBD binds estradiol with high affinity (dissociation constant Kd = 5.3 nm; Fig. 3D). Taken together, these data establish that annelid ERs are estrogen sensitive and estrogen specific, with hormone-dependent functions similar to classic vertebrate estrogen receptors.
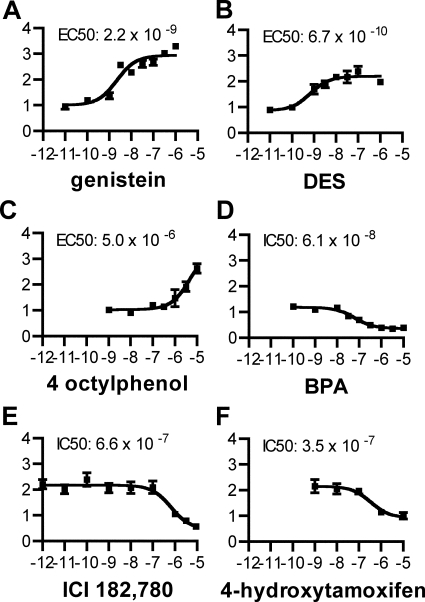
The estrogen dependence of the annelid ERs suggested that these receptors may also be sensitive to endocrine disrupting chemicals. We screened a number of known xenoestrogens and antiestrogens (14,15,16,17,62,63,64,65) for activation and inhibition of PdER (Fig. 4) and human ERα (Fig. S6). The phytoestrogen genistein and the pharmaceutical DES were strong PdER activators, and 4-octylphenol, an additive in detergents and plastics, was a weak activator. These results are consistent with the effects of these compounds on human ERα (supplemental Fig. 6). In contrast, bisphenol-A, a component of plastics, repressed the baseline activity of PdER, contrasting with its agonistic effect on human ERα. The human ER antagonists ICI 182,780 and 4-hydroxytamoxifen, both pharmaceuticals, inhibited activation of PdER LBD when administered in mixture with 17β-estradiol. These results demonstrate that PdER's activity can be activated or antagonized by exogenous substances. Some of the responses can be predicted from the effects of each chemical on human ERs, but some differ between phyla.
Figure 4.
PdER is activated and antagonized by endocrine disrupting chemicals. Fold activation of a luciferase reporter gene by a PdER-LBD fusion relative to vehicle-only control is shown in the presence of increasing concentrations (in log M) of agonists and antagonists: genistein (A), DES (B), 4-octylphenol (C), and bisphenol A (BPA; D). E and F, Cells were treated with varying concentrations of inhibitor and estradiol at its EC80. Similar results were found for the full-length PdER and human ERα (supplemental Fig. 6). Mean ± se of three replicates is shown. EC50 and IC50, concentrations of activator and inhibitor, respectively, at which half-maximal activation or inhibition is achieved.
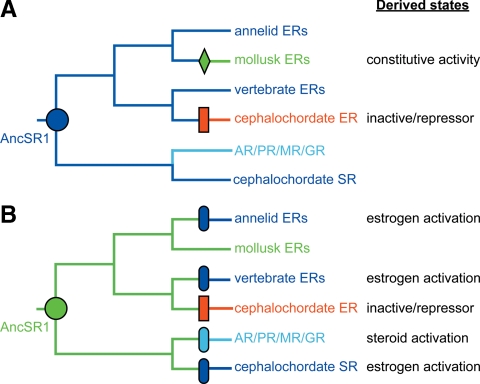
To understand the evolutionary implications of estrogen sensitivity in the annelid ERs, we plotted the response to ligand on the SR phylogeny (Fig. 5). The hypothesis that AncSR1 was estrogen activated is considerably more parsimonious than the hypothesis that AncSR1 was a constitutive activator. Under the former scenario, all ligand-activated SRs inherit their hormone sensitivity directly from the ancestor, including three separate lineages of estrogen-sensitive receptors (vertebrate ERs, annelid ERs, and BfSR), and the vertebrate AR/PR/GR/MR group, which are also steroid activated but prefer steroids that have a keto instead of a hydroxyl group at the 3-position on the steroid backbone (66). This scenario implies just two major shifts of function during evolution: a gain of constitutive activity after mollusks diverged from annelids, and a loss of transcriptional activity in the cephalochordate ER. In contrast, the alternative hypothesis of a constitutive AncSR1 (Fig. 5B) requires independent gains of ligand sensitivity in each of the four lineages of liganded receptors, plus a loss of transcriptional activity in the cephalochordate. Under this scenario, the only present-day phenotype explained as due to descent from a common ancestor is that observed in the mollusk ERs. The rescaled consistency index, a measure of how well a phylogenetic reconstruction explains the evolution of a character, is 1.0 (the highest possible score) if AncSR1 is ligand activated and 0.0 (the worst possible) if AncSR1 is constitutive (supplemental Table 2).
Figure 5.
Evolution of receptor functional diversity from an ancestral ER (AncSR1, circle). Present-day receptors are colored according to their transcriptional functions (blue, ligand-activated; green, constitutively active; red, inactive; estrogen-activated receptors are dark blue, and those activated by 3-ketosteroids are light blue). A, If AncSR1 was ligand dependent, two changes of function are required: one loss of transcriptional activity (red box) and one loss of ligand-dependence (green diamond). B, If AncSR1 was constitutively active, four independent gains of ligand dependence are required (blue ovals) plus loss of transcriptional activity (red box). The rescaled consistency index, a measure of how parsimoniously a phylogenetic scenario explains the distribution of character states among extant species, is 1.0 for the hypothesis in A (the maximum possible) and 0.0 for the hypothesis in B (the minimum possible).
When the AncSR1 sequence was previously reconstructed and experimentally characterized (44), only one invertebrate SR sequence was available. It has been suggested that the estrogen sensitivity of AncSR1 might be an artifact of this limited species sampling (50). We repeated the reconstruction using a dramatically expanded database of 210 SR and related sequences, including numerous receptors from mollusks, annelids, and cephalochordates. The revised ML sequence of AncSR1 is even more similar to that of human and other estrogen-dependent ERs than before (supplemental Table 1). In the new AncSR1, 16 of the 18 residues that line the ligand pocket (67) are conserved with the human ERα and/or ERβ (two more than in the original reconstruction); the two exceptions are conserved with the annelid receptors and are therefore insufficient to abolish estrogen response (Fig. 1B). The three sites known to be critical for estrogen-specific binding (E353, R394, and H524, using human ERα numbering) remain conserved in the human ER-like state in AncSR1 but now have posterior probability 0.999 or greater (Fig. 1B and supplemental Table 1). The reconstruction of AncSR1 as ER-like is therefore strengthened, not weakened, by improved taxon sampling.
Discussion
Our data indicate that annelid ERs share the functional characteristics of vertebrate ERs. PdER recognizes classic EREs, binds estrogen with high affinity, and activates transcription in response to low doses of estrogens. Its transcriptional activity can be disrupted by known endocrine-disrupting substances. Estrogen responsiveness is not limited to P. dumerilii because the ER from C. capitata is also highly sensitive to estrogens. This is the first report of hormone-activated invertebrate ERs and the first example of an invertebrate ER that can be disrupted by xenobiotics.
Our results indicate that invertebrates are subject to ER-mediated endocrine disruption. We found that annelid ERs can be disrupted by some of the same synthetic and plant-derived compounds that activate vertebrate ERs and cause developmental and reproductive impairment. In some cases, the effects on the annelid ERs occur at nanomolar or subnanomolar concentrations, raising the possibility that annelid development and reproduction might be disrupted by exogenous substances in the environment. The effects of some but not all substances on the annelid ER could be predicted from their effects on the human ER. If our results exemplify a general pattern, then testing programs to identify endocrine disrupting substances using vertebrate models will be inadequate to identify all such substances in invertebrates.
Because the loss of the ancestral receptor's estrogen sensitivity appears to be unique to the mollusks, other phyla in the same phylogenetic lineage as the annelids, including platyhelminthes, brachiopods, nemerteans, phoronids, and echiura, should be expected to contain estrogen-sensitive ERs and therefore to be potentially subject to interference by endocrine-disrupting chemicals. Endocrine disruption of some invertebrates has been observed, but the effects have been mediated through other mechanisms (38). Our findings indicate that the potential scope of ER-mediated endocrine disruption is considerably greater than appreciated by current programs for the identification and control of such substances. Because the diverse phyla potentially subject to endocrine disruption play important ecological roles, the possibility and extent of functional impairment in invertebrates by endocrine-disrupting chemicals is a worthy focus for further research.
Our experiments allow us to speculate about the ancient roles in animal physiology of estrogen and its receptors. Annelids produce estrogens, and these hormones regulate the provisioning of oocytes with vitellogenin during female reproduction (53,54). Our work suggests the testable hypothesis that the annelid ER mediates these effects of estrogens. In cephalochordates, the estrogen-sensitive BfSR is expressed primarily in gonads, particularly in maturing germ cells. In vertebrates, ERs also regulate ovarian function and germ cell development (68,69). Together, these data suggest that the ancestral bilaterian animal may have used estrogen/ER signaling to regulate reproduction, including germ cell maturation.
The estrogen sensitivity of annelid ERs, together with that of the cephalochordate BfSR, adds strong support to the hypothesis that the ancestral steroid receptor was an estrogen-sensitive transcriptional activator. This scenario explains the ligand dependence observed throughout the gene family as due to descent from AncSR1 and requires just two shifts of function during evolution: a gain of constitutive activity in the mollusk ERs after mollusks diverged from annelids and a loss of transcriptional activity in the cephalochordate ER. The radically different modes of action of these two receptors clearly indicate that their lack of estrogen sensitivity was independently acquired, not shared with the common ancestor. The alternative hypothesis, that the ancestor was a constitutive activator, is far less parsimonious and explains almost nothing about the phylogenetic distribution of functions in the SR family (Fig. 5B). Under this scenario, only the mollusks retain the ancestral phenotype, requiring a shift in function in every other lineage, including independent gains of ligand sensitivity in all four lineages of liganded receptors (annelid ERs, vertebrate ERs, BfSR, and vertebrate SRs), plus a loss of transcriptional activity in the cephalochordate ER.
Our results therefore corroborate experimental work showing that the reconstructed AncSR1 is specifically activated by estrogens (44). They indicate that the major mode of evolution in the SR family has been partial loss or subtle modification of function from a hormone-sensitive ancestor. The cephalochordate ER has been shown to have gained its function as a competitive repressor of BfSR by a single simple mutation that compromised the functions of the LBD without affecting those of the DBD (49). The mollusk ER gained constitutive activity when the LBD's allosteric switch became stuck in the agonist position, as has been observed for other nuclear receptors (70). And the vertebrate receptors for androgens, progestins, and corticosteroids evolved by subtle remodeling of the estrogen-sensitive ligand pocket to recognize steroids with different substituents around the same structural backbone (71). Taken together, these findings indicate that the marked functional diversification of the SR gene family evolved through relatively simple paths, without de novo engineering of radically new structures or functions.
Supplementary Material
Acknowledgments
We thank Geeta Eick for computational analysis; Anna Reye for research assistance; Stephan Schneider, Bill Gillis, and Carola Noji for annelid animals and libraries; and Patrick Phillips, Jamie Bridgham, and members of the Thornton laboratory for comments.
Footnotes
This work was supported by National Institutes of Health Grants R01-GM081592, NSF-IOB-0546906, and IOB-0508948 and a Sloan fellowship (to J.W.T.); National Science Foundation (NSF) Grant IGERT DGE-9972830; and an NSF graduate research fellowship (to J.K.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 26, 2008
Abbreviations: AF-2, Activation function 2; CcER, Capitella capitata ER; DBD, DNA-binding domain; DES, diethylstilbestrol; ER, estrogen receptor; ERE, estrogen response element; BfSR, Branchiostoma floridae SR; LBD, ligand-binding domain; ML, maximum likelihood; PdER, Platynereis dumerilii ER; RACE, rapid amplification of cDNA ends; SR, steroid receptor; TEGDK, buffer of Tris-HCl, EDTA, KCl, glycerol, and dithiothreitol.
References
- Bentley PJ 1998 Comparative vertebrate endocrinology. 3rd ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Laudet V, Gronemeyer H 2001 The nuclear receptor factsbook. London, UK: Academic Press [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J 2004 Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol 33:387–410 [DOI] [PubMed] [Google Scholar]
- Beekman JM, Allan GF, Tsai SY, Tsai MJ, O'Malley BW 1993 Transcriptional activation by the estrogen receptor requires a conformational change in the ligand binding domain. Mol Endocrinol 7:1266–1274 [DOI] [PubMed] [Google Scholar]
- Berrevoets CA, Doesburg P, Steketee K, Trapman J, Brinkmann AO 1998 Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor 2). Mol Endocrinol 12:1172–1183 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL 2001 Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S 2002 Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE 2002:RE9 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Gore AC 2007 Endocrine-disrupting chemicals: from basic research to clinical practice. Totowa, NJ: Humana Press [Google Scholar]
- 2003 Implications of endocrine active substances for humans and wildlife: a SCOPE/IUPAC Project. Pure Appl Chem 75:1617–2615 [Google Scholar]
- Cheek AO, Vonier PM, Oberdorster E, Burow BC, McLachlan JA 1998 Environmental signaling: a biological context for endocrine disruption. Environ Health Perspect 106(Suppl 1):5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM 2000 The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci 54:138–153 [DOI] [PubMed] [Google Scholar]
- Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ 1997 Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect 105:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, McLachlan JA, Arnold SF 1997 The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. Steroids 62:365–372 [DOI] [PubMed] [Google Scholar]
- Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM 2000 Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect 108:723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutendorf B, Westendorf J 2001 Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 166:79–89 [DOI] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF 1996 Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect 104:1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K 2004 Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112:524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Kwack SJ, Kwon O, Kim HS, Kim SS, Kim SH, Sohn KH, Lee RD, Park CH, Jeung EB, An BS, Park KL 2002 Comparative evaluation of alkylphenolic compounds on estrogenic activity in vitro and in vivo. J Toxicol Environ Health A 65:419–431 [DOI] [PubMed] [Google Scholar]
- McLachlan JA 2001 Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22:319–341 [DOI] [PubMed] [Google Scholar]
- Mueller SO 2004 Xenoestrogens: mechanisms of action and detection methods. Anal Bioanal Chem 378:582–587 [DOI] [PubMed] [Google Scholar]
- Guillette Jr LJ, Moore BC 2006 Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med 24:134–141 [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM 2001 Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978 [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM 1993 Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW 2000 Pandora's poison: chlorine, health, and a new environmental strategy. Cambridge, MA: MIT Press [Google Scholar]
- McLachlan JA, Simpson E, Martin M 2006 Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab 20:63–75 [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Jobling S 1995 Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103(Suppl 7):173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter JP 1995 Feminized responses in fish to environmental estrogens. Toxicol Lett 82–83:737–742 [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC 1971 Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881 [DOI] [PubMed] [Google Scholar]
- Lauver D, Nelles KK, Hanson K 2005 The health effects of diethylstilbestrol revisited. J Obstet Gynecol Neonatal Nurs 34:494–499 [DOI] [PubMed] [Google Scholar]
- Veurink M, Koster M, Berg LT 2005 The history of DES, lessons to be learned. Pharm World Sci 27:139–143 [DOI] [PubMed] [Google Scholar]
- Swan SH 2000 Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS 108:793–804 [DOI] [PubMed] [Google Scholar]
- Oetken M, Bachmann J, Schulte-Oehlmann U, Oehlmann J 2004 Evidence for endocrine disruption in invertebrates. Int Rev Cytol 236:1–44 [DOI] [PubMed] [Google Scholar]
- McClellan-Green P, Romano J, Oberdorster E 2006 Does gender really matter in contaminant exposure? A case study using invertebrate models. Environ Res 104:183–191 [DOI] [PubMed] [Google Scholar]
- Oehlmann J, Di Benedetto P, Tillmann M, Duft M, Oetken M, Schulte-Oehlmann U 2007 Endocrine disruption in prosobranch molluscs: evidence and ecological relevance. Ecotoxicology 16:29–43 [DOI] [PubMed] [Google Scholar]
- Janer G, C Porte 2007 Sex steroids and potential mechanisms of nongenomic endocrine disruption in invertebrates. Ecotoxicology 16:145–160 [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M,et al. 2002 The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298:2157–2167 [DOI] [PubMed] [Google Scholar]
- Baker ME 2003 Evolution of adrenal and sex steroid action in vertebrates: a ligand-based mechanism for complexity. Bioessays 25:396–400 [DOI] [PubMed] [Google Scholar]
- Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT 2001 Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol 2:RESEARCH0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Ashby M, Materna SC, Brown CT, Chen L, Cameron RA, Davidson EH 2006 Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev Biol 300:90–107 [DOI] [PubMed] [Google Scholar]
- Yagi K, Satou Y, Mazet F, Shimeld SM, Degnan B, Rokhsar D, Levine M, Kohara Y, Satoh N 2003 A genomewide survey of developmentally relevant genes in Ciona intestinalis. III. Genes for Fox, ETS, nuclear receptors and NFκB. Dev Genes Evol 213:235–244 [DOI] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D 2003 Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 301:1714–1717 [DOI] [PubMed] [Google Scholar]
- Keay J, Bridgham JT, Thornton JW 2006 The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology 147:3861–3869 [DOI] [PubMed] [Google Scholar]
- Kajiwara M, Kuraku S, Kurokawa T, Kato K, Toda S, Hirose H, Takahashi S, Shibata Y, Iguchi T, Matsumoto T, Miyata T, Miura T, Takahashi Y 2006 Tissue preferential expression of estrogen receptor gene in the marine snail, Thais clavigera. Gen Comp Endocrinol 148:315–326 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Nakamura AM, Mori K, Akiyama I, Hirose H, Takahashi Y 2007 Oyster estrogen receptor: cDNA cloning and immunolocalization. Gen Comp Endocrinol 151:195–201 [DOI] [PubMed] [Google Scholar]
- Castro LF, Melo C, Guillot R, Mendes I, Queiros S, Lima D, Reis-Henriques MA, Santos MM 2007 The estrogen receptor of the gastropod Nucella lapillus: modulation following exposure to an estrogenic effluent? Aquat Toxicol 84:465–468 [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Brown JE, Rodriguez-Mari A, Catchen JM, Thornton JW 2008 Evolution of a new function by degenerative mutation in cephalochordate steroid receptors. PLoS Genet 4:e1000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M, Pettersson K, Schubert M, Bertrand S, Pongratz I, Escriva H, Laudet V 2008 An amphioxus orthologue of the estrogen receptor that does not bind estradiol: insights into estrogen receptor evolution. BMC Evol Biol 8:219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW 2001 Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA 98:5671–5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Safi R, Hanni C, Langlois MC, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V 1997 Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA 94:6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso J, Rebscher N 2005 Estradiol signalling in Nereis virens reproduction. Invertebr Reprod Dev 48:95–100 [Google Scholar]
- Garcia-Alonso J, Hoeger U, Rebscher N 2006 Regulation of vitellogenesis in Nereis virens (Annelida: Polychaeta): effect of estradiol-17β on eleocytes. Comp Biochem Physiol A Mol Integr Physiol 143:55–61 [DOI] [PubMed] [Google Scholar]
- O'Sullivan O, Suhre K, Abergel C, Higgins DG, Notredame C 2004 3DCoffee: combining protein sequences and structures within multiple sequence alignments. J Mol Biol 340:385–395 [DOI] [PubMed] [Google Scholar]
- Anisimova M, Gascuel O 2006 Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552 [DOI] [PubMed] [Google Scholar]
- Yang Z, Kumar S, Nei M 1995 A new method of inference of ancestral nucleotide and amino acid sequences. Genetics 141:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WM 1971 Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20:406–416 [Google Scholar]
- Farris JS 1989 The retention index and the rescaled consistency index. Cladistics 5:417–419 [DOI] [PubMed] [Google Scholar]
- Zilliacus J, Carlstedt-Duke J, Gustafsson JA, Wright AP 1994 Evolution of distinct DNA-binding specificities within the nuclear receptor family of transcription factors. Proc Natl Acad Sci USA 91:4175–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG 2007 Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. [DOI] [PubMed] [Google Scholar]
- Henley DV, Korach KS 2006 Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 147:S25–S32 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 2004 Estrogen receptor-α mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology 205:55–63 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D 1993 Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132:2279–2286 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA 1998 Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252–4263 [DOI] [PubMed] [Google Scholar]
- Freedman LP 1998 Molecular biology of steroid and nuclear hormone receptors. Boston, MA: Birkhauser [Google Scholar]
- Tanenbaum DM, Wang Y, Williams SP, Sigler PB 1998 Crystallographic comparison of the estrogen and progesterone receptor's ligand binding domains. Proc Natl Acad Sci USA 95:5998–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G 2007 Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod 22:1885–1892 [DOI] [PubMed] [Google Scholar]
- Miura T, Miura C, Ohta T, Nader MR, Todo T, Yamauchi K 1999 Estradiol-17β stimulates the renewal of spermatogonial stem cells in males. Biochem Biophys Res Commun 264:230–234 [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA 2005 Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- Ortlund EA, Bridgham JT, Redinbo MR, Thornton JW 2007 Crystal structure of an ancient protein: evolution by conformational epistasis. Science 317:1544–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F 2001 MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP 2003 MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O 2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. System Biol 52:696–704 [DOI] [PubMed] [Google Scholar]
- Yang, Z 1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl BioSci 13:555–556 [DOI] [PubMed] [Google Scholar]
- Yang, Z 2007 PAML 4: a program package for phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.