More than 800 G protein-coupled receptors (GPRs) are transcribed in the human genome (1). For many of these receptors, no endogenous ligand has been found, and, therefore, they are currently designated as orphan receptors. One such protein, GPR30, has been proposed to be an estrogen receptor (ER) (2,3).
GPR30 has been localized either to the cell plasma membrane (2) or to the endoplasmic reticulum (3) by two investigative groups, and has been proposed to mediate estrogen action as a steroid receptor. At the cellular level, the early experimental support for this idea is simply not strong. 17-β-Estradiol (E2) shows minimal (arguably background) binding to GPR30 and quite modest signal transduction (4). This is compared with dramatically stronger actions of physiological E2 at classical endogenous (5) or exogenous ERα (6). A recent report indicates that radioactive E2 fails to bind GPR30 in a saturable or specific fashion, and E2 does not stimulate cAMP or calcium release (7). Thus, there is scant support that E2 directly binds GPR30. In addition, a putative GPR30 agonist, G1 (8), does not stimulate estrogen-like effects in the uterus or mammary gland of mice (7). It is puzzling that the receptor pharmacology is quite different, in that ICI182780 (fulvestrant; AstraZeneca Sverige AB, Södertälje Sweden), an ER antagonist, is proposed to strongly activate GPR30 (2). Importantly, it has not been demonstrated that fulvestrant actually binds GPR30. Finally, GPR30 is incapable of mediating any actions of estrogen in cells in which ERα and ERβ are absent (5), and several laboratories have shown that E2 effects are unaltered when GPR30 has been knocked down with interfering RNA (5,9).
To test rigorously the hypothesis that GPR30 is a functional ER, Isensee et al. (10) created a GPR30-lacZ reporter mouse, described in this issue of Endocrinology. The lacZ reporter was inserted into the GPR30 gene locus, causing a partial deletion of the GPR30 coding sequence but identifying where GPR30 is expressed in the mouse. The tissue distribution showed extensive expression in the brain and endocrine organs, consistent with GPR30 production in many tissues.
Isensee et al. (10) then performed extensive phenotyping of female mice. No reproductive tract or mammary gland abnormalities were seen, and the mice were fertile. Body weight and fat mass were comparable to wild-type littermates, even upon challenge with a high-fat diet. Under normal or high-fat feeding conditions, glucose tolerance testing was indistinguishable between wild-type and GPR30-lacZ mice. The authors then performed an exhaustive screen of behavior, assessment of the physiology of many organs, and blood chemistries. The only difference found between wild-type and GPR30-lacZ mice was a moderate decrease in CD4+ and CD8+T lymphocyte cells in the blood of the GPR30-lacZ mice. Thus, most biological functions were not influenced by the loss of GPR30 function. Importantly, there were no similarities of the GPR30-lacZ mouse to the extraordinary phenotype of female ERα knockout (KO) mice; the latter mice demonstrate a wide variety of abnormal organ development and functions (11,12).
The lack of phenotype in the GPR30-lacZ mouse nicely complements several other recent reports investigating different GPR30 KO mice. Otto et al. (13) created a complete GPR30 KO mouse by deleting exon 3. As a result, GPR30 was not expressed in any reproductive tract or mammary tissues, compared with extensive expression of this orphan receptor in these same organs from wild-type littermates. In female GPR30 KO mice, reproductive tract and mammary gland development was indistinguishable from that of wild-type littermates. In addition, fertility in the two mice was comparable, and uterine responses to injected E2 showed similar epithelial cell proliferation and gene expression. The authors concluded that GPR30 does not mediate these classical actions of E2 in female mice. In work from Wang et al. (14), the only estrogen-related phenotype of a GPR30 KO mouse reported was a decrease in thymus size. Moreover, some of the effects of E2 on thymocyte development were reported as mediated through ERα. In vivo, E2 induced less cell death of F-CD4/CD8 double-negative thymocytes from GPR30 KO mice compared with wild-type littermates. However, GPR30 expression in wild-type mice was not found in the F-CD4/CD8 double-negative thymocytes that underwent E2-induced apoptosis, although the cells showed ERα expression. The authors stated that they found no obvious physical, immunological, reproductive, or neurological abnormalities in these GPR30 KO female mice. In contrast to the aforementioned reports, Martensson et al. (15) found female GPR30 KO mice to be hyperglycemic or show impaired glucose tolerance and reduced IGF-I levels in serum. Insulin expression and release from isolated islets or in vivo in response to estrogen was also reported as impaired in this model. These mice also were reported to show decreased skeletal development and growth, and elevated blood pressure and vascular resistance, but these abnormalities were not attributed to a loss of estrogen action. This is different than the other three GPR30 KO mouse models, and may rather reflect the genetic background and techniques used to create these mice.
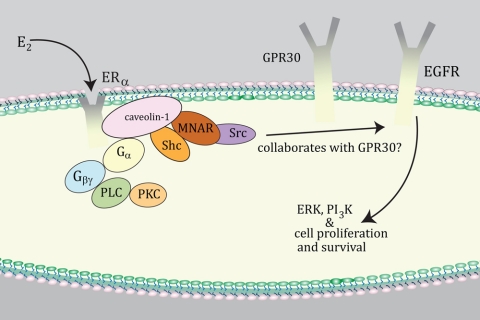
How can we coherently understand these disparate views on the role of GPR30 in E2 signaling, particularly from isolated cell studies? One possibility is that E2 binds and activates classical ERα at the membrane, and this communicates to membrane-localized GPR30 (Fig. 1). The ability of E2 and membrane ERα to signal to cell biology requires the assembly of a large protein signalsome at the plasma membrane (reviewed in Ref. 16). This complex consists of scaffold proteins (e.g. caveolin-1, striatin, modulator of nongenomic activity of estrogen receptor), linker proteins (Shc), nonreceptor tyrosine kinases (Src), threonine/serine kinases [p85 subunit of phosphatidylinositol 3 (PI3) kinase, Akt], and in some situations, growth factor receptor tyrosine kinases [epidermal growth factor receptor (EGFR), IGF-I receptor]. The number and variety of proteins involved convey specificity to the signaling from membrane ERα, in cell and context-dependent fashion.
Figure 1.
Possible collaboration between GPR30 and membrane ERα. GPR30 may transmit early signals from membrane ERα in cancer cells to downstream signal molecules (e.g. growth factor receptors). This occurs in cell and context-specific fashion for some signal cascades that modulate cell biology. MNAR, Modulator of nongenomic activity of estrogen receptor; PI3K, PI3 kinase; PKC, protein kinase C; PLC, phospholipase C.
There is some support that seven-transmembrane spanning GPRs can collaborate in the setting of E2/membrane ERα signaling. The sphingosine-1 phosphate receptor, Edg-3, is recruited to participate in a rapid signaling amplification schema, but only after E2/ER stimulates ERK MAPK from the membrane of breast cancer cells (17). GPR30 could occupy a similar role in some contexts. Support for such an interaction with GPR30 has been seen in various cancer cells, in which blocking ER or GPR30 action diminishes E2 signaling (18,19,20). For instance, the ability of E2 to stimulate Fos and cell cycle gene transcription and ERK activation in ovarian cancer cells or the inhibition of TGF-β action in MCF-7 cells has required both ERα and GPR30 (19,20). These authors note that GPR30 is not acting as an ER and that classical ERα is required for the actions of E2. In contrast, gene transcription that occurs in response to membrane ERα signaling in MCF-7 cells does not require GPR30 (9). Therefore, we might view GPR30 as one of numerous links in a chain that sometimes conveys initial signaling by membrane ERα to specific downstream effector kinases that alter cell biology (Fig. 1). The loss of any of the linked signalsome proteins then disrupts the signaling cascade.
Unfortunately, few details are known about how GPR30 potentially collaborates with membrane ERα. Initial reports suggested that GPR30 in some way facilitates the generation of ligands for transactivation of the EGFR, leading to downstream signaling (2). It is not clear that G protein activation, a very early event in membrane ERα signaling, requires GPR30. In contrast, membrane-localized ERα directly binds and activates various Gα and Gβγ subunits that are required for signal transduction (6,21,22).
Whether membrane-localized ERα may recruit GPR30 from the endoplasmic reticulum or signal to this pool of orphan receptors is even less well understood. In the original description of endoplasmic reticulum GPR30 mediating E2 action, the authors noted that E2 could also activate calcium signaling in this organelle through endogenous ERα present in the cells investigated (3). Selective loss of GPR30 from any discrete cellular pool has not been described, limiting conclusions in this regard. The use of E2-BSA to presumably bind endoplasmic reticulum GPR30 is inappropriate because intact E2-BSA does not pass the plasma membrane.
Recently, a transgenic mouse model was constructed expressing the E domain of ERα at the plasma membrane of many organs in the absence of nuclear ERα (membrane only estrogen receptor α or MOER) (23). Expression of the E domain alone at the plasma membrane rescued ERK and PI3 kinase activation by E2 that was absent in organs/cells from the Strasbourg ERαKO mouse, the background for the MOER mouse. Thus, in vivo rapid signaling by E2 requires membrane-localized ERα. Whether GPR30 facilitates signal transduction by the membrane-localized E domain of ERα is unknown at present.
A problem with many of the studies of E2 and GPR30 is that the experiments rely exclusively on cell-based assays, sometimes in cells that are manipulated to overexpress GPR30. Overexpression potentially results in protein mislocalization creating artifacts. Even when endogenous GPR30 is examined, cell-based assays may not recapitulate E2 functions in whole animals. A recent paper indicated that GPR30 is required in some way for E2 stimulation of primordial follicle formation (24). The conclusion was based upon counting the percentage of follicles relative to oocytes in ovaries cultured with E2, in the presence or absence of GPR30 small interfering RNA. However, as noted previously, none of the four GPR30 KO models shows cycling or fertility abnormalities in female mice. Thus, we suggest that cell-based studies indicating a collaborative role for GPR30 in estrogen biological actions should be accompanied by strong support from animal models. Clearly, at this point, we can say that GPR30 is not an ER, and may facilitate membrane-initiated steroid signaling under limited circumstances.
Footnotes
Disclosure Summary: The author has nothing to disclose.
For article see page 1722
Abbreviations: EGFR, Epidermal growth factor receptor; E2, 17-β-estradiol; ER, estrogen receptor; GPR, G protein-coupled receptor; KO, knockout; PI3, phosphatidylinositol 3.
References
- Kroeze WK, Sheffler DJ, Roth BL 2003 G-protein-coupled receptors at a glance. J Cell Sci 116(Pt 24):4867–4869 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2004 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 164:624–632 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH 2008 G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856 [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Svchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, Hrabe de Angelis MH, Irgang M, Otto C, Noppinger PR 2009 Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150:1722–1730 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH 2009 GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H 2008 GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22:636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM 2009 Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–692 [DOI] [PubMed] [Google Scholar]
- Levin ER 2008 Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol 295:R1425–R1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P 2006 Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol 173:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M 2007 G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Kleuser B, Malek D, Gust R, Pertz HH, Potteck H 2008 17-β-Estradiol inhibits transforming growth factor-β signaling and function in breast cancer cells via activation of extracellular signal-regulated kinase through the G protein-coupled receptor 30. Mol Pharmacol 74:1533–1543 [DOI] [PubMed] [Google Scholar]
- Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW 2001 Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Gα(i). J Biol Chem 276:27071–27076 [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW 2007 Direct interactions with G α I and G βγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol 21:1370–1380 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EYHP, Luderer U, Levin ER 3 December 2008 Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem 10.1074/jbc.M806249200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK 2008 G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology 149:4452–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]