Abstract
Each of the three members of the p160 steroid receptor coactivator (SRC) family of coactivators (SRC-1, SRC-2 and SRC-3) stimulates estrogen receptor (ER)-α function in trans-activation assays. Consequently, we sought to elucidate their contributions to the ER-regulated processes of cell proliferation, apoptosis, and the expression of ERα target genes in MCF-7 breast cancer cells. The small interfering RNA depletion of SRC-2 or SRC-3 but not SRC-1 inhibited growth of MCF-7 cells, and this was reflected in decreased cell cycle progression and increased apoptosis in SRC-2- or SRC-3-depleted cells as well as a reduction in ERα transcriptional activity measured on a synthetic reporter gene. However, only SRC-3 depletion blocked estradiol stimulated cell proliferation. Depletion of SRC-1 did not affect these events, and together this reveals functional differences between each of the three SRC family coactivators. Regulation of the endogenous ERα target gene, c-myc was not affected by depletion of any of the p160 coactivators although depletion of each of them decreased pS2 mRNA expression in estradiol-treated MCF-7 cells. Moreover, progesterone receptor and cyclin D1 gene expression were decreased in SRC-3 small interfering RNA-treated cells. Expression of mRNA and protein levels for the antiapoptotic gene, Bcl-2 was dependent on SRC-3 expression, whereas Bcl-2 protein but not mRNA expression also was sensitive to SRC-1 depletion. Together these data indicate that the closely related p160 coactivators are not functionally redundant in breast cancer cells because they play gene-specific roles in regulating mRNA and protein expression, and they therefore are likely to make unique contributions to breast tumorigenesis.
The p160 family of coactivators plays gene-specific roles in regulating mRNA and protein expression in breast cancer cells.
The biological contribution of the steroid hormone 17β-estradiol (E2) to the normal development and maintenance of reproductive function as well as the initiation and progression of breast cancer has been widely accepted (1). Classically, estrogens act by binding to nuclear estrogen receptors (ERs), ERα or ERβ, which function as ligand-regulated transcription factors. Liganded ERs undergo a change in conformation followed by dimerization and binding to estrogen response elements (EREs) upstream of E2-responsive genes (2). The ER complex is then able to recruit coactivators that are involved in enhancing ER-mediated gene transcription and the expression of target genes. The best-characterized coactivator proteins associated with ER signaling belong to the p160/steroid receptor coactivator (SRC) family. This family includes SRC-1, the first p160 family member to be cloned, as well as SRC-2 (also known as transcription intermediary factor TIF2 and glucocorticoid receptor interacting protein-1 GRIP-1) and SRC-3 (also known as amplified in breast cancer-1 AIB1, receptor-associated coactivator 3 RAC3, activator of thyroid and retinoic acid receptor ACTR, thyroid hormone receptor activator molecule 1 TRAM-1 and p300/CBP cointegrator-associated protein p/CIP), which was identified based on its amplification in breast cancer (3). These coactivators interact with ERs in a ligand-dependent manner and enhance transcriptional activation of the ER via histone acetylation and recruitment of additional coactivators, such as CARM-1 and cAMP response element-binding protein/p300 (2,3).
In estrogen-responsive breast cancer cells, E2 induces cell proliferation by stimulating progression through the G1 phase of the cell cycle (4). Many genes involved in cell growth, growth factor signaling, and cell cycle control are estrogen responsive (e.g. TGF-β, c-myc, and c-fos), and inappropriate expression or activity of a number of these molecules have been implicated in breast cancer (5,6). For instance, cyclin D1 amplification is found in 10–15% of invasive breast carcinomas and appears to be an early event in the development of breast carcinoma (7). Estrogens also can regulate the expression of genes involved in apoptosis such as the antiapoptotic gene, Bcl-2 (8), and through coordinated regulation of cell cycle progression and apoptosis, agonist-bound ERα has a strong positive effect on cell proliferation and tissue growth (9). An imbalance between cell proliferation and death has been implicated in tumorigenesis, and use of the ERα antagonist, tamoxifen, to inhibit ERα-dependent gene expression and consequently cell growth is used therapeutically for breast cancer (10).
The transcriptional activity of ERα bound to agonist as well as antagonists is influenced by the quantity and activity of coactivators present in the cell and the ability of receptors and coactivators to bind to one another (11). The p160 family of coactivators (SRC-1, SRC-2, and SRC-3) shares a significant degree of sequence identity and overexpression of each stimulates the activity of ERα in trans-activation assays (12). Moreover, each of the SRC family coactivators has been shown to contribute to the E2-dependent expression of the well-characterized ERα target gene, pS2 (13). However, results from studies of each of the p160 coactivator knockout mice indicates that these molecules are not functionally identical (12), and several instances of differential effects of these coactivators on steroid receptor transcriptional activity have been documented. For instance, in a mouse mammary tumor virus model system, progesterone receptor activity is preferentially regulated by SRC-1, whereas glucocorticoid receptors preferentially use SRC-2 (14). It has also been demonstrated that SRC-2/ glucocorticoid receptor interacting protein-1 but not SRC-3 can repress gene expression via glucocorticoid receptor and ER (15,16) Thus, whereas studies suggest that the p160 coactivators have similar and redundant transcriptional effects in model systems of ER-dependent gene expression, there is a significant and largely unexplored potential for these coactivators to differentially regulate transcription.
Both SRC-1 and SRC-3 coactivators contribute to growth responses in mammary gland as evidenced by a compromised growth phenotype in knockout mice treated with estrogen and progesterone (12), but the specific roles of each coactivator in this complex response are poorly defined. In in vitro studies, overexpression of SRC-1 has been associated with enhanced growth of MCF-7 breast cancer cells (17), and depletion of SRC-1 and SRC-2 by antisense oligonucleotides reduced [3H]thymidine incorporation, suggesting a role for these coactivators in cell proliferation (18). More detailed studies reveal that SRC-3 promotes cell proliferation via ER-dependent and ER-independent mechanisms (19,20). Several studies also suggest that SRC-1 and SRC-3 play a role in limiting apoptotic responses (19,21). However, the extent to which these effects are common to the three p160 coactivators has not been directly compared. Thus, the goal of the research described in this report was to better understand the contribution of each of the p160 family coactivators in the control of estrogen-stimulated MCF-7 cell proliferation and regulation of ERα-dependent gene expression. Our results indicate that each of the three p160 coactivators contributes to ERα transcriptional activity in MCF-7 cells but that there are differential requirements for these coactivators for cell growth, apoptosis and gene expression.
Materials and Methods
Chemicals, small interfering (si) RNAs and cell culture
E2 and the antiestrogen, 4-hydroxytamoxifen (4HT) were obtained from Sigma Chemical Co. (St. Louis, MO). All siRNAs were chemically synthesized by Ambion (Austin, TX) as oligonucleotide duplexes, using previously defined target sequences for SRC-1 (14), SRC-2 (14), and SRC-3 (22). A combination of two SRC-1 siRNAs was used to achieve optimal results (13,14). A sequence targeting luciferase (sense: 5′-CGT ACG CGG AAT ACT TCG A-3′) was used as nonspecific control in all cases except for luciferase reporter gene experiments, which used Ambion's Silencer no. 2 as the negative control. The MCF-7 human breast cancer cell line was obtained from American Type Culture Collection (Manassas, VA), whereas the LCC2 cell line was a kind gift of Dr. Robert Clarke (Georgetown University, Washington, DC).
Growth assays
One day before transfection, 1 × 105 cells were plated/well of a 6-well plate and grown overnight in DMEM with 10% fetal bovine serum (FBS). The next day, cells were transfected with 10 pmol siRNA targeting luciferase (siCont), SRC-1 (5 pmol siSRC-1a and 5 pmol siSRC-1b; siSRC-1), SRC-2 (siSRC-2), or SRC-3 (siSRC-3) using Oligofectamine reagent (Invitrogen, Carlsbad, CA). After 4 h, cells were fed with phenol red-free DMEM containing 10% stripped fetal bovine serum (sFBS) and treated with vehicle (VEH; 0.1% ethanol), 1 nm E2, or 100 nm 4HT. Two days later, media were replaced and fresh hormone was added. On d 5 after transfection, cells were harvested, and cell number was determined with a Beckman Z1 dual Coulter counter (Beckman Coulter, Fullerton, CA). Cell lysates prepared from parallel cultures were assessed by Western blot to verify coactivator protein level reductions by siRNA.
Cell cycle distribution and apoptosis assays
For cell cycle experiments, 3 × 105 MCF-7 cells were plated/well of a 6-well plate, and after siRNA transfection, cells were synchronized in phenol red-free DMEM plus 2.5% sFBS for 48 h. For apoptosis assays, 1 × 106 cells were plated in a 100-mm dish and 24 h later were transfected with 60 pmol siRNA and synchronized as above. After treatment with VEH (0.1% ethanol), 1 nm E2, or 100 nm 4HT for 24 h, adherent cells were collected for flow cytometric analysis, and both adherent and nonadherent cells were collected for apoptosis assay.
Flow cytometry
Adherent cells were fixed with 100% ethanol and then resuspended in PBS containing 50 μg/ml propidium iodide (Sigma) and incubated with ribonuclease A for 30 min at 37 C. Samples were analyzed on a Beckman Coulter EPICS XL-MCLs, and the resulting data were examined using WinMDI free flow cytometric analysis program (Scripps Research Institute, La Jolla, CA).
Cell death ELISA
Apoptosis was detected by the cell death ELISA kit from Roche Applied Sciences (Indianapolis, IN). Briefly, cells were lysed, and supernatants were assayed for levels of histone-associated DNA fragments using an Original MultiSkan PLUS plate reader (Thermo Electron Corp., Milford, MA).
Trans-activation assays for ER activity
Three × 105 MCF-7 cells were plated/well of a six-well plate and grown overnight in DMEM without phenol red containing 10% sFBS. The following day, siSRC-1, siSRC-2, or siSRC-3 was transfected as described above and Ambion's Silencer no. 2 was used as a negative control (siCont). Twenty-four hours later, cells were transfected with 1 μg of the ERE-E1b-LUC reporter using Trans-IT-LT1 (Mirus, Madison, WI), and 1 d later, cells were treated with VEH (0.1% ethanol) or 1 nm E2 for 24 h. Cell extracts were prepared using the luciferase assay system kit (Promega, Madison, WI) and assessed with a Luminoskan Ascent Thermo Labsystems (Thermo Electron). Relative luciferase units were normalized to total cellular protein measured by Bio-Rad protein assay (Bio-Rad, Hercules, CA). A third well was harvested in parallel to verify coactivator down-regulation by Western blot analysis.
Endogenous target gene expression
For monitoring endogenous ER-responsive genes, MCF-7 cells were plated in phenol red-free DMEM with 10% FBS. Cells were transfected with specified siRNAs as described above and placed in phenol red-free DMEM with 10% sFBS. Twenty-four hours later they were treated with VEH (0.1% ethanol), 1 nm E2, or 100 nm 4HT for 90 min (c-myc), 3 h (cyclin D1), or 24 h (progesterone receptor (PR), pS2, and Bcl-2) and then harvested for RNA isolation using Trizol (Invitrogen). In parallel, cells were collected and whole cell lysates were used to determine the protein expression by Western blot analysis (see below).
Reverse transcription and quantitative real-time PCR analysis
RNA was reverse transcribed with random primers and SuperScript II reverse transcriptase according to Invitrogen's protocol. Reactions for quantitative PCRs (qPCRs) were conducted using TaqMan (PR, Bcl-2, c-myc, 18S) or Power SYBR Green (SRC-1, SRC-2, SRC-3, cyclin D1, and pS2) PCR master mixes (Applied Biosystems, Foster City, CA), and samples were amplified with the ABI Prism 7700 sequence detector (Applied Biosystems). Primers for pS2 (13), SRC-1 (18), SRC-2 (18), SRC-3 (23), cyclin D1 (24), and primer and probe sequences for PR have been published (24). Bcl-2 and c-myc levels were analyzed using a predesigned primer-probe mix (Applied Biosystems). All mRNA quantities were normalized against 18S RNA using Eukaryotic 18S rRNA endogenous control reagent (Applied Biosystems).
Western blot analysis
Cells were harvested, resuspended in lysis buffer [50 mm Tris (pH 8.0) containing 400 mm NaCl, 5 mm EDTA, 1.0% Nonidet P-40, 0.2% Sarkosyl, and Complete minitablets protease inhibitors tablet (Roche Applied Sciences), incubated on ice for 20 min, and centrifuged to pellet cellular debris. The protein content of the whole cell lysate was determined by Bio-Rad protein assay. Proteins were then separated by SDS-PAGE using Invitrogen's precast 3–8% Tris-acetate (SRC-1, SRC-2, SRC-3, ERα) or 4-12% Bis-Tris (PR, c-myc, Bcl-2) gels and transferred to nitrocellulose membranes by electrotransfer. Nonspecific sites were saturated by incubating the blots in blocking buffer [PBS containing 1% Tween 20 and 5% (wt/vol) dried nonfat milk powder] for 1 h at room temperature and probed overnight with primary antibodies. After incubation with the appropriate horseradish peroxidase-conjugated antirabbit or antimouse antibody (Amersham Biosciences, Piscataway, NJ) diluted in PBS containing 1% Tween 20 containing 1% milk powder, detection of specifically bound proteins was carried out by ECL+PLUS according to the manufacturer's protocol (Amersham Pharmacia Biotech, Arlington Heights, IL) using XL-1 Blue film (Kodak, Branchburg, NJ). The primary antibodies are anti-SRC-1 (612378), anti-SRC-2 (610985), anti-SRC-3 (611105), anti-cyclin D1 (554180), and anti-Bcl-2 (610538) (all from BD Biosciences, San Jose, CA); anti-c-myc (9E10; Santa Cruz Biotechnology, Santa Cruz, CA); anti-PR (1294; a kind gift from Dr. Dean Edwards, Baylor College of Medicine, Houston, TX); and anti-actin (MAB1501R; Chemicon, Temecula, CA).
Results
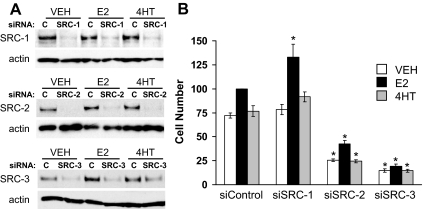
The first objective of these studies was to determine the relative contributions of each of the p160 coactivators to growth of MCF-7 cells using siRNA technology to ablate their expression. Previously we demonstrated by Western blot that these siRNAs effectively reduced p160 coactivator expression in MCF-7 cells when measured 12 h after transfection (13), and the goal of this experiment was to examine the ability of these siRNAs to suppress coactivator expression for an extended duration. As shown in representative blots (Fig. 1A, top panel) transfection of siRNAs for SRC-1 reduced this coactivator's protein in MCF-7 cells for at least 5 d, regardless of whether cells were treated with VEH, 1 nm E2, or 100 nm of the antiestrogen, 4HT. Similar decreases in the expression of SRC-2 and SRC-3 were observed for cells treated with the siRNAs for each respective coactivator (Fig 1A, middle and bottom panels). These experiments indicate that coactivator levels are suppressed for a length of time sufficient to conduct cell proliferation assays and that hormone treatment does not influence the siRNA-induced down-regulation of p160 coactivator expression.
Figure 1.
Prolonged suppression of p160 coactivator expression by siRNA transfection and effect on MCF-7 cell growth. MCF-7 cells were transfected with 10 pmol total siRNA Silencer-negative control (siControl; C), SRC-1, SRC-2,or SRC-3 and then treated with VEH (0.1% ethanol), 1 nm E2, or 100 nm 4HT for 5 d. Subsequently expression of SRC-1, SRC-2, SRC-3, and actin was assessed by Western blotting (A) and cell number was determined by Coulter counter (B). Representative blots are shown and demonstrate that coactivator depletion is apparent 5 d after siRNA transfection and hormone treatment. The cell number data are normalized to values obtained for control siRNA-transfected and E2-treated cultures and represent the mean ± sem of three to four experiments. Statistical analyses were by ANOVA. *, P < 0.05 vs. similarly treated (VEH, E2, or 4HT), control siRNA-transfected cells.
To determine the contribution of p160 coactivators to cell growth, siRNAs targeting SRC-1, SRC-2, or SRC-3 were transfected into MCF-7 cells, which were subsequently cultured in media containing stripped serum and treated with VEH, 1 nm E2, or 100 nm 4HT for 5 d. For the control siRNA MCF-7 experiment (Fig. 1B), cell number was increased for cultures treated with E2. 4HT does not exert a significant growth-inhibitory effect on these cultures because they are grown in the absence of E2. Depletion of SRC-1 expression modestly increased E2 stimulation of MCF-7 cell growth but had no impact on the proliferation of 4HT-treated MCF-7 cells. In contrast, depletion of SRC-2 and SRC-3 attenuated the growth of MCF-7 cells for all treatment conditions in comparison with the control siRNA groups. Notably, depletion of SRC-3 but not SRC-2 blocked E2 stimulation of cell growth. Collectively, these results demonstrate that SRC-2 and SRC-3 but not SRC-1 play a positive role in regulating the growth of MCF-7 cells.
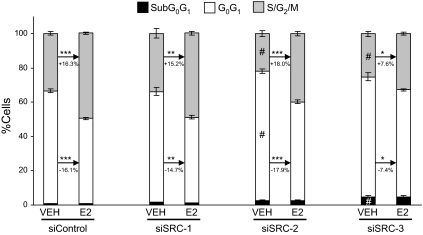
Because p160 coactivator depletion had a significant effect on MCF-7 proliferation, we subsequently determined the cell cycle distribution of p160-depleted cells. To study this, cells were transfected with siRNA targeting SRC-1, SRC-2, or SRC-3, and then media were changed to 2.5% sFBS to synchronize the cells. After 48 h, cultures were treated with either VEH or 1 nm E2 for an additional 24 h. In control siRNA-transfected MCF-7 cells, E2 treatment produced the expected result of a reduced percentage of cells in the sub-G0 and G0G1 phases of the cell cycle, and an increased number of cells in the S/G2M phase (Fig. 2). A similar effect is observed for cells depleted of SRC-1. When SRC-2 or SRC-3 is depleted, several differences are noted. First, the percentage of cells in the sub-G0 peak is not decreased by E2 treatment, and the fraction of cells in the S/G2M peaks is significantly diminished. Thus, depletion of SRC-2 or SRC-3 results in a significantly larger percentage of cells in the sub-G0 peak as well as a smaller percentage of cells in the S/G2M phases of the cell cycle. Both of these findings are consistent with the reduced proliferation of cells depleted of either SRC-2 or SRC-3. Cells lacking SRC-2 differed from those lacking SRC-3 with respect to the ability of E2 treatment to decrease the percentage of cells in G0G1 and increase the proportion of cells in S/G2M. For instance, E2 treatment caused a 18.0% increase in the number of cells in S/G2M in cells pretreated with siRNA to SRC-2, whereas in SRC-3-depleted cells, only a 7.6% increase in cells was observed. This is consistent with the greater ability of E2 to increase cell number in SRC-2-depleted cells in comparison with those lacking SRC-3.
Figure 2.
Effect of p160 coactivator depletion on cell cycle distribution in MCF-7 cells. MCF-7 cells were transfected with either control siRNA or siRNA against SRC1, SRC-2, or SRC-3 and subsequently treated with VEH (0.1% ethanol) or 10 nm E2 for 24 h. Thereafter cells were harvested, stained with propidium iodide, and subjected to flow-cytometric analysis. The data represent the mean ± sem of three to four experiments. Comparisons between VEH and E2-treated samples within the same siRNA treatment group were made by student t test (***, P < 0.001; **, P < 0.01; *, P < 0.05). Comparisons of the effect of siRNA treatments on VEH or E2 groups were done by one-way ANOVA (#, P < 0.05 vs. the respective siControl group).
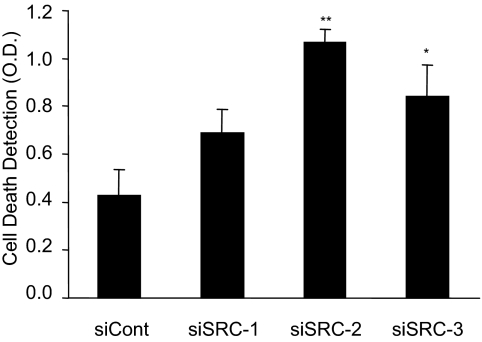
As noted above, when performing cell cycle analysis of propidium iodide stained cells, the subG0 peak, which can be indicative of cells undergoing apoptosis, was increased by depletion of SRC-2 and SRC-3 but not SRC-1 in both cell lines. Consequently, we performed a cell death ELISA to determine whether coactivator depletion influenced apoptosis under our assay conditions. The results of the cell death assay indicate that compared with the control siRNA, depletion of SRC-2 or SRC-3 increased programmed cell death (Fig. 3). This observation is consistent with the increased sub-G0 peak seen in the cell cycle experiments for SRC-2- or SRC-3-depleted cells (Fig. 2), and the reduction in the proliferation of SRC-2- or SRC-3-deficient cultures (Fig. 1B). Thus, the reduction in the ability of E2 to stimulate cell proliferation of SRC-2- or SRC-3-depleted MCF-7 cells is a consequence of a reduction in the ability of hormone to stimulate cell cycle progression and an increase in the number of cells undergoing apoptosis.
Figure 3.
Effect of p160 depletion on apoptosis of MCF-7 cells. MCF-7 cells were plated in media containing 10% FBS and 24 h later were transfected with siRNA targeting luciferase as a negative control (siCont), SRC-1 (siSRC-1), SRC-2 (siSRC-2), or SRC-3 (siSRC-3). After transfection, media were replaced with phenol-red-free DMEM and 2.5% sFBS for 48 h, and cells were exposed to VEH (0.1% ethanol) for an additional 24 h. Apoptosis was detected in cells using a cell death ELISA, and the data represent the mean OD ± sem of nine experiments. Statistical analyses were by one-way ANOVA: *, P < 0.05; or **, P < 0.01 vs. control siRNA-transfected cells.
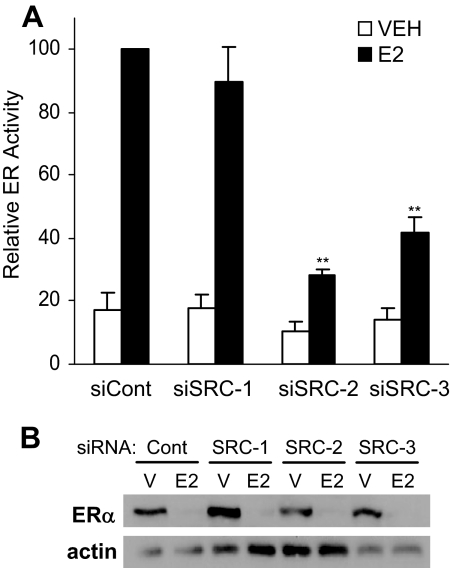
Because the activity of ERα contributes to the regulation of genes that play a role in cell cycle progression as well as apoptosis, the ability of SRC coactivator depletion to influence ERα transcriptional activity was examined. Expression of the p160 coactivators was depleted in MCF-7 cells by siRNA and 1 d later cells were first transfected with an ERE-E1b-LUC reporter construct and then treated with VEH or 1 nm E2 for an additional 24 h. Figure 4A shows that depletion of SRC-2 or SRC-3 abrogated the extent of E2-induced transcriptional activity compared with control transfected cells. In contrast, depletion of SRC-1 did not alter E2-induced transcriptional activity. Previous studies suggested that ER transcriptional activity is required for ligand-induced receptor down-regulation (25,26). Results obtained in these studies demonstrate that E2 treatment induces degradation of ERα protein levels, regardless of SRC coactivator depletion (Fig. 4B), even when the receptor's transcriptional activity is significantly compromised in SRC-2- or SRC-3-depleted cells.
Figure 4.
ERα transcriptional activity and expression in MCF-7 cells after depletion of SRC coactivators. A, MCF-7 cells were transfected with 10 pmol total siRNA specific for Ambion's negative control siRNA no. 2 (siCont), SRC-1 (siSRC-1), SRC-2 (siSRC-2), or SRC-3 (siSRC-3). Twenty-four hours later, cells were transfected with 1 μg ERE-E1b-LUC reporter plasmid, and 1 d later cells were exposed to VEH (0.1% ethanol) or 1 nm E2 for an additional 24 h. Luciferase activity was assessed and normalized to total protein. Data are presented as ER activity relative to values obtained for E2-treated, control siRNA-transfected samples and represent the mean ± sem of six experiments. Statistical analyses were by one-way ANOVA: *, P < 0.05; or **, P < 0.01 vs. similarly treated (VEH or E2) control siRNA-transfected cells. Parallel MCF-7 cells (B) treated with either vehicle (V) or E2 were assessed for ERα and actin expression by Western blot analysis. A representative Western blot is shown.
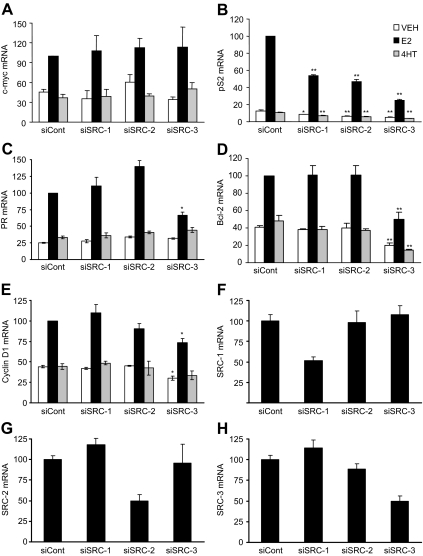
To determine whether the distinct contributions of each of the three p160 coactivators to regulate cell proliferation, apoptosis, and ER transcriptional activity was reflected in the regulation of endogenous target genes, the impact of SRC coactivator depletion on the expression of five well-characterized ER-regulated genes (c-myc, PR, Bcl-2, cyclin D1, and pS2) was investigated in MCF-7 cells treated with VEH, 1 nm E2, or 100 nm 4HT for times ranging from 90 min to 24 h, depending on the gene. Treatment with E2 increased the mRNA levels of all the genes tested (Fig. 5, A–E) in comparison with the VEH-treated controls. Among the five genes, only c-myc was unaffected by individual depletion of any of the SRCs. In contrast, the E2-induced mRNA expression of the well characterized ERα target gene, pS2, was significantly compromised in cells depleted of each of the individual SRCs in comparison with control cells (Fig. 5B). An intermediate effect was seen in the case of PR, Bcl-2, and cyclin D1 mRNA expression, which were found to be significantly decreased only in SRC-3-depleted cells (Fig. 5, C–E). Basal expression of the pS2 and Bcl-2 genes were reduced in all coactivator-depleted cells and SRC-3-depleted cells, respectively, implying a role for these coactivators in regulating the expression of these genes, even in the absence of E2. The specificity of siRNA treatment was determined by RT-qPCR. In each case there was an approximately 50% decrease in the target mRNA and no impact on the expression of other members of the p160 family (Fig. 5, F and G). These results indicate that the ability of coactivators to regulate transcription is gene specific.
Figure 5.
Effect of p160 coactivator depletion on mRNA expression of ERα target genes. MCF-7 cells were transfected with siRNA targeting luciferase-negative control (siCont), SRC-1 (siSRC-1), SRC-2 (siSRC-2), or SRC-3 (siSRC-3) and 24 h later were treated with VEH (0.1% ethanol), 1 nm E2, or 100 nm 4HT for 90 min (c-myc; A), 3 h (cyclin D1, E), or 24 h (pS2, PR, and Bcl-2; B–D, respectively). Cells were then harvested for RNA isolation and measurement of the indicated mRNAs by RT-qPCR. The data are normalized to E2-treated control siRNA samples and represent the mean ± sem of three to four experiments. Statistical analyses were by one-way ANOVA: *, P < 0.05; or **, P < 0.01 vs. similarly treated (VEH, E2, or 4HT) control siRNA-transfected cells. Levels of SRC-1 (F), SRC-2 (G), and SRC-3 (H) mRNAs are shown for MCF-7 cells transfected with control or p160 coactivator siRNAs and treated with VEH for 24 h. Values represent mean ± sem of three experiments.
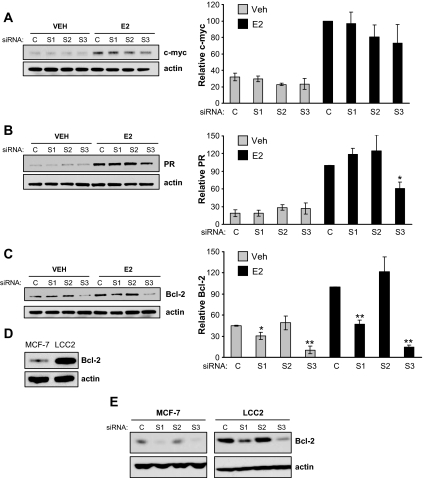
The impact of p160 coactivator depletion on the protein level of these genes was also determined. Western blot analyses revealed that c-myc, PR, and Bcl-2 protein levels are elevated by E2 treatment of MCF-7 cells (Fig. 6, A–C). Consistent with mRNA data, protein levels of c-myc were unaffected by depletion of any of the coactivators (Fig. 6A), whereas PR levels were reduced in cells depleted of SRC-3 but not SRC-1 or SRC-2 (Fig. 6B). More interestingly, protein levels of Bcl-2 were lower after SRC-1 or SRC-3 siRNAs (Fig. 6C), in contrast to the mRNA levels that were found to be affected by SRC-3 depletion only. Because the decrease in basal Bcl-2 protein expression in SRC-1-depleted, E2-treated cells was not reflected at the mRNA level, this reduction likely reflects a posttranscriptional effect of SRC-1.
Figure 6.
Effect of p160 coactivator depletion on protein expression of ERα target genes. MCF-7 cells transfected with control (C), SRC-1 (S1), SRC-2 (S2), or SRC-3 (S3) siRNAs were treated with VEH or E2 for 90 min (c-myc) or 24 h (PR and Bcl-2), harvested and whole-cell lysates assessed for c-myc (A), PR (B), and Bcl-2 protein (C) levels by Western blotting. Blots obtained from three to four independent experiments were quantitated by densitometry and expressed as mean ± sem. Statistical analyses by t test: *, P < 0.05; or **, P < 0.001 vs. similarly treated (VEH or E2) control siRNA-transfected cells. Western blot analysis of constitutive Bcl-2 protein expression for untreated MCF-7 and LCC2 cells (D) or MCF-7 and LCC2 cells treated with siRNAs and exposed to E2 (E) were as described for C. Blots are representative of three separate experiments.
To further explore the role of p160 coactivators in regulation of Bcl-2 expression, we turned to LCC2 breast cancer cells, a tamoxifen-resistant line derived from MCF-7 cells that grow in the absence of estrogen or presence of tamoxifen (27). It has been demonstrated previously (28) that LCC2 cells express higher levels of Bcl-2 than MCF-7 cells (Fig. 6D). Depletion experiments assessed whether SRC coactivators also played a role in the regulation of high Bcl-2 expression in LCC2 cells. Similar to the results obtained for MCF-7 cells, depletion of SRC-1 or SRC-3 reduced Bcl-2 protein expression in E2-treated LCC2 cells (Fig. 6E). This indicates that despite the increased protein expression of Bcl-2 in LCC2 compared with MCF-7 cells, SRC-1 and SRC-3 make similar contributions to the regulation of Bcl-2 protein expression.
Discussion
Numerous studies have sought to elucidate the mechanisms by which agonists and antagonists influence growth and survival of ER-positive breast cancer cells, and SRC coactivators as mediators of ER action are predicted to make important contributions to these events. In our studies, we discovered that depletion of SRC-2 or SRC-3, but not SRC-1, decreased proliferation of MCF-7 cells, indicating that there are differences between these related coactivators with respect to cell growth. Moreover, our data indicate that the reduced proliferation of SRC-2- or SRC-3-depleted cells was due to their decreased progression into the S and G2M phases of the cell cycle as well as an increase in the number of cells undergoing apoptosis. We further noted that SRC-3 but not SRC-2 is required for E2 regulation of cell proliferation. This is particularly striking because both SRC-2 and SRC-3 depletion significantly decreased E2-stimulated, ERα-dependent gene expression measured by reporter gene assay. Thus, analysis of hormone-dependent and hormone-independent regulation of transcription clearly distinguishes the biological specificity of SRC coactivators within this breast cancer cell environment.
The ability of SRC-3 to contribute to breast cancer cell growth has been relatively well characterized. Early experiments demonstrated that depletion of SRC-3 by ribozyme reduced MCF-7 cell growth (19), and more recent experiments have implicated a role for SRC-3 in regulating cell proliferation via the effects of this coactivator on ER as well as the E2F1 transcriptional factor (20). The latter acts directly with SRC-3 to stimulate the expression of genes important for DNA replication, and hence, fibroblasts lacking SRC-3 do not effectively enter the S phase of the cell cycle (29). Our experiment demonstrating a loss of cells in the S/G2M phase of the cell cycle for SRC-3-depleted MCF-7 cells is consistent with that finding. A role for SRC-3 in prostate cancer cell proliferation has also been documented; SRC-3 overexpression stimulates, whereas siRNA depletion of SRC-3 decreases, cell growth and cell size (30). In these studies, changes in SRC-3 expression were correlated with modulation of the AKT signaling pathway, which is involved in antiapoptosis, cell survival, and cell proliferation in many cancers (30,31). Other investigators have demonstrated that SRC-3 but not SRC-2 is required for proliferation of androgen-dependent and androgen-independent prostate cancer cell and tumor growth, and as for fibroblast cells, SRC-3 stimulates the expression of cyclin E and cdk2 (29,32).
The ability of SRC-1 and SRC-2 to affect cell proliferation is less well studied. An early experiment conducted with MCF-7 cells stably overexpressing SRC-1 indicated that this coactivator increased both cell growth and pS2 gene expression (17). Whereas our data support a stimulatory role for SRC-1 on pS2 expression, our growth assay indicated a mild inhibitory role in cell growth, and this apparent difference may be a reflection of the stable overexpression used in the prior report vs. the acute depletion methodology used in these studies. The cell cycle and apoptosis experiments were not informative as to the cause of the increased number of cells in SRC-1 depleted cultures, perhaps because they reflect cellular processes 24 h after E2 treatment in contrast to the 5-d growth assays.
Our data indicate that SRC-2 depletion reduces overall cell growth but not the ability of E2 to stimulate cell proliferation and progression through the cell cycle, and this is interesting in light of the trans-activation data indicating a requirement of SRC-2 for maximal ERα transcriptional activity measured by synthetic reporter gene. In prostate cancer cells, the role for SRC-2 in regulation of proliferation has been less clear, with one study suggesting a role for SRC-2 in AR-induced target gene expression and proliferation of androgen receptor-dependent and androgen receptor-independent prostate cancer cells (33), whereas another study did not find a role for SRC-2 in prostate cancer proliferation (32). Little is known about the mechanisms by which SRC-2 contributes to cell growth. Our studies indicate that under vehicle conditions SRC-2-depleted cells undergo an increased rate of apoptosis and are found in greater proportion in the G0G1 phase of the cell cycle in comparison with control cells. Together, these findings suggest that SRC-2 exerts effects on cell proliferation independent of estrogens. This is further supported by the demonstration that E2 retains the ability to promote MCF-7 cell progression into the S/G2M phase of the cell cycle. Depletion of SRC-2 therefore is phenotypically similar to the estrogen-independent growth effects observed for SRC-3 depletion, and this raises the possibility that SRC-2 also may regulate the expression of some of the cell cycle genes known to be regulated by SRC-3 in an ERα-independent manner (29,32). In this regard it would be interesting to compare the effect of SRC-2 vs. SRC-3 depletion on global gene expression to determine the extent to which these coactivators overlap in their regulation of genes.
It has been suggested that proteasomal degradation of ERα is important for its efficient ligand-stimulated transcriptional activity (25). Mutational analyses of the ERα ligand binding domain suggests that E2-stimulated down-regulation of ERα depends on the receptor's interaction with a protein(s) via its coactivator binding groove (25). Depletion of SRC-3 in MCF-7 cells by Shao et al. (26) suggested that this coactivator was required for agonist-induced, but not antagonist-induced ERα down regulation, and we therefore determined in our studies whether inhibition of E2-dependent ERα turnover was related to the loss of ERα transcriptional activity and cell growth observed for MCF-7 cells. However, individual depletion of the p160 coactivators did not influence E2-induced down-regulation of ERα, and loss of receptor turnover is therefore unlikely to contribute to the phenotypes observed for SRC-3- or SRC-2-depleted cells.
Loss of SRC-2 and SRC-3 expression resulted in increased levels of apoptosis in MCF-7 cells, and this is reminiscent of results from another laboratory that found that overexpression of SRC-1 and SRC-3, and to a lesser extent SRC-2, suppressed TNF-α-induced cell death in MCF-7 cells (21); the involvement of SRC-1 in the latter studies may reflect use of cytokine to induce apoptosis. One of the molecules involved in negative regulation of apoptosis by estrogen is Bcl-2 (8,34), and because E2 regulation of the Bcl-2 gene is direct (35), we examined the impact of p160 coactivator depletion on its expression. Depletion of SRC-3 significantly decreased levels of Bcl-2 mRNA and protein in MCF-7 cells, suggesting that this coactivator is required for maximal expression of this gene. This also was found for LCC2 cells, suggesting that p160 coactivator regulation of this gene is maintained, even though its basal Bcl-2 expression is dramatically higher in these tamoxifen-resistant cells.
Our data also revealed a reduction in the levels of Bcl-2 protein by siRNA specific to SRC-1 that was not reflected at the mRNA level, and this suggests that the regulatory function of SRC-1 may extend beyond the level of transcriptional control. Although, to the best of our knowledge, this is a novel finding for SRC-1, it has been demonstrated that SRC-3 can repress the translation of mRNAs involved in inflammatory responses induced by TNFα and IL-1β (36), and it therefore appears likely that the ability of coactivators to regulate cytoplasmic events associated with the overall process of gene expression may be a more common feature than previously appreciated. Because SRC-1 depletion does not induce apoptosis, it is likely that other antiapoptotic genes compensate for loss of Bcl-2 expression, and/or the impact of the decreased Bcl-2 expression is below the sensitivity of detection for the cell death ELISA.
Overall, our studies of gene expression revealed that the effect of p160 coactivator depletion on ERα target gene expression was gene-specific. Previous overexpression studies of transiently transfected CHO-K1 cells suggested that there could be ERE sequence-dependent differences in ER functional interactions with SRC-1, SRC-2, and SRC-3 (37), and our data demonstrate the specificity of endogenous coactivator action in human breast cancer cells. As expected (13), depletion of each of the p160 coactivators decreased pS2 mRNA expression in E2-treated MCF-7 cells. Moreover, the reductions in PR and cyclin D1 mRNA expression in SRC-3-depleted cells were consistent with chromatin immunoprecipitation (ChIP) experiments demonstrating E2-induced SRC-3 recruitment to each of these genes (data not shown). Somewhat surprisingly, however, the expression of c-myc, which is a well-defined estrogen target gene in breast cancer cells important for progression through the cell cycle (4,38), was not affected by p160 coactivator depletion in our experiments. It is possible that other coactivators may be critical for the expression of this gene in MCF-7 cells or that depletion of a single p160 coactivator is insufficient to perturb its expression. In support of the latter concept, ChIP-re-ChIP experiments demonstrated simultaneous binding of more than one p160 coactivator to some but not all ERα target genes (39).
Taken together, our data demonstrate that the p160 coactivators play distinct roles in regulating breast cancer cell proliferation and estrogen target gene expression. Allosteric changes in ERα conformation resulting from the receptor's interactions with distinct DNA responses elements has been suggested to contribute to the variable contributions of individual coactivators to the regulation of specific genes by altering the ER-coactivator binding affinity (37,40). Our data also suggest that coregulators can exert control at a posttranscriptional level, and this has the potential to significantly extend the mechanisms used by SRC family coactivators to exert their biological effects. These are likely to be quite varied and clinically important because studies have linked elevated coactivator levels to breast cancer pathology and the development of tamoxifen-resistant tumors. For instance, elevated SRC-3 levels in conjunction with increased HER2 expression were predictors of a poorer outcome for women on tamoxifen therapy compared with women not on tamoxifen therapy (41), and this finding has recently been confirmed by another study that found high SRC-3 expression was correlated with early disease recurrence in tamoxifen-treated patients (42). In addition, several but not all breast cancer studies noted a correlation with elevated SRC-1 expression and resistance to tamoxifen (43,44,45). Thus, greater insight into p160 coactivator action provides an opportunity to better understand breast tumorigenesis and response to endocrine therapy.
Acknowledgments
We thank Judy Roscoe for technical assistance and David Lonard for providing reagents for the PR qPCR analyses.
Footnotes
This work was supported by Public Health Service Grants DK53002 and DK64038 and Department of Defense Award W81XWH-04-1-0424 (to C.L.S.). S.K. was supported by a postdoctoral fellowship award (PDF 0707868) from the Susan G. Komen for the Cure Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 18, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; FBS, fetal bovine serum; 4HT, 4-hydroxytamoxifen; PR, progesterone receptor; qPCR, quantitative PCR; si, small interfering; siCont, control siRNA sFBS, stripped fetal bovine serum; SRC, steroid receptor coactivator; VEH, vehicle.
References
- Henderson BE, Ross RK, Bernstein L 1988 Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res 48:246–253 [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J-Å 2001 Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL 1997 Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem 272:10882–10894 [DOI] [PubMed] [Google Scholar]
- Dubik D, Shiu RP 1992 Mechanism of estrogen activation of c-myc oncogene expression. Oncogene 7:1587–1594 [PubMed] [Google Scholar]
- Hodges LC, Cook JD, Lobenhofer EK, Li L, Bennett L, Bushel PR, Aldaz CM, Afshari CA, Walker CL 2003 Tamoxifen functions as a molecular agonist inducing cell cycle-associated genes in breast cancer cells. Mol Cancer Res 1:300–311 [PubMed] [Google Scholar]
- Vos CB, Ter Haar NT, Peterse JL, Cornelisse CJ, van de Vijver MJ 1999 Cyclin D1 gene amplification and overexpression are present in ductal carcinoma in situ of the breast. J Pathol 187:279–284 [DOI] [PubMed] [Google Scholar]
- Teixeira C, Reed JC, Pratt MA 1995 Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res 55:3902–3907 [PubMed] [Google Scholar]
- Song RX-D, Santen RJ 2003 Apoptotic action of estrogen. Apoptosis 8:55–60 [DOI] [PubMed] [Google Scholar]
- Kumar A, Vadlamudi RK, Adam L 2000 Apoptosis in mammary gland and cancer. Endocr Relat Cancer 7:257–269 [DOI] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW 2004 Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- Labhart P, Karmakar S, Salicru EM, Egan BS, Alexiadis V, O'Malley BW, Smith CL 2005 Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci USA 102:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wong J, Tsai SY, Tsai M-J, O'Malley BW 2003 Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol 23:3763–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR 2002 Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci USA 99:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyli S, Fox MS, Leitman DC 2006 Distinct roles of unliganded estrogen receptors in transcriptional repression. Mol Cell 21:555–564 [DOI] [PubMed] [Google Scholar]
- Tai H, Kubota M, Kato S 2000 Involvement of nuclear receptor coactivator SRC-1 in estrogen-dependent cell growth of MCF-7 cells. Biochem Biophys Res Commun 267:311–316 [DOI] [PubMed] [Google Scholar]
- Cavarretta ITR, Mukopadhyay R, Lonard DM, Cowsert LM, Bennett CF, O'Malley BW, Smith CL 2002 Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERα transcriptional activity and MCF-7 proliferation. Mol Endocrinol 16:253–270 [DOI] [PubMed] [Google Scholar]
- List H-J, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT 2001 Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem 276:23763–23768 [DOI] [PubMed] [Google Scholar]
- Louie MC, Zou JX, Rabinovich A, Chen H-W 2004 ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24:5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon CB, Elliott S, Zhu Y, Clayton JL, Curiel TJ, Jaffe BM, Burow ME 2004 Regulation of estrogen-mediated cell survival and proliferation by p160 coactivators. Surgery 136:346–354 [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Tsai SY, O'Malley BW 2004 Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol 24:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL 2007 The silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full estrogen receptor-α transcriptional activity. Mol Cell Biol 27:5933–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turn-over and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Shao W, Keeton EK, McDonnell DP, Brown M 2004 Coactivator AIBI links estrogen receptor transcriptional activity and stability. Proc Natl Acad Sci USA 101:11599–11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Brunner N, Katzenellenbogen BS, Thompson EW, Norman MJ, Koppi C, Paik S, Lippman ME, Dickson RB 1989 Progression of human breast cancer cells from hormone-dependent to hormone-independent growth both in vitro and in vivo. Proc Natl Acad Sci USA 86:3649–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilling G, Hacohen H, Nordenberg J, Livnat T, Rotter V, Sidi Y 2000 Differential sensitivity of MCF-7 and LCC2 cells, to multiple growth inhibitory agents: possible relation to high bcl-2/bax ratio? Cancer Lett 161:27–34 [DOI] [PubMed] [Google Scholar]
- Louie MC, Revenko AS, Zou JX, Yao J, Chen H-W 2006 Direct control of cell cycle gene expression by proto-oncogene product ACTR, and its autoregulation underlies its transforming activity. Mol Cell Biol 26:3810–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai M-J 2003 Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol 23:7742–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL 2002 The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501 [DOI] [PubMed] [Google Scholar]
- Zou JX, Zhong Z, Shi XB, Tepper CG, DeVere White RW, Kung HJ, Chen H 2006 ACTR/AIB1/SRC-3 and androgen receptor control prostate cancer cell proliferation and tumor growth through direct control of cell cycle genes. Prostate 66:1474–1486 [DOI] [PubMed] [Google Scholar]
- Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WEI, Erdem H, Frolov A, Smith CL, Ayala GE, Ittmann MM, Weigel NL 2006 Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res 66:10594–10602 [DOI] [PubMed] [Google Scholar]
- Kandouz M, Lombet A, Perrot J-Y, Jacob D, Carvajal S, Kazem A, Rostene W, Therwath A, Gompel A 1999 Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. J Steroid Biochem Mol Biol 69:463–471 [DOI] [PubMed] [Google Scholar]
- Perillo B, Sasso A, Abbondanza C, Palumbo G 2000 17β-Estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol 20:2890–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW 2007 An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell 25:765–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J 2004 Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol 33:387–410 [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Weinberg RA 1997 Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol 17:4059–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yi X, Sun X, Yin N, Shi B, Wu H, Wang D, Wu G, Shang Y 2004 Differential gene regulation by the SRC family of coactivators. Genes Dev 18:1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Likhite VS, Loven MA, Nardulli AM 2001 Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Mol Endocrinol 15:1114–1126 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SAW, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Dihge L, Bendahl PO, Grabau D, Isola J, Lovgren K, Ryden L, Ferno M 2008 Epidermal growth factor receptor (EGFR) and the estrogen receptor modulator amplified in breast cancer (AIB1) for predicting clinical outcome after adjuvant tamoxifen in breast cancer. Breast Cancer Res Treat 109:255–262 [DOI] [PubMed] [Google Scholar]
- Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O'Higgins NJ, Hill AD, Young LS 2004 Inverse relationship between ER-β and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer 91:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AJ, Herrick RE, Heinsohn EC, Schiff R, Osborne CK 2003 Dominant-negative nuclear receptor corepressor relieves transcriptional inhibition of retinoic acid receptor but does not alter the agonist/antagonist activities of the tamoxifen-bound estrogen receptor. Mol Endocrinol 17:1543–1554 [DOI] [PubMed] [Google Scholar]
- Sarvilinna N, Eronen H, Miettinen S, Vienonen A, Ylikomi T 2006 Steroid hormone receptors and coregulators in endocrine-resistant and estrogen-independent breast cancer cells. Int J Cancer 118:832–840 [DOI] [PubMed] [Google Scholar]