Abstract
Estrogens have been shown to have positive and negative effects on anxiety and depressive-like behaviors, perhaps explained by the existence of two distinct estrogen receptor (ER) systems, ERα and ERβ. The ERβ agonist, diarylpropionitrile (DPN) has been shown to have anxiolytic properties in rats. DPN exists as a racemic mixture of two enantiomers, R-DPN and S-DPN. In this study, we compared R-DPN and S-DPN for their in vitro binding affinity, ability to activate transcription in vitro at an estrogen response element, and in vivo endocrine and behavioral responses. In vitro binding studies using recombinant rat ERβ revealed that S-DPN has a severalfold greater relative binding affinity for ERβ than does R-DPN. Furthermore, cotransfection of N-38 immortalized hypothalamic cells with an estrogen response element-luc reporter and ERβ revealed that S-DPN is a potent activator of transcription in vitro, whereas R-DPN is not. Subsequently, we examined anxiety-like behaviors using the open-field test and elevated plus maze or depressive-like behaviors, using the forced swim test. Ovariectomized young adult female Sprague Dawley rats treated with racemic DPN, S-DPN, and the ERβ agonist, WAY-200070, showed significantly decreased anxiety-like behaviors in both the open-field and elevated plus maze and significantly less depressive-like behaviors in the forced swim test compared with vehicle-, R-DPN-, or propylpyrazoletriol (ERα agonist)-treated animals. In concordance with the relative binding affinity and transcriptional potency, these results demonstrate that the S-enantiomer is the biologically active form of DPN. These studies also indicate that estrogen's positive effects on mood, including its anxiolytic and antidepressive actions, are due to its actions at ERβ.
Diarylpropionitrile regulates stress response through S-enantiomer.
Estrogens have diverse and powerful biological actions due in part to the existence of two estrogen receptor (ER) subtypes, ERα and ERβ (1,2). Adding to this diversity is the existence of multiple splice variants (for review see Refs. 3 and 4) and differential expression of each receptor subtype in various tissues (5,6). ERα predominates, whereas ERβ plays a minor role, in classical estrogen-sensitive tissues such as uterus, mammary glands, pituitary, and bone (7). However, a role for ERβ has been found in the ovary, prostate, cardiovascular system, and central nervous system (7,8).
The development of ERα and ERβ knockout animals (αERKO and βERKO, respectively) as well as the discovery of subtype selective ligands has provided initial insight into the biological function of each receptor. Importantly, subtype selective high affinity agonists and antagonists allow for the pharmacological examination of receptor-mediated functions in a normal wild-type animal (for review see Refs. 9 and 10). For example, propylpyrazoletriol (PPT) has a relative binding affinity (RBA; compared with estradiol = 100) for ERα of 49 and binding is 410-fold more selective for ERα than ERβ. In contrast, diarylpropionitrile (DPN) has a RBA for ERβ of 18 and binding is 72-fold more selective for ERβ than ERα (11,12). In addition, WAY-200070 (Wyeth, Princeton, NJ), an aryl diphenolic azole, exhibits high affinity and selectivity for ERβ (IC50 = 2.3 nm; RBA = 68) (9,13). Transcriptional selectivity of these compounds are greater than that of binding, with DPN having a 170-fold greater relative potency in transcription assays for ERβ (11). Other ligands including (1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole ERα antagonist), (PHTPP; 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol ERβ antagonist), and plant-derived phytoestrogens (genistein, coumesterol, and equol) all exhibit some selectivity for ERβ (14,15). Interestingly, the dihydrotestosterone metabolite, 5α-androstane, 3β, 17β-diol, is also a somewhat selective ERβ agonist and can activate estrogen response element (ERE)-mediated transcription in the presence of ERβ at a much greater selectivity than binding would predict (16).
Studies using knockout animals as well as subtype selective agonists and antagonists have been critical in determining the biological actions specific to ERα or ERβ. These studies have implicated ERβ signaling in alleviating several pathologies including inflammation, inflammatory bowel syndrome, endometriosis, and heart disease (for reviews see Refs. 9, 17 and 18). Important to the studies described here, these studies also indicated a role for ERβ signaling in anxiety- and depressive-like behaviors. For instance, estradiol's antidepressant actions in the forced swim test (FST) are absent in βERKO animals (19), and these animals exhibit increased anxiety-type behaviors in the elevated plus maze (EPM) relative to wild-type animals (20,21). Studies using receptor subtype selective agonists are in concordance with the experiments using knockout animals. Treatment of gonadectomized males and female rats with the ERβ agonist DPN results in less depressive-like behavior in the FST and horizontal crossing task and decreased anxiety-type behaviors in the EPM (22,23,24).
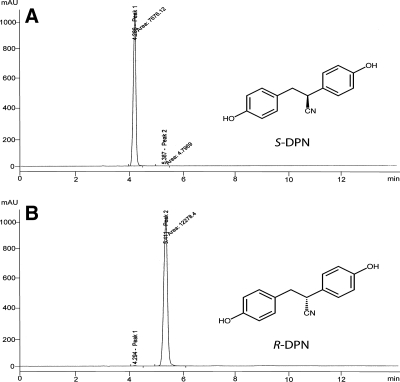
Contrary to other ERβ selective ligands such as WAY-200070, DPN exists as a racemic mixture of two enantiomers, S-DPN and R-DPN (Fig. 1) (11). Furthermore, examination of the ligand-receptor interaction by molecular modeling predicts the S enantiomer to be responsible for DPN′s selectivity for ERβ (25). The stereochemistry of S-DPN allows the nitrile group of DPN to positively interact with Met-336 in the ligand-binding pocket of ERβ, much like the azole in WAY-200070. The nitrile group in R-DPN points away from this residue and thus may not form as stable of a complex with ERβ. Additionally, the modeling indicates that R-DPN forms a lower energy (more favorable) complex with ERα than ERβ. Thus, the S-enantiomer likely has higher affinity and selectivity for ERβ than the R-enantiomer. To date, asymmetric synthesis and/or chromatographic separation of the two enantiomers have not been reported. Studies using DPN use a racemic mixture of two stereoisomers, which likely have differing pharmacological effects. To this end, in the studies described here, we separated S-DPN and R-DPN by chiral HPLC (Fig. 1) and compared their action in ER binding, transcriptional activation of an estrogen-responsive reporter gene, and anxiety-type and learned helplessness behaviors. Our studies indicate that S-DPN is indeed the biologically active enantiomer of DPN. Furthermore, R-DPN is nonselective and has little activity at ERβ. Therefore, future studies using DPN should carefully consider using an enantiomeric-pure preparation.
Figure 1.
Separation of DPN enantiomers by reverse-phase chiral HPLC. A, Chromatogram of S-DPN. B, Chromatogram of R-DPN.
Materials and Methods
Saturation isotherms
In vitro ligand binding assays were performed as previously described (26,27), with a minimum of four assays per ER and saturation isotherms constructed as follows. Full-length rat ERβ1 and human ERα were synthesized in vitro using the TnT-coupled reticulocyte lystate system (Promega, Madison, WI; according to manufacturer's protocol) with T7-RNA polymerase from their respective plasmid expression vectors (pcDNA3.0-ERβ; T. T. Brown, Pfizer, Groton, CT; PSG5-ERα; R. Price, University of California, San Francisco, San Francisco, CA). Aliquots (100 μl) of the translation reaction mixture were incubated 90 min at room temperature (ERβ1) or 18 h at 4 C (ERα) with increasing (0.01–1000 nm) concentrations of 17β-estradiol (E2), DPN, S-DPN, or R-DPN and 1.0 nm [3H]E2 in duplicate. These incubation conditions were previously shown to be optimal for these receptor types (24). Nonspecific binding was determined using 200-fold excess of the ER agonist, diethylstilbestrol. After incubation, bound and unbound [3H]E2 were separated by passing the incubation reaction through a 1-ml lipophilic Sephadex LH-20 (Sigma, St. Louis, MO) column. IC50 was calculated from a nonlinear regression of the log-transformed data using GraphPad Prism (version 3.0; San Diego, CA), and inhibitory constant (Ki) was determined by using the Cheng and Prusoff equation [Ki = (inhibitory concentration 50) ÷ (1 + (concentration of radioligand ÷ dissociation constant of radioligand))].
Transcriptional activation assay
The mouse-derived hypothalamic cell line N-38 (28) (CELLutions Biosystems, Inc., Toronto, CA) was used for all transcriptional assays. Cells were maintained in 1× DMEM (Cellgro, Manassas, VA) containing 1× nonessential amino acids, l-glutamine, 4.5% glucose, and 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA) at 37 C with 5% CO2. Cells were plated at 100,000 cells/well in 24-well plates, grown to 60–70% confluency before transfection, and used before passage 25. The ERE-thymidine kinase (tk)-luciferase reporter construct contained two ERE sequences coupled to a minimal tk promoter subcloned into pGL2-Basic plasmid (Promega). The β-galactosidase-expressing Simian virus 40-LacZ reporter (PJ3, generously provided by Dr. Rosalie Uht, University of Virginia, Charlottesville, VA) was used as an internal control for plasmid transfer efficiency. Plasmid expression vectors for ERα and ERβ were the same as used in the saturation isotherms. Empty vector controls were used with each plasmid. Constructs were transfected in triplicate, and each assay was repeated four to six times. Transfections were carried out using a lipid-mediated reagent (Fugene 6; Roche Diagnostics, Indianapolis, IN) according to manufacturer's protocol using a total DNA concentration of 0.33 μg/well and 1 μl/well Fugene 6. Optimal total DNA amounts and Fugene concentrations were determined empirically. Cells were incubated with the transfection complex for 16 h, media were replaced with fresh media containing 10% charcoal-stripped fetal bovine serum (to ensure estrogen-free culture conditions) for 8 h, and then media were replaced with treatment media for 16 h. Hormone treatments were made by diluting E2 (Sigma), DPN, S-DPN, and R-DPN in ethanol and used at a final concentration of 1, 10, or 100 nm (ethanol ≤ 0.001%). Vehicle contained the equivalent amount of ethanol. After 16 h of treatment, cells were washed with 1× PBS and lysed. To measure luciferase activity, 20 μl lysate were added to 100 μl luciferin substrate (Promega), and to measure β-galactosidase activity, 40 μl lysate were added to 200 μl galactin substrate (Tropix-GalactoLight; Applied Biosystems, Foster City, CA). Relative light units (RLUs) were measured using as 20/20 TD luminometer (Turner Designs, Sunnyvale, CA). RLUs for each treatment were normalized to the respective empty expression vector control, and data are expressed as percent change compared with vehicle-treated, empty expression vector controls.
Animals
Sixty-day-old female Sprague Dawley rats were obtained from Harlan Laboratories (San Diego, CA), housed individually, and maintained on a 12-h light, 12-h dark schedule (lights on at 0600 h) in temperature- and humidity-controlled rooms at the Laboratory Animal Research Facility at Colorado State University. Animals had ad libitum access to a soy-free diet (modified AIN-93G, DYET no. 101591; DYETS, Inc., Allentown, PA; with corn oil substituted for soy oil). One week after arrival, animals were ovariectomized under isofluorane anesthesia. After ovariectomy, animals were handled daily (5 min each animal) by the same experimenter. All animal studies were previously approved by the Animal Care and Use Committee at Colorado State University.
Hormone/drug treatments
Beginning 1 wk after ovariectomy, animals were given a single daily sc injection of hydroxypropyl betacyclodextran [vehicle; 27% (wt/vol) in saline; CTD Inc., High Springs, FL], DPN (2.0 mg/kg), S-DPN (2.0 mg/kg), R-DPN (2.0 mg/kg), WAY-200070-3 (2.0 mg/kg), or PPT (1.0 mg/kg) in a total volume of 0.2 ml. Injections occurred at 0800 h for 7 d. DPN was synthesized de novo as previously described [Lund et al. (24)], WAY-200070-3 was provided by Wyeth Discovery Neuroscience, and PPT and PHTPP were purchased from Tocris Inc. (Ellisville, MO). R-DPN and S-DPN were obtained in greater than 99% enantiomeric excess by separation of racemic DPN via reverse-phase chiral HPLC (Chiralpack IC column; hexanes-isopropanol, 70:30 mobile phase; UV detection, 250 nm). Purity of the resulting compounds was confirmed by nuclear magnetic resonance. Three hours after the daily treatment injection on d 4–7, animals underwent behavioral testing consisting of two paradigms established as indicators of anxiety [EPM, open field (OF)], and one paradigm established as an indicator of helplessness (FST), as described below.
OF
The OF test was performed on the fourth day of treatment and was conducted as previously described (29). Testing commenced at 1100 h, and light intensity was 80 lux in center of arena. Behavioral measures included activity (total square crossings), latency to exit middle squares, interactions with novel objects, rearing, head dips, and total time spent grooming.
EPM
Maze performance was evaluated on the fifth day of treatment and was run as previously described (30). Testing commenced at 1100 h, and light intensity was 80 lux in the open arms of the maze. Behaviors measured included number of entries into open and closed arms, total time spent in open and closed arms, rearing, head dips, total time spent grooming, and the number of fecal boli.
FST
The FST was evaluated on the seventh day of treatment and was a modified version of the Porsolt swim test (31). Total time spent swimming, struggling, and immobile in a glass cylindrical container (40 cm × 27 cm) filled with 30 cm of 25 C fresh water was recorded for 5 min. Animals were acclimated to the test 1 d prior (sixth day of treatment) via a 10-min swim test. Swimming was defined as movement of the forelimbs and hind limbs that does not break the surface of the water, struggling was defined as movement of the forelimbs rapidly to break the surface of the water and/or attempting to climb against the wall of the container, and immobility was defined as absence of any movement except for slight movements necessary for the animal to keep its head above water.
Plasma corticosterone (CORT) analysis
Twenty minutes after the FST, animals were rapidly decapitated and trunk blood collected into ice-chilled tubes containing 0.5 m EDTA and aprotinin (4 mg/ml; Sigma). Plasma levels of CORT were determined by RIA as previously described (24). Intra- and interassay variance were 4.3 and 8.9%, respectively.
In situ hybridization (ISH)
Twenty minutes after the FST, brains were harvested and frozen in cold 2-methylbutane (−40 C) and stored at −80 C until processed for ISH. ISH for c-fos mRNA was performed as previously described (32) using a 48-bp oligonucleotide probe (5′-GCAGCGGGAGGATGACGCCTCGTAGTCCGCGTTGAAACCCGAGAACAT-3′) targeted to the rat c-fos gene.
Statistics
ANOVA was performed on data from each experiment using the Statview data analysis software (Abacus Concepts, Inc., Berkeley, CA). Fisher's protected least significant difference analysis was used post hoc where appropriate. Differences were considered significant when P < 0.05. Data are expressed as group means ± sem. Transfection data are expressed as percent change compared with vehicle-treated, empty vector controls.
Results
RBAs of S-DPN and R-DPN for ERs
RBAs for the enantiomers of diarylpropionitrile for in vitro transcribed ERα and ERβ were determined via competitive binding assays. Displacement of [3H]E2 from each receptor was quantitated to determine the affinity of the two enantiomers for each receptor subtype (Table 1). At optimal binding conditions with respect to each receptor, the S enantiomer had greater affinity for ERβ (Ki = 0.27 ± 0.05) than did the R-enantiomer (Ki = 1.82 ± 0.21). Selectivity for ERβ was similar for the two enantiomers (∼80-fold). S-DPN exhibited an affinity for ERβ that was close to that of the cognate ligand, E2 (Ki = 0.13 ± 0.02), and had good binding selectivity for ERβ over ERα. Thus, by binding parameters, S-DPN is a high-affinity, partially selective ERβ agonist.
Table 1.
Affinities of ER subtypes for the enantiomers of DPN in comparison with estradiol
| ERα | ERβ | |
|---|---|---|
| Estradiol | 0.10 ± 0.02 | 0.13 ± 0.02 |
| DPN | 48.1 ± 3.5 | 0.61 ± 0.07 |
| S-DPN | 20.8 ± 2.9 | 0.27 ± 0.05 |
| R-DPN | 147 ± 12.3 | 1.82 ± 0.21 |
Data shown as mean ± sd Ki (nm).
Comparison of S-DPN and R-DPN on ERβ-dependent ERE-luciferase activity
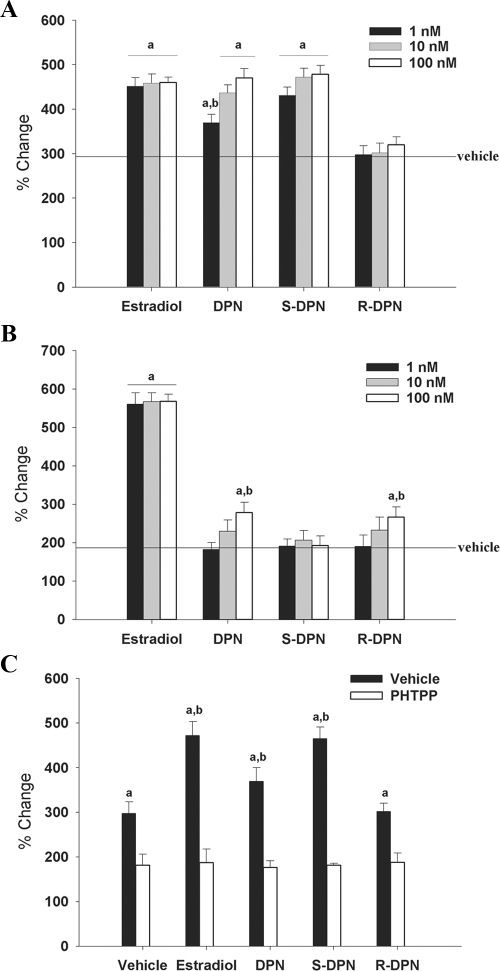
To determine whether DPN exhibits enantiomer-specific activation of ERE-dependent transcription through ERβ or ERα, we cotransfected an ERE-luciferase reporter plasmid with an expression vector for ERβ into the mouse hypothalamic cell line, N-38. These cells were chosen because the hypothalamus is a brain region rich in ER expression and is important in reproduction and homeostasis (both targets of estrogen action in the brain). N-38 cells do express low levels of ERα and ERβ [Belsham et al. (33)]; however, when transfected with an ERE-luciferase promoter construct, estradiol did not stimulate luciferase expression (data not shown). Thus, in these experiments the cells were cotransfected with ERα or ERβ expression vectors. In cells cotransfected with ERβ, there was a significant (P < 0.01) ligand-independent (vehicle) effect (∼300% of empty vector control) (Fig. 2A). The racemic mixture of DPN significantly (P < 0.01) activated transcription above the observed constitutive level in a dose-dependent manner in which 10 and 100 nm DPN were statistically identical with the effect seen with 1 nm estradiol. S-DPN stimulated ERE-luciferase activity in a similar fashion to estradiol at all doses and was significantly higher than racemic DPN at the 1 nm dose (P < 0.02). In contrast, R-DPN did not stimulate luciferase expression to levels greater than that seen in the absence of ligand.
Figure 2.
Effects of ER ligands on ERE-luc promoter activity mediated by ERβ (A and C) or ERα (B). N-38 cells were cotransfected with an ERE-tk-luc reporter construct and an expression vector containing ERα or ERβ. After transfection, cells were treated with vehicle (0.001% EtOH), estradiol, DPN, S-DPN, or R-DPN for 16 h. Data are represented as percent change in relative light units (RLU) from vehicle-treated empty vector controls (set at 100%). a, Significant differences (P < 0.05) from vehicle-treated controls; b, significant differences (P < 0.05) within treatment groups. Solid horizontal lines indicate constitutive activity of receptor in absence of ligand. A, Effect of 1, 10, and 100 nm concentrations of ligands on ERE-luc promoter activity in cells cotransfected with ERβ. B, Effect of 1, 10, and 100 nm concentrations of ligands on ERE-luc promoter activity in cells cotransfected with ERα. C, Effect of ligands (1 nm) on ERE-luc promoter activity in cells cotransfected with ERβ in the presence or absence of the ERβ antagonist PHTPP (10 μm).
Comparison of S-DPN and R-DPN on ERα-dependent ERE-luciferase activity
In cells cotransfected with ERα, there was a smaller but significant constitutive activity observed (∼185% of empty vector control) (Fig. 2B). The racemic mixture of DPN did not stimulate transcription at the 1 nm or 10 nm concentrations; however, a small but significant increase (P < 0.01) in activity was observed at the 100 nm concentration. However, this activation was at a much lower level than any dose of estradiol (278% of empty vector control for 100 nm DPN vs. 560% for 1 nm estradiol). The same effect was observed with R-DPN, in which a significant increase in ERE-luciferase activity was seen only at the 100 nm concentration (P < 0.05). Interestingly, S-DPN did not stimulate luciferase activity at any of the doses. This suggests that the ERα-dependent activity of the racemate is due to the R-enantiomer of DPN.
Comparison of S-DPN and R-DPN on ERβ-dependent ERE-luciferase activity in the presence of an ERβ antagonist
To inhibit ERβ-dependent transcription, we used an ERβ antagonist (PHTPP) in N-38 cells cotransfected with an ERE-luciferase reporter construct and an ERβ expression vector. All treatments were given at 1 nm concentration, with or without 10 μm PHTPP [concentration required to inhibit the effect of 1 nm estradiol (15)]. Interestingly, the antagonist significantly (P < 0.02) decreased the ligand-independent activation of ERβ but not to the levels seen in the empty vector controls (297 to 181% of empty vector control) (Fig. 2C). Racemic DPN significantly increased transcriptional activity, but E2 and S-DPN activated transcription to a much greater level than racemic DPN. R-DPN was similar to the vehicle control. When the agonists were given in conjunction with the antagonist, PHTPP, ERE-luciferase levels were similar to treatment with the antagonist alone. Thus, S-DPN is a potent and selective activator of ERE-dependent transcription in N-38 cells, and this activation can be abolished with concomitant treatment with an ERβ antagonist.
Anxiety-type behaviors and learned helplessness in S-DPN- and R-DPN-treated animals
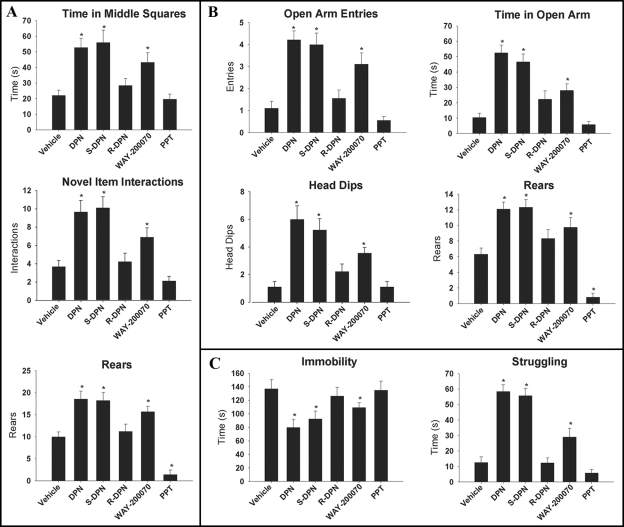
To determine whether DPN′s anxiolytic and antidepressant effects are via the S-enantiomer, we examined the effect of each enantiomer on anxiety-like behaviors using the EPM and OF test. We used the FST to evaluate depressive-like behaviors. Rats treated with racemic DPN, S-DPN, and WAY-200070 showed significantly decreased anxiety-like behaviors in both the OF and EPM. In the OF, these animals made more rears, interacted more with a novel object, and spent more time in the middle squares of the OF arena than did vehicle-, PPT-, or R-DPN-treated animals (P < 0.01; Fig. 3A). No differences were observed in total square crossings, an indicator of locomoter activity. In the EPM, racemic DPN-, S-DPN-, and WAY-200070-treated females had significantly higher open arm entries, open arm time, rearing, and head dips than did control or PPT- or R-DPN-treated animals (P < 0.01; Fig. 3B). No differences were seen in total arm entries, an indicator of locomoter activity. Rats treated with racemic DPN, S-DPN, and WAY-200070 showed significantly less depressive-like behaviors in the FST (Fig. 3C). These animals spent significantly more time struggling and less time immobile than did control or PPT- or R-DPN-treated animals (P < 0.01). There were no differences in time swimming, an indicator of overall locomotion. Thus, S-DPN is the behaviorally active enantiomer of DPN with respect to its anxiolytic and antidepressant activity.
Figure 3.
Effects of ER ligands on anxiety-related behavior in the OF (A) and EPM (B), and learned helplessness in the FST (C). Behaviors measured include time spent in the middle squares, novel item interactions, and rearing in the OF; open arm entries, time spent in open arm, and rearing in the EPM; head dips, time spent immobile, and time spent struggling in the FST. Data are represented as mean ± sem (n = 9 animals per treatment group). *, Significant difference (P < 0.05) compared with vehicle treatment.
Comparison of the hypothalamic-pituitary-adrenal axis response after the FST in S-DPN- and R-DPN-treated animals
To determine the effect of S-DPN and R-DPN on hypothalamus-pituitary-adrenal (HPA) axis activation, serum CORT was measured from blood samples taken 20 min after the start of the swim test. Animals treated with DPN, S-DPN, and WAY-200070 had significantly lower serum CORT than vehicle-treated animals, whereas the R-enantiomer had no effect compared with controls (Fig. 4). Conversely, treatment with the ERα agonist PPT significantly (P < 0.05) increased serum CORT.
Figure 4.
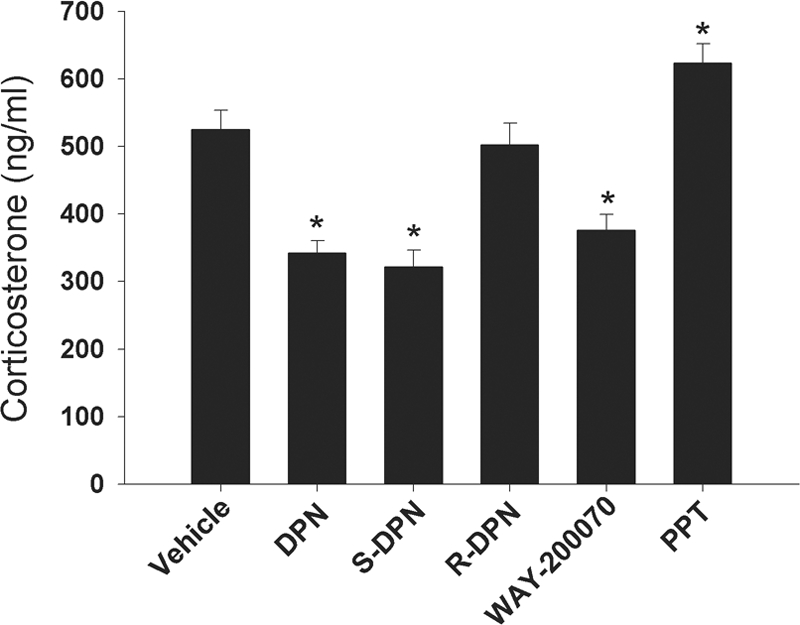
Effects of ER ligands on plasma CORT after the FST. Plasma samples were obtained 20 min after the start of the swim test. Data are represented as mean plasma CORT ± sem (n = 6 animals per treatment group). *, Significant difference (P < 0.05) compared with vehicle treatment.
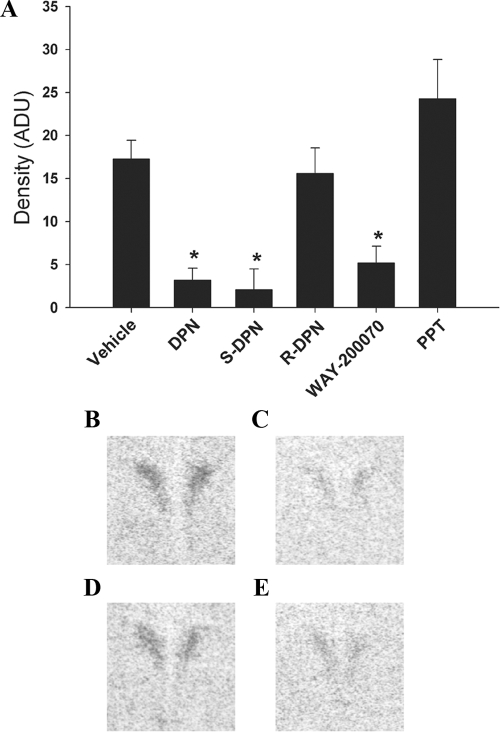
Activation of paraventricular nucleus of the hypothalamus (PVN) neurons by stress is inhibited by S-DPN
c-fos mRNA expression was examined in the PVN of brains taken from animals subjected to the FST. c-fos mRNA was expressed in PVN neurons, but expression was significantly (P < 0.05) lower in DPN-, S-DPN-, and WAY-200070-treated animals compared with treatment with vehicle, R-DPN, or PPT (Fig. 5). Thus, the effects of racemic DPN on HPA axis activation can be attributed to the S-enantiomer and not the R-enantiomer. Furthermore, the comparable effect seen with WAY-200070 supports an ERβ-dependent mechanism.
Figure 5.
Effects of ERβ ligands on PVN c-fos mRNA levels after the FST as measured by ISH. A, Relative levels of c-fos mRNA expression. Data are represented as mean arbitrary density units (ADU) ± sem (n = 6 animals per treatment group). *, Significant difference (P < 0.05) compared with vehicle treatment. B–E, Representative film autoradiograms for vehicle (B), S-DPN (C), R-DPN (D), and WAY-200070 (E)-treated animals.
Discussion
Since the discovery and characterization of DPN (11) as a potent and selective ERβ agonist, it has been used in numerous in vitro and in vivo studies aimed at determining ERβ's biological function. DPN is a chiral molecule and thus is synthesized as two enantiomeric forms, S-DPN and R-DPN, which may have different binding and transactivational properties. Molecular modeling predicts that S-DPN associates with the ligand binding pocket of ERβ in a much more favorable fashion compared with R-DPN (25). The proximal phenol ring (β-ring) of S-DPN mimics the A-ring of E2, and it likely engages in hydrogen-bonding with the same residues as E2 (Glu-305, Arg-346, and His-475). Furthermore, the positioning of the nitrile group in S-DPN is more favorable for engaging in stabilizing interactions with the sulfur of Met-336, and S-DPN may be able to engage in H-bonding with Thr-299 that cannot occur with R-DPN. To better interpret results of studies using the racemic mixture of DPN, we sought to determine the biological profile for each enantiomer and determine whether one form was the preferred ERβ agonist.
S-DPN is a high-affinity ERβ agonist
In competitive binding assays using recombinant receptor, S-DPN had a 6.7-fold higher affinity for ERβ compared with R-DPN. Like the endogenous ligand for ERβ, E2, S-DPN has a subnanomolar affinity for ERβ. Additionally, it has greater affinity for ERα compared with R-DPN, but it is more than 200-fold less potent at ERα than E2, and it retains similar selectivity for ERβ as the racemic mixture (80-fold selective). It is important to note that binding to a receptor does not necessarily lead to functional activation of the receptor, and thus, transcriptional activity was tested in further studies. DPN binds ERα with submicromolar affinity, and it can cross the blood-brain barrier, yet when peripherally administered (1 mg/kg, sc) it does not activate ERα in the brain (24). These binding results do not directly conform to the modeling predictions that R-DPN has a lower energy state conformation (stronger affiliation) with ERα than ERβ. However, we did find that S-DPN is a more potent ERβ ligand as predicted by the modeling.
S-DPN is a potent activator of ERβ-dependent transcription at an ERE
To determine whether the binding profiles correlated to functional activity, we compared the ability of the two enantiomers to stimulate transcription through an ERE-luciferase promoter reporter construct. Rather than use a cell type that does not normally express ERs, for these studies we chose the immortalized neural cell line N-38 that expresses both ER subtypes (28). Because these cells are derived from the mouse hypothalamus and express several neuropeptides like oxytocin and neuropeptide Y, they provide an excellent model to study neuroendocrine systems in vitro (33). Additionally, N-38 cells most likely have all the transcriptional machinery present to respond genomically to hormonal stimulation, whether it is through ER or another hormone receptor. Interestingly, when transfected with an ERE-luciferase reporter gene and treated with 100 nm E2 or DPN, there is no stimulation seen above the empty vector control. One possible explanation for this lack of response may be that the endogenous estrogen receptors do not bind to the promoter region of our transfected reporter construct or are not expressed in sufficient levels to elicit a detectible response. However, when expression vectors for either ERα or ERβ are cotransfected with the reporter construct, there are significant ligand-independent and ligand-dependent increases in luciferase activity seen in these cells. Therefore, we were able to separately determine the ERα- and ERβ-dependent transcriptional activities for both enantiomers in a neuroendocrine cell model.
Our results confirm those of Pak et al. (34), that when ERβ is cotransfected with an ERE-luciferase reporter gene, there is a significant ligand-independent effect of ERβ (∼300%) vs. empty vector controls (34). This constitutive effect has been reported across various cell types and promoters including the GnRH, arginine vasopressin, and CRH promoters (35,36,37). S-DPN further stimulates luciferase activity above the constitutive level for ERβ and to a level statistically identical with E2 at all doses tested (1, 10, 100 nm). At the 1-nm dose, S-DPN significantly increased promoter activity to a level greater than racemic DPN. By contrast, R-DPN did not stimulate promoter activity above the constitutive level at any dose tested. Therefore, S-DPN appears to be similar to estradiol at inducing ERβ-induced transcription mediated by an ERE promoter site, whereas R-DPN is ineffective.
When ERα is cotransfected with an ERE-luciferase promoter reporter construct, we also found a significant ligand-independent effect of ERα (∼185%) vs. empty vector controls, albeit not to the levels seen with ERβ. S-DPN was not effective at stimulating ERα-mediated transcription at any dose, thus demonstrating its selectivity for ERβ. Interestingly, we found that R-DPN did increase luciferase expression at the 100-nm dose, as did the racemic mixture. Racemic DPN has been previously shown to induce ERα-dependent transcription through an ERE promoter at higher doses (11). Thus, our data demonstrate that it is the R enantiomer that provides loss of selective activity to the racemic compound.
Because N-38 cells also have been reported to contain ERα and ERβ (28), although they appear to be nonfunctional in our hands, we further confirmed the ERβ specificity of S-DPN by using a selective ERβ antagonist, PHTPP, which has been shown to fully antagonize the effect of E2 on ERβ initiated transcription through an ERE promoter site. PHTPP completely blocked the effect of E2, S-DPN, and racemic DPN, suggesting that the induced transcriptional activity is indeed ERβ dependent. Interestingly, PHTPP did not fully block the ligand-independent effect of ERβ. This is in contrast to the nonselective ER antagonist 4-hydroxy tamoxifen, which has been shown to fully inhibit the constitutive effect of ERβ in HT-22 cells (34). It is possible that ERβ undergoes a conformational change upon binding PHTPP that still allows it to engage DNA with a low-level constitutive activity, whereas tamoxifen cannot. In this state the ligand-receptor complex may not be able to bind DPN or E2, or it blocks the ability of agonists to alter the conformation of the receptor to one that is more transcriptionally active.
S-DPN treatment decreases anxiety-type behaviors and learned helplessness
Previous work from our laboratory has shown that peripherally administered racemic DPN can cross the blood-brain barrier, bind ER, and decrease anxiety and fear in both female and male adult rats in the EPM and OF test (24). These behavioral paradigms take advantage of the natural conflict of exploration of a novel environment vs. avoidance of a brightly lit and exposed environment (30). The anxiolytic effect of DPN seen in these tests is abolished by cotreatment with the ER antagonist tamoxifen, indicating an ERβ-dependent mechanism. Other studies have also indicated an antidepressant effect of DPN in the FST (23,38) and that this effect is lost on βERKO animals (39). The FST provides a measure of despair as measured by the propensity to remain immobile, which can be decreased with antidepressant treatment (31).
In this study, we confirmed the findings that racemic DPN exhibits anxiolytic and antidepressant activity. We now show that S-DPN is the enantiomer solely responsible for the behavioral effects seen with DPN. Furthermore, treatment with the selective ERβ agonist WAY-200070 mimicked the effects seen with DPN and S-DPN. WAY-200070 has a RBA of 133 and is 68-fold selective for ERβ (13). Recent studies have shown WAY-200070 to be anxiolytic in the four-plate test and antidepressant in the tail suspension test when peripherally administered to male mice (40). Therefore, the similarities seen across the three behavioral paradigms (OF, EPM, and FST), and among the different ERβ agonists, all point to an ERβ-dependent mechanism.
S-DPN treatment attenuates HPA reactivity to stress
Estradiol has been shown to potentiate CORT secretion after a stressor whether administered peripherally or locally within the hypothalamus (32,41). This appears to be largely due to the activation of ERα because PPT treatment mimics this effect. Peripheral and central administration of the ERβ agonist DPN has an opposing, inhibitory effect on stress-induced CORT secretion. Furthermore, these same effects on CORT secretion are observed after the EPM (24). In agreement with these previous studies, our results show that DPN decreased plasma CORT levels after the FST. Furthermore, S-DPN seems to be the enantiomer responsible for reducing stress-induced activation of the HPA axis because S-DPN inhibited CORT secretion, whereas R-DPN treatment had no effect. Selectivity for ERβ in this test was further demonstrated by the administration of another ERβ agonist, WAY-200070, which mimicked the effect seen with DPN and S-DPN, further confirming an ERβ-dependent mode of action. In all of these studies, the effect of WAY 200070 was less than that of S-DPN. This is likely due to the lower affinity of WAY-200070 for ERβ compared with S-DPN.
S-DPN acts to inhibit activation of PVN neurons
The expression of the immediate early gene c-fos is a well-described reporter for neuronal activation. Estradiol has been shown to increase the expression of c-fos mRNA in the PVN after a stressor (32). This increase appears to be due to activation of ERα because PPT mimics this effect, whereas DPN decreases stress-induced c-fos mRNA in the PVN (41). Our results are in accordance with these studies because PPT increased, whereas DPN decreased, PVN c-fos expression 20 min after the start of the FST. Additionally, the effect of DPN is due to the S-enantiomer because S-DPN-treated animals had significantly lower, whereas R-DPN-treated animals had similar, levels of c-fos mRNA in the PVN compared with controls. One might expect R-DPN to augment c-fos mRNA expression due to its transcriptional activity via ERα at high doses; however, these effects are likely minimal at the administered dose (2.0 mg/kg).
The ERβ agonist WAY-200070 also mimicked DPN and S-DPN, providing further support for an ERβ-specific mechanism. Whether this is a direct or indirect effect of ERβ remains to be determined. There are populations, albeit small, of ERβ-containing CRH neurons in the medial parvocellular division of the PVN (42,43), providing a potential direct mode of action. However, ERβ is also expressed in other cell types in the PVN and brain regions like the amygdala, hippocampus, dorsal raphe nucleus, suprachiasmatic nucleus, and the bed nucleus of the stria terminalis that send glutamatergic and secretion of γ-aminobutyric acid projections to the PVN, providing potential indirect modes of action (5,44,45).
In summary, the ERβ agonist DPN exists as two enantiomers with different biological activities. S-DPN provides the ERβ potency and selectivity in both receptor binding and transcriptional activation. Furthermore, S-DPN is the active enantiomer in ERβ-dependent effects seen in anxiety and learned helplessness behavioral paradigms. Currently DPN is the standard ERβ agonist of choice for in vivo and in vitro studies. Our results indicate that an enantiomeric-pure preparation should be considered in future studies using DPN. The effects of a pure ERβ agonist, S-DPN, on stress-related behaviors are in accordance with previous reports using ERβ-specific agonists and underline the importance of ERβ-dependent signaling in anxiety-like and depressive-like behavior.
Footnotes
This work was supported by the Public Health Service, National Institutes of Health National Institute of Neurological Disorders and Strokes Grant R01-NS039951 (to R.J.H.) and Department of Defense, United States Army Medical Research and Material Command Grant 04182001 (to T.J.W.).
Disclosure Summary: M.J.W., T.J.W., and R.J.H. have nothing to disclose.
First Published Online December 12, 2008
Abbreviations: CORT, Corticosterone; DPN, diarylpropionitrile; E2, 17β-estradiol; EPM, elevated plus maze; ER, estrogen receptor; ERE, estrogen response element; ERKO, ER knockout; FST, forced swim test; HPA, hypothalamus-pituitary-adrenal; ISH, in situ hybridization; OF, open field; PHTPP, 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol; PPT, propylpyrazoletriol; PVN, paraventricular nucleus of the hypothalamus; RBA, relative binding affinity; RLU, relative light unit; tk, thymidine kinase.
References
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P 1986 Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ 2008 Estrogen receptor β in the brain: from form to function. Brain Res Rev 57:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Toresson G, Xu L, Koehler KF, Gustafsson JA, Dahlman-Wright K 2005 Mouse estrogen receptor β isoforms exhibit differences in ligand selectivity and coactivator recruitment. Biochemistry 44:7936–7944 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I 1998 Comparative distribution of estrogen receptor-α (ER-α) and β (ER-β) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids 63:498–504 [DOI] [PubMed] [Google Scholar]
- Gustafsson JA 2000 Novel aspects of estrogen action. J Soc Gynecol Investig 7:S8–S9 [DOI] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K, Gustafsson JA 2008 Estrogen receptor β: an overview and update. Nucl Recept Signal 6:e003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA 2007 Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- Veeneman GH 2005 Non-steroidal subtype selective estrogens. Curr Med Chem 12:1077–1136 [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA 2001 Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2000 Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith Jr JC, Harris HA 2004 Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-β ligands. J Med Chem 47:5021–5040 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA 1998 Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139:4252–4263 [DOI] [PubMed] [Google Scholar]
- Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA 2004 Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor β antagonist activity. J Med Chem 47:5872–5893 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L 2008 An alternate pathway for androgen regulation of brain function: activation of estrogen receptor β by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA 2006 The unexpected science of estrogen receptor-β selective agonists: a new class of anti-inflammatory agents? Nucl Recept Signal 4:e012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HA 2006 Preclinical characterization of selective estrogen receptor β agonists: new insights into their therapeutic potential. Ernst Schering Found Symp Proc:149–161 [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ 2005 17β-Estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology (Berl) 179:637–643 [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF 2005 Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav 84:157–163 [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF 2001 Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci USA 98:12278–12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2005 ERβ-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology 30:1598–1609 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA 2004 Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav 78:523–529 [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ 2005 Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- Sun J, Baudry J, Katzenellenbogen JA, Katzenellenbogen BS 2003 Molecular basis for the subtype discrimination of the estrogen receptor-β-selective ligand, diarylpropionitrile. Mol Endocrinol 17:247–258 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Reid DL, Resko JA 1986 Androgen receptors in brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod 34:293–303 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Stadelman HL, Resko JA 1987 Effect of estrogen on androgen receptor dynamics in female rat pituitary. Endocrinology 121:84–89 [DOI] [PubMed] [Google Scholar]
- Titolo D, Cai F, Belsham DD 2006 Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol Endocrinol 20:2080–2092 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Cross MK, George M, Gordon BH, Burgess LH, Cabrera TM, Hata N, Campbell DB, Lorens SA 1993 Neuroendocrine and neurochemical responses to novelty stress in young and old male F344 rats: effects of d-fenfluramine treatment. Pharmacol Biochem Behav 46:101–109 [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW 1993 An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods 29:129–138 [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M 1977 Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732 [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ 2004 Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 16:272–278 [DOI] [PubMed] [Google Scholar]
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L 2004 Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 145:393–400 [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ 2005 The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology 146:147–155 [DOI] [PubMed] [Google Scholar]
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM 2004 Estrogen receptor (ER)β isoforms rather than ERα regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci 24:10628–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak T, Chung W, Handa R 2007 Estrogen receptor-β mediates DHT-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology 148:3371–3382 [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Roberts JL, Handa RJ 2006 Ligand-independent effects of estrogen receptor β on mouse gonadotropin-releasing hormone promoter activity. Endocrinology 147:1924–1931 [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2007 Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav 86:407–414 [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA, Adult female wildtype, but not oestrogen receptor β knockout, mice have decreased depression-like behaviour during pro-oestrus and following administration of oestradiol or diarylpropionitrile. J Psychopharmacol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang G, Schechter LE, Rosenzweig-Lipson S, Day M 2008 WAY-200070, a selective agonist of estrogen receptor β as a potential novel anxiolytic/antidepressant agent. Neuropharmacology 54:1136–1142 [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ 2006 The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci 26:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S 1998 Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36:357–378 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ 2005 Estrogen receptor-β, but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol 484:28–42 [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE 2002 Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav 71:457–468 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I 2001 Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol 436:64–81 [PubMed] [Google Scholar]