Abstract
Of the central nervous system receptors that could mediate the energy balance effects of leptin, those of the hypothalamic arcuate nucleus receive the greatest attention. Melanocortin receptors (MC-Rs) contribute to the feeding and energetic effects of hypothalamically delivered leptin. Energy balance effects of leptin are also mediated by extrahypothalamic neurons including the hindbrain nucleus tractus solitarius. Hindbrain leptin receptors play a role in leptin's anorectic effects, but their contribution to its energetic effects and their functional interaction with melanocortin systems within the hindbrain remains unexplored. Here rats implanted with telemetric devices for recording energetic/cardiovascular responses were examined to determine whether: 1) hindbrain (fourth ventricular) leptin receptor stimulation triggers energetic and cardiovascular effects, 2) these effects are altered by a 6-wk high-fat diet maintenance, and 3) hindbrain MC-Rs mediate the thermogenic, cardiovascular, and anorexic effects of hindbrain leptin delivery. Results show that hindbrain leptin receptor stimulation produced long-lasting (>6 h) increases in core temperature and heart rate and also decreased food intake and body weight. These responses were not altered by high-fat maintenance, in contrast to what has been reported for forebrain leptin delivery. Fourth ventricular pretreatment with MC-R antagonist SHU 9119 completely abolished the hyperthermia, anorexia, and body weight loss seen with hindbrain-directed leptin but had no effects of its own. These data highlight a role for hindbrain leptin receptors in the initiation of energetic and anorexic responses and show that MCRs are part of the downstream mediation of hindbrain leptin-induced energy balance effects, paralleling effects observed for hypothalamic leptin receptors.
Hindbrain selective leptin stimulation increases temperature and heart rate; the effects are mediated by hindbrain melanocortin receptors and are also observed in leptin-resistant high-fat fed rats.
Disruption in central nervous system (CNS) leptin signaling results in severe obesity (1). Exogenous leptin delivery reverses the hyperphagia and reductions in activity, metabolism, and body temperature that contribute to the obesity observed in ob/ob mice (1,2,3). Central leptin receptors mediate these energy balance effects (4). Although leptin receptors (Ob-Rbs) are anatomically distributed across nuclei in the basal forebrain, midbrain, and hindbrain of relevance to energy balance control (5), the literature is dominated by a focus on the role of Ob-Rb-bearing arcuate hypothalamic neurons. Recent experiments establish contributions to the control of energy intake from leptin signaling in the ventromedial hypothalamus (6), ventral tegmental area (7), and nucleus tractus solitarius (NTS) (8). The neural mediation of leptin's energy expenditure effects receives considerably less attention than its energy intake effects (9), yet the focus here too is on hypothalamic leptin signaling (10). This paper addresses the contribution of caudal brainstem leptin receptors in mediating the energetic effects of leptin.
The energy balance effects of basal forebrain-directed, third ventricular leptin delivery are mediated, in part, by downstream effects on hypothalamic proopiomelanocortin (POMC) neurons and melanocortin receptors (MC-Rs) (11,12,13). This perspective is supported by experiments that show that leptin signaling in these neurons increases POMC gene expression (14,15) and pretreatment with MC-R antagonist attenuates the anorexic effects, as well as some of the sympathetically mediated energetic effects of third ventricular leptin delivery (11,12). Therefore, a second aim of this paper was to examine whether caudal brain stem MC-Rs and NTS POMC neurons contribute to the energy balance effects triggered by leptin signaling in the caudal brainstem.
High-fat (HF) diet feeding in rodents and humans results in obesity, hyperleptinemia, resistance to the effects of exogenous leptin and insulin delivery, and glucose intolerance (16,17). Leptin resistance is characterized by a decreased sensitivity to the anorexic and body weight effects of leptin (16). As noted above, there is a developing consensus that the effect of leptin on energy balance is mediated by several brain nuclei. By contrast, the neuroanatomical basis of leptin resistance is less clear. Leptin resistance may result from alterations in leptin signaling across several CNS sites or by contrast may be attributable to specific populations of Ob-Rb-expressing neurons in the CNS (18,19). A variety of experimental evidence is consistent in revealing that forebrain ventricular delivery of leptin results in diminished hypophagia in rats maintained on HF diet than chow-maintained controls (20,21,22). Whereas it is generally assumed that hypothalamic Ob-Rb-expressing neurons are the principal target of forebrain ventricular delivery, it is unclear whether other Ob-Rb-expressing nuclei accessed by this injection also contribute to the reduction in response magnitude observed in HF diet-maintained rodents. Unexamined are the effects of HF diet maintenance on the hypophagia to hindbrain ventricular delivery of leptin. It also remains to be determined whether some, but possibly not all, responses induced by central leptin delivery are attenuated by HF diet maintenance because sympathetic nervous system activation is observed in leptin-treated animals that are otherwise leptin resistant (23,24). For these reasons we pursue the functional consequences of HF diet maintenance on the energy intake as well as energy expenditure responses triggered by hindbrain-delivered, fourth ventricle leptin.
Collectively, the results described here establish a role for hindbrain Ob-Rb in mediating elevations in core temperature and heart rate. These energetic and cardiovascular responses as well as anorexic and body weight responses triggered by hindbrain leptin delivery were found to be unaltered in rats fed HF diet. In addition, our studies provide support for the hypothesis that hindbrain MC-Rs are downstream mediators of hindbrain leptin-induced energetic and anorexic responses.
Materials and Methods
Subjects
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–400 g at the time of surgery, were housed individually in plastic bins and maintained under a 12-h light, 12-h dark cycle (0800 h lights on). Pelleted food (Purina 5001; St. Louis, MO) and water were available ad libitum unless otherwise noted. All procedures conformed to the institutional standards of animal care and use (University of Pennsylvania).
Surgery
Rats were anesthetized with ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) delivered im.
Fourth intracerebroventricular (4th v) cannulation
All rats received a 4th v guide cannula (22-gauge; Plastics One, Roanoke, VA) with its tip stereotaxically positioned 2.0 mm above the 4th v (coordinates: on the midline, 2.5 mm anterior to the occipital suture, and 4.5 mm ventral to the dura, with injector aimed 6.5 mm ventral from dura). Cannulas were attached to the skull with dental acrylic and jeweler's screws and closed with an obturator.
Telemetric transponder surgery
At the time of ventricular surgery, telemetric transponders (HRC 4000 Mini-Mitter, VitalView; Philips Respironics, Bend, OR) for recording core temperature (TC), heart rate (HR), and spontaneous physical activity (SPA) were inserted into the abdominal cavity, and external leads were positioned sc and secured to the muscles on either side of the heart with sutures [as previously described (25)].
Experimental procedure
Cannula position verification
At least 7 d after surgery, 4th v cannula placement was assessed by measurement of the sympathoadrenal-mediated hyperglycemic response to the cytoglucopenia induced by 5-thio-d-glucose [210 μg in 2 μl of artificial cerebral spinal fluid (aCSF)] (26). A postinjection elevation of at least 100% of baseline glycemia was required for subject inclusion.
Habituation training
Before the start of experimental testing, rats were acclimated to handling, 4th v injections, and gastric gavages for oral glucose tolerance tests (OGTT) testing.
Food intake and body weight monitoring
Food was removed 1 h before treatment (1 h into the light cycle) and returned 8 h later, late in the light phase. Thereby food was not available during the period of energetic response measurement as feeding can affect these responses. Food intake and body weight measurements were made 24 h after the injection of drug. Food was available during the dark cycle and a minimum of 48 h was allotted between experimental testing.
Experiment 1: effects of 4th v leptin delivery on energy expenditure and food intake in chow-fed and HF-fed rats
Feeding maintenance
Rats were randomly assigned to standard chow (n = 8) or HF diet (no. D12266B; Research Diets, New Brunswick, NJ; 4.47 kcal/g with 21% of the metabolizable energy content as protein, 31% as fat, and 48% as carbohydrate, n = 16) maintenance 1 wk after surgery. All testing began after 5 wk of maintenance on the respective diets. Food intake and body weight of all rats were monitored daily. The physiological alterations induced by HF diet maintenance are well established and include hyperleptinemia-leptin resistance and hyperinsulinemia-insulin resistance (see for review Refs. 16 and 17). The OGTT was administered before HF exposure and 1, 4, and 5 wk after diet maintenance to provide one correlate of the physiological effects of HF diet maintenance. Food was withheld for a 6-h period (with access to water) during the first half of the light cycle; an oral glucose load (2 g/kg) was delivered to each rat by gavage. Blood glucose was measured before gavage and at 30, 60, 90, 120, and 180 min after glucose loading by collecting a drop of tail blood and placing it in a standard glucometer strip (Accucheck; Roche Diagnostics, Indianapolis, IN).
Experimental testing
All rats received 4th v injections early in the light cycle. Due to the caudal flow of the cerebral spinal fluid, low volume injections through the 4th v cannula under specific experimental conditions provide selective stimulation of hindbrain CNS regions without affecting hypothalamic and other forebrain regions as shown elsewhere (27,28,29,30). Three conditions were run in a counterbalanced fashion across separate days. Energetic responses were examined in chow- and HF-fed rats after a control condition with 4th v vehicle (1 μl sodium bicarbonate) and two doses of leptin: 3 and 10 μg [dose selection based on other reports (20,21,31)]. HR, TC, and SPA were continuously monitored for 8 h at 5-min intervals (TC and SPA) or 30-sec intervals (HR) in rats with implanted HRC-4000 transponders (Philips Respironics).
Experiment 2: effect of hindbrain-delivered MC-R antagonist on the energy balance effects of leptin delivered to hindbrain
All rats (n = 9) received 4th v injections early in the light cycle. All conditions were separated by 2 d and run in a counterbalanced fashion. Responses were examined after four conditions as follows: 1) control condition with 4th v vehicle [1 μl of aCSF (vehicle for MC3/4-R antagonist, SHU 9119) with 1 μl of sodium bicarbonate (vehicle for leptin)], 2) aCSF +3 μg leptin, 3) 0.1 nmol SHU 9119 + sodium bicarbonate, and 4) SHU 9119 with leptin. SHU 9119/vehicle was injected 20 min before leptin/vehicle treatment. SHU 9119 dose was selected from results of a pilot study that tested the efficacy of SHU doses in blocking the energy balance effects of 4th v MC-R agonist (MTII; Phoenix Pharmaceutical, St. Joseph, MO) without producing any independent effects alone. HR, TC, and SPA were continuously monitored as in experiment 1.
Statistical analysis
All energy expenditure parameters were analyzed by one- or two-way ANOVAs on 6 h postinjection averages and followed by post hoc t tests and Tukey's honestly significant difference test as appropriate. Twenty-four-hour food intake and body weight were analyzed by ANOVA followed by post hoc t tests and Tukey's honestly significant difference test as appropriate. All statistical analysis was conducted using Statistica software (Tulsa. Oklahoma). Differences were considered significant at P < 0.05.
Results
Experiment 1: stimulation of hindbrain Ob-Rb increases energy expenditure and decreases food intake in chow- and HF-fed rats
Chow-fed group: core temperature
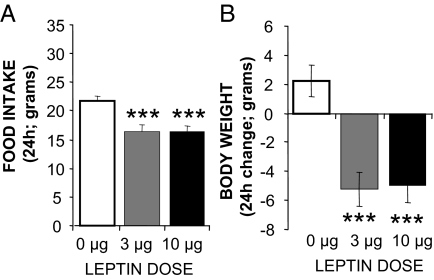
Figure 1A shows that hindbrain-delivered leptin injection increased TC for the 6 h postinjection period. A one-way ANOVA examining treatment effects on average postinjection TC values revealed a significant drug treatment effect [F (2,10) = 13.89, P < 0.005]. Post hoc analysis showed a significant effect of both leptin doses on TC (3 μg; P < 0.005, 10 μg: P < 0.005). Heart rate (Fig. 1B) shows that leptin increased HR, compared with control treatment. A one-way ANOVA of average HR values for the 6-h postinjection period yielded a significant drug effect [F (2,10) = 6.40, P < 0.05); post hoc analysis showed a significant effect of the lower dose of leptin on HR (3 μg; P < 0.05, 10 μg: P < 0.323). Spontaneous physical activity was not significantly increased by leptin treatment [F (2,10) = 1.79, P = 0.216; Fig. 1C]. For food intake and body weight, 4th v injection of leptin significantly decreased 24-h food intake [F (2,14) = 6.883, P < 0.01; post hoc: 3 μg; P < 0.28, 10 μg: P < 0.05] and body weight [F (2,14) = 4.155, P < 0.05; post hoc: 3 μg; P < 0.82, 10 μg: P < 0.05] (Fig. 2, A and B).
Figure 1.
Effect of caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on core temperature (A), HR (B), and spontaneous activity (C) in chow-fed (CHOW) rats. Line graphs represent across-rat average parameter measurements through the 8 h-recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms provide 6-h postinjection averages ± sem for each parameter at each dose. *, P < 0.05; **, P < 0.005 (n = 6). ICV, Intracerebroventricular.
Figure 2.
Effect of caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on food intake (A) and body weight (B) in chow-fed rats. The histograms provide average values ± sem. *, P < 0.05 (n = 8).
HF-fed group
OGTTs were performed before HF-diet exposure [area under the curve ± se: 315.4 ± 15.8 chow fed (CH) vs. 320.3 ± 7.4 HF] and after 1 wk (CH: 312.6 ± 16.2 vs. HF: 312.9 ± 5.5), 4 wk (CH: 313.1 ± 8.9 vs. HF: 348.6 ± 6.5 ± 8.9) and 5 wk (CH: 316.7 ± 8.0 vs. HF: 361.7 ± 6.4) on HF diet maintenance in comparison with CH control rats. HF-fed animals showed impairment in glucose clearance relative to CH control rats after 4 wk (P < 0.005) and 5 wk (P < 0.0005) of high-fat exposure. All rats on HF diet showed reduced glucose clearance after 5 wk on the HF diet; therefore, all were included in the energy expenditure analysis. For core temperature, Fig. 3A shows that 4th v injection of each dose of leptin increased TC for the 6 h postinjection period. A one-way ANOVA examining treatment effects on average postinjection TC values yielded a significant drug treatment effect [F (2,28) = 7.00, P < 0.005]. Post hoc analysis revealed a significant effect of both leptin doses on TC (3 μg; P < 0.01, 10 μg: P < 0.01). For HR, Fig. 3B shows that leptin increased HR, compared with the aCSF vehicle treatment. A one-way ANOVA of average HR values for the 6-h postinjection period yielded a significant drug effect [F (2,28) = 5.47, P < 0.01]; post hoc analysis showed a significant effect of both doses of leptin on HR (3 μg; P < 0.05, 10 μg: P < 0.05). Spontaneous physical activity was not significantly increased [F (2,28) = 0.029, P = 0.972] by leptin treatment (Fig. 3C). For food intake and body weight, both leptin doses significantly decreased 24-h food intake [F (2,30) = 18.79, P < 0.0001; post hoc: 3 μg; P < 0.0005, 10 μg: P < 0.0005] and body weight [F (2,30) = 13.75, P < 0.0001; post hoc: 3 μg; P < 0.0005, 10 μg: P < 0.0005] (Fig. 4, A and B). Between-group comparisons (CH vs. HF fed) were made for all parameters. No significant differences in TC, HR, SPA, and food intake of CH- and HF-fed groups were found for any doses. Body weight responses were significantly different at the 0 and 10 μg doses, but when responses were expressed as change from vehicle, no significant differences were found.
Figure 3.
Effect of caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on (A) core temperature, (B) HR, and (C) spontaneous activity in HF-fed rats. Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms provide 6-h postinjection averages ± sem for each parameter at each dose. *, P < 0.05 (n = 15). ICV, Intracerebroventricular.
Figure 4.
Effect of caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on food intake (A) and body weight (B) in HF-fed rats. The histograms provide average values ±sem. ***, P < 0.0005 (n = 16).
Experiment 2: hindbrain-delivered MC-R antagonist blocks the energy balance effects of hindbrain leptin delivery
Hindbrain pretreatment with a MC-R antagonist reversed the effects of 4th v leptin treatment on energetic/cardiovascular responses, food intake, and body weight but was without effects on its own (Figs. 5 and 6). For core temperature, Fig. 5A shows that the leptin-induced increase in TC for the 6-h postinjection period was reversed by pretreatment with the MC-R antagonist SHU 9119. A two-way ANOVA examining the interaction of leptin and SHU 9119 on average postinjection TC values revealed a significant interaction [F (1,8) = 9.25, P < 0.05]. Post hoc analysis revealed a significant effect of only leptin alone on TC (3 μg; P < 0.05) compared with vehicle. The leptin + SHU 9119 condition was not significantly different from vehicle (P = 0.88). For HR, Fig. 5B shows that there was a trend for leptin to increase HR, although the effect was not significant compared with the control treatment. A one-way ANOVA of average HR values for the 6-h postinjection period yielded a significant drug effect [F (3,24) = 3.714, P < 0.05]; post hoc analysis showed a trend for leptin to increase HR (3 μg; P < 0.078), but SHU 9119 pretreatment attenuated the effect of leptin on HR (P = 0.999). A two-way ANOVA of average HR values for the 6-h postinjection period examining the interaction of leptin and SHU 9119 did not reach significance [F (1,8) = 2.61, P = 0.14]; spontaneous physical activity was not significantly affected by leptin, SHU 9119, or their combination. There was no significant interaction [F (1,8) = 2.06, P = 0.19] (Fig. 5C). For food intake and body weight, hindbrain-directed leptin delivery significantly decreased 24-h food intake. Two-way ANOVA revealed significant interaction of leptin and MC-R antagonist [F (1,8) = 7.83, P < 0.05]; post hoc Tukey tests revealed that leptin decreased food intake (P < 0.005), and pretreatment with SHU 9119 blocked the effects of leptin [leptin + SHU 9119: P = 0.97] (Fig. 6A). Body weight was also significantly decreased by leptin treatment alone, but pretreatment with SHU 9119 reversed this effect [F (1,8) = 11.55, P < 0.05; post hoc Tukey test for leptin alone: P < 0.05, and for leptin SHU 9119: P = 0.79] (Fig. 6B). SHU9119 application itself had no effect on any of the parameters measured.
Figure 5.
Effect of caudal brainstem MC-R blockade [1 μl, SHU9119 (SHU), 4th v delivery] on caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on core temperature (A), HR (B), and (C) spontaneous activity in chow-fed rats. Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. The histograms provide 6-h postinjection averages ± sem for each parameter at each dose. *, P < 0.05 (n = 9). ICV, Intracerebroventricular.
Figure 6.
Effect of caudal brainstem MC-R blockade [1 μl, SHU 9119 (SHU), 4th v delivery] on caudal brainstem leptin receptor stimulation (1 μl, 4th v delivery) on food intake (A) and body weight (B) in chow-fed rats. The histograms provide average values ± sem. *, P < 0.05; **, P < 0.005 (n = 9).
Discussion
The data gathered indicate that hindbrain leptin receptors contribute to the energy balance effects of CNS leptin delivery. These are the first data to show that stimulation of hindbrain leptin receptors drives thermogenic and tachycardic responses. The hypophagia and body weight reduction also observed with hindbrain leptin delivery confirm our earlier work in lean chow-fed rats (32). The present study also shows that hindbrain leptin delivery in HF diet maintained rats with reduced glucose clearance triggers anorexic and energetic responses of the same magnitude as those observed in chow-fed controls. These data indicate that alterations in hindbrain Ob-Rb signaling do not contribute to the physiology of leptin resistance.
A role for hypothalamic melanocortin gene expression and MC-Rs in the downstream effects of hypothalamic leptin signaling is well established (11,12). Our experiments address a parallel but neglected question: whether hindbrain melanocortin signaling is downstream of the functional effects of hindbrain leptin signaling. We show that hindbrain delivery of the MC3/4-R antagonist SHU9119 blocked the food intake, body weight, and energetic responses triggered by 4th v leptin. This pattern of results is parallel with data generated for forebrain ventricular delivery using a similar strategy (11,12,13). Systemic leptin treatment stimulates Ob-Rb on arcuate POMC neurons, induces phosphorylated signal transducer and activator of transcription-3 in arcuate POMC neurons, and increases POMC gene expression. These effects are mediated by direct action of leptin on these POMC neurons. Some controversy surrounds whether NTS POMC neurons express leptin receptors in mouse models (33,34). Whether the effects observed here are mediated by leptin receptor expression in rat NTS POMC neurons or leptin receptors on other neurons that in turn affect NTS POMC gene expression and melanocortin ligand release remains to be investigated.
Obesity induced by multiweek HF-diet maintenance did not reduce the magnitude of either the anorexic or energetic-sympathetic responses elicited by hindbrain leptin administration. These functional results are consistent with the findings of Munzberg et al. (19), who showed that multiweek, HF diet maintenance did not reduce Ob-Rb signaling (phosphorylated signal transducer and activator of transcription-3 immunohistochemistry) in NTS neurons or in the neurons of the ventromedial, dorsomedial, or premammillary hypothalamus (see also Ref. 35). Of the brain regions sampled in their study, an attenuation of leptin signaling with diet-induced obesity was observed only in the hypothalamic arcuate nucleus; this reduced arcuate-leptin signaling was detected as early as 6 d after the start of HF diet feeding (19). Whereas we have not examined forebrain-directed leptin responses, other investigators established that lateral ventricular leptin delivery (doses overlapping with those used here) in rats made obese with HF feeding (20,21) or in obese aged rats (36) result in markedly attenuated food intake and body weight effects of leptin. These examples of attenuated functional effects of leptin delivered to the forebrain ventricles of rats with some degree of leptin resistance contrast with what we observed in similarly maintained rats receiving 4th v leptin. These contrasting data sets suggest that specific CNS Ob-Rb-expressing regions respond differently to the obesity resulting from HF diet maintenance and also that lack of alterations in NTS leptin signaling (19) correlate with preserved effects on feeding and energy expenditure. Hindbrain Ob-Rbs, due to their lack of molecular and functional leptin resistance, might therefore account for the preserved sympathetic nervous system activation observed after peripheral leptin injections in animals otherwise leptin resistant (23,24). Ob-Rbs in the hindbrain might also contribute to partial anorexic responses observed after lateral ventricle leptin application because the drug can diffuse with the caudal CSF flow and stimulate Ob-Rb-expressing hindbrain neurons.
Our studies focused attention on the functional effects of hindbrain leptin receptors and, in addition, identified a downstream mediator of these effects, POMC neurons, and hindbrain MC-Rs. Furthermore, our data along with findings by Munzberg et al. (19) suggest that this hindbrain projection pathway may, unlike its hypothalamic counterpart, be less subject to the effect of the reduction in leptin signaling observed with diet-induced obesity. The details of the mediating neural circuitry, however, still remain to be delineated. Neurons of the NTS and parabrachial nucleus express Ob-Rbs (5) and contribute to the control of feeding behavior and sympathetic outflows (37,38). It is likely that the responses observed here reflect NTS stimulation. Studies using targeted leptin delivery to NTS as well as parabrachial nucleus are necessary to resolve this issue. Results from our current studies do not exclude the possibility that projections to forebrain neurons are required for response production. Little is known about the neurochemical phenotype and anatomic projections of Ob-Rb-expressing hindbrain neurons. NTS neurons are known to project to hypothalamic neurons including those in arcuate and paraventricular hypothalamus. NTS neurons may project to POMC-expressing arcuate neurons that, in turn, project back to the NTS to provide for the release of melanocortin ligand in the NTS from a hypothalamic origin (39,40). The requirement for forebrain-hindbrain neural communication for leptin-induced increases in energy expenditure might be suggested by a study of Harris et al. (41). They showed that peripheral leptin stimulation in chronic decerebrate rats, which lack neural forebrain-hindbrain communication, does not increase energy expenditure; in fact, a decrease in oxygen consumption in these rats is noted under certain experimental conditions. On the other hand, in its simplest form, the mediating circuitry may be placed entirely within hindbrain with circulating leptin stimulating hindbrain Ob-Rbs, which could in turn activate hindbrain POMC and MC-Rs, yielding behavioral and autonomic responses. We have already shown (25) that the circuitry required for MC-R stimulation-induced hyperthermia and tachycardia is contained entirely within the hindbrain. Future experiments are needed to determine whether the neural link from hindbrain leptin to hindbrain MC-Rs is contained entirely within the hindbrain or whether the connection is mediated by circuitry involving forebrain neurons.
Acknowledgments
We thank Christian Bjorbaek, Ph.D., (Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, MA) and Matt Hayes, Ph.D., (University of Pennsylvania), for editorial comments and Jolanta Jozefara for her technical assistance.
Footnotes
This work was supported by the National Institutes of Health Research Grant DK-21397 (to H.J.G.) and National Research Service Award NS-059254 (to K.P.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2008
Abbreviations: aCSF, Artificial cerebral spinal fluid; CH, chow fed; CNS, central nervous system; HF, high-fat; HR, heart rate; MC-R, melanocortin receptor; NTS, nucleus tractus solitarius; Ob-Rb, leptin receptor; OGTT, oral glucose tolerance test; POMC, proopiomelanocortin; SPA, spontaneous physical activity; TC, core temperature; 4th v, fourth intracerebroventricular.
References
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Friedman JM 1997 Leptin and its receptor. J Endocrinol 155:215–216 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB 1997 Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138:839–842 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB 1998 Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ 2006 Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- Huo L, Maeng L, Bjorbaek C, Grill HJ 2007 Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148:2189–2197 [DOI] [PubMed] [Google Scholar]
- Hermann GE, Barnes MJ, Rogers RC 2006 Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res 1117:118–124 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA 2007 Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49:647–652 [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL 1999 Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33:542–547 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW 1997 Melanocortin receptors in leptin effects. Nature 390:349 [DOI] [PubMed] [Google Scholar]
- Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K 1998 Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett 249:107–110 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG 1997 Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- Elmquist JK 2001 Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int J Obes Relat Metab Disord 25(Suppl 5):S78–S82 [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H 2008 Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556 [DOI] [PubMed] [Google Scholar]
- Gleason CE, Gross DN, Birnbaum MJ 2007 When the usual insulin is just not enough. Proc Natl Acad Sci USA 104:8681–8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA 2007 Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C 2004 Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Upton R, Buckingham R, Arch J, Williams G 1997 Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 46:1782–1785 [DOI] [PubMed] [Google Scholar]
- Tulipano G, Vergoni AV, Soldi D, Muller EE, Cocchi D 2004 Characterization of the resistance to the anorectic and endocrine effects of leptin in obesity-prone and obesity-resistant rats fed a high-fat diet. J Endocrinol 183:289–298 [DOI] [PubMed] [Google Scholar]
- Fam BC, Morris MJ, Hansen MJ, Kebede M, Andrikopoulos S, Proietto J, Thorburn AW 2007 Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes Metab 9:840–852 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Mark AL 2002 Cardiovascular and sympathetic effects of leptin. Curr Hypertens Rep 4:119–125 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC 2008 Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ 2008 Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149:3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ 1985 Fourth ventricular phlorizin dissociates feeding from hyperglycemia in rats. Brain Res 341:331–336 [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ 2002 The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD 2004 Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7:335–336 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG 2004 Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brainstem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA 2002 Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283:R941–R948 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Halatchev IG, Cone RD 2006 Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147:3190–3195 [DOI] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C 2006 Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55:567–573 [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR 2004 Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145:3704–3711 [DOI] [PubMed] [Google Scholar]
- Shek EW, Scarpace PJ 2000 Resistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged rats. Regul Pept 92:65–71 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2001 Interoceptive and integrative contributions of forebrain and brainstem to energy balance control. Int J Obes Relat Metab Disord 25(Suppl 5):S73–S77 [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF 2003 Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460:303–326 [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL 1987 Pro-opiomelanocortin-derived peptides (ACTH/β-endorphin/α-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436:323–338 [DOI] [PubMed] [Google Scholar]
- Berthoud HR BK, Wan S, Patterson LM, Babic T, Townsend RL, Sutton G, Skibicka KP, Butler A, Grill HJ, Travagli RA, Zheng H, Brainstem melanocortin signaling in the control of food intake and energy balance. Proc Keystone Symposia, Banff, Canada, 2008 [Google Scholar]
- Harris RB, Bartness TJ, Grill HJ 2007 Leptin responsiveness in chronically decerebrate rats. Endocrinology 148:4623–4633 [DOI] [PubMed] [Google Scholar]