Abstract
To understand whether bone morphogenetic protein plays any role in the formation of primordial follicles in the hamster, we examined the temporal and spatial expression of bone morphogenetic protein receptor (BMPR) mRNA and protein in embryonic (E) 13 through postnatal day (P) 15 ovarian cells and a possible regulation by FSH during the formation of primordial follicles on P8. BMPRIA and BMPRII mRNA levels were significantly higher than that of BMPR1B throughout ovary development. BMPRIA and BMPRII mRNA levels increased significantly on E14 and declined by P5 through P6. Whereas BMPRII mRNA increased again by P7, BMPRIA mRNA levels increased through P8 concurrent with primordial follicle formation. In contrast, BMPRIB mRNA levels increased greater than 10-fold on P7-9, with a further 3-fold increase by P10. BMPR proteins were low in the somatic cells and oocytes on E13 but increased progressively during postnatal development. BMPR expression in somatic cells increased markedly on P8. Whereas BMPRII expression declined by P10 and remained steady thereafter, BMPRIA protein expression fluctuated until P15 when it became low and steady. Overall, BMPRIB immunoreactivity also declined by P10 and then remained low in the interstitial cells through P15. FSH antiserum treatment on E12 significantly attenuated receptor mRNA and protein levels by P8, but equine chorionic gonadotropin replacement on P1 reversed the inhibition. Furthermore, FSH in vitro up-regulated BMPR levels in P4 ovaries. This unique pattern of BMPR expression in the oocytes and somatic cells during perinatal ovary development suggests that BMP may play a regulatory role in primordial follicle formation. Furthermore, FSH may regulate BMP action by modulating the expression of its receptors.
Bone-morphogenetic protein (BMP) may play a regulatory role in primordial follicle formation, and FSH may regulate BMP action by modulating the expression of its receptors.
Bone morphogenetic proteins (BMPs) belong to the TGFβ superfamily and play a critical role in tissue morphogenesis and function (1). Similar to TGFβ, BMPs have been shown to act via type I and type II receptors, namely, BMPR-IA, BMPR-IB, and BMPR-II (1). Despite certain degree of cross-reactivity among different BMPs and type I receptor, ligand receptor preferences have also been reported (1,2). Among the BMP ligands, BMP2 binds to BMPR-IA, BMP4 binds to BMPR-IB whereas BMP6 binds to activin receptor-IA, but all of them allow the respective type I receptor to heterodimerize with the BMPR-II for downstream signaling (1,3,4,5). Using in situ hybridization, the definitive presence of BMPRIB mRNA has been shown in rat granulosa cells of follicles in all classes of development, whereas consistent expression of BMPRIA mRNA is observed from primary follicles onward (1). In contrast, weak expression of BMPRII mRNA is present in the rat granulosa cells regardless of the follicle size (1), and no BMPRIB expression is observed in the granulosa cells of mouse primordial follicles (6). Although BMPRIB null mice show no apparent difference in follicular development relative to the wild type, the animals are infertile due to defects in cumulus cell expansion and endometrial development (6).
BMP-4 has been shown to promote primordial to primary follicle transition and a BMP-4 antibody markedly reduces the number of primordial follicles in the rat (7). Recently using rat granulosa cells in vitro, Edwards et al. (8) have shown that similar to TGFβ ligands, ovine growth differentiation factor (GDF)-9 or ovine BMP15 first binds to BMPRII, which recruits type I component. GDF 9 plays an important role in primary to secondary follicle transition in mice (9). In contrast to mouse, GDF9 protein expression in the hamster oocytes occurs long before the first cohort of primordial follicles appear in the ovary (10). Furthermore, GDF9 action is critical for hamster primordial follicle formation (11). All these lines of evidence indicate that GDF9 and BMP family of ligands have an important role in ovarian follicular development and function. We have shown that FSH regulates the expression of GDF9 (10), estrogen receptor (12), and CYP19 mRNA in ovarian cells during perinatal ovary development and plays an essential role in primordial follicle formation (13). The objective of the present study was to determine whether the expression of BMPRIA, BMPRIB, or BMPRII during perinatal ovarian morphogenesis in the hamster relates to the formation of primordial follicles and whether FSH action might influence the expression of BMPR during primordial follicle formation. We used golden hamsters as the animal model because morphologically distinct primordial follicles first appeared in in vivo grown ovaries in the morning of postnatal day (P) 8 (13). This unique developmental program allowed us examine the expression patterns of BMP receptors coinciding with the formation of first cohort of primordial follicles, thus identifying the probable physiological relevance of BMP action in ovarian somatic cell differentiation into primordial granulosa cells.
Materials and Methods
Adult golden male and female hamsters were purchased from Charles River Laboratories (Charles River, MA) and maintained in a climate-controlled room with 14 h light and 10 h dark with free access to food and water according to the Institutional Animal Care and Use Committee and the U.S. Department of Agriculture guidelines. The use of hamsters for this study was approved by the Institutional Animal Care and Use Committee. Females with at least three consecutive estrous cycles were mated with males in the evening of proestrous, and the presence of sperm in the vagina the next morning was considered d 1 of pregnancy. Hamster gestation lasts for 16 d, and pups are born on 16th day of gestation, which we considered the first day of postnatal life.
The rabbit polyclonal antibodies to human BMPRIA, BMPRIB, and BMPRII were generously provided by Dr. Carl H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). The antibodies were thoroughly characterized previously (14) and also optimized for hamster tissues using appropriate antibody controls. A rabbit polyclonal FSH antibody that neutralized hamster FSH was prepared in the laboratory and tested for its efficiency during perinatal ovary development (13). Second antibody conjugated to Alexa 488 was purchased from Molecular Probes/Invitrogen (Carlsbad, CA), equine chorionic gonadotropin (eCG; 2000 IU/mg solid) was purchased from Sigma Chemical Company (St. Louis, MO), ovine FSH-20 was obtained from Dr. A. F. Parlow (National Pituitary hormone Program, Harbor-UCLA, Torrance, CA), PCR chemicals were from Roche Molecular Biochemicals (Indianapolis, IN), Pharmacia Biotech Boehringer (Piscataway, NJ), and Invitrogen. Real-time PCR primers and probes were synthesized in the Eppley DNA synthesis Core Facility (University of Nebraska Medical Center, Omaha, NE). All other fine chemicals were purchased from Fisher Scientific Co. (Pittsburgh, PA).
Partial cloning of hamster BMPRIA, BMPRIB, and BMPRII
Hamster BMPRIA, BMPRIB, and BMPRII cDNA were cloned using reverse transcription and PCR. cDNA was amplified from total RNA from P8 ovaries according to the protocol described previously (15). The sequences for forward (F) and reverse primers (R) for generating hamster BMPR cDNA were: BMPRIA F, 5′-ATTTGCAGCCTACACTGCC′3′, R, 5′-ACCCATCCATACTTCTCCATAC-3′ corresponding to 398-753 bases of rat BMPRIA (accession no. D38082); BMPRIB F, 5′-AAGACCTACACCCTACACTGCC-3′, R, 5′-CACTTTCCCATCCAAACTTCCC-3′ corresponding to 311-668 bases of human BMPRIB (accession no. U89326); and BMPRII F, 5′-AACCACCACAAACACCACC-3′, R, 5′-TGTGTGAAGTCCTGTTGTCC-3′, corresponding to 1836-2174 bases of mouse BMPRII (accession no. U78048). The primers were designed using Amplify (Dr. Bill Engels, Genetics Department, University of Wisconsin, Madison, WI) and Vector NTI primer design software version 10 (Invitrogen). The PCR was done for 35 cycles with an annealing temperature of 55 C, and the cDNA for each receptor was cloned into a PCR 4-TOPO plasmid vector, sequenced in the DNA sequencing core facility (University of Nebraska at Lincoln, Lincoln, NE), and BLAST searched in the National Institutes of Health (NIH) GenBank (Bethesda, MD). After confirmation, hamster BMPR sequences were compared with corresponding rat, mouse, and human BMPR sequences using a Vector NTI gene analysis software version 10 (Molecular Analysis Core, University of Nebraska Medical Center). Partial amino acid sequences were deduced from the cDNA and all sequences were deposited in the National Center for Biotechnology Information gene bank (Bethesda, MD).
Determination of BMPR expression patterns in perinatal hamster ovaries
Ovaries were collected from E age 13–15 and P1-15 hamsters, placed in the optimum cutting temperature compound, snap frozen in liquid nitrogen-cooled methylisopentene, and sectioned at 6 μm in a Leica cryostat microtome for immunofluorescence detection of BMPRIA, BMPRIB, and BMPRII protein.
In a parallel experiment, three pairs of ovaries for each day of development were collected for RNA preparation, which was used for real-time quantification of the levels of BMPRIA, BMPRIB, and BMPRII mRNA.
Effect of anti-FSH serum on ovarian BMPR expression
Pregnant hamsters were injected sc with 200 μl of an anti-FSH serum on 12th day of gestation because previous studies indicated this time to be ideal for blocking the formation of primordial follicles (13). After the pups were born, one set of pups received 0.5% BSA in saline (vehicle for eCG), whereas the others were injected sc with 20 IU eCG on P1. Ovaries were collected on P8 for real-time RT-PCR and immunofluorescence detection of BMPRIA, BMPRIB, and BMPRII mRNA and protein expression, respectively. eCG was used because of its longer half-life in vivo, thus avoiding repeated injections to newborn pups and its ability to stimulate follicular development in the hamster (16,17,18). Moreover, eCG did not cross-react with the FSH antiserum; hence, could bypass the antibody neutralization, and LH receptors did not appear in postnatal hamster ovarian cells until P13 (Roy, S. K., unpublished observation), so contribution of LH receptors was not a concern.
Effect of FSH in vitro on ovarian BMPR mRNA expression
Ovaries were collected from P4 animals and cultured for 24 h in the absence of presence of 1 ng/ml ovine FSH-20 (NIH) on 3 μm pore-insert in DMEM supplemented with 0.1 mg/ml bovine insulin (Sigma), 1.25 μg/ml human transferrin (BD Biosciences, San Jose, CA), 1.25 ng/ml selenious acid, 0.25 mg/ml BSA, 1.07 μg/ml linoleic acid, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml Fungizone (Invitrogen, Carlsbad, CA) under 5% CO2 in air as described previously (11,19). Ovaries were retrieved and used for quantification of BMPR mRNA levels.
Spatiotemporal effect of eCG in vivo on ovarian BMPR mRNA expression
Because eCG acted like FSH in the hamster with a longer half-life, it helped avoiding repeated injections to small pups, which would have been necessary had we used FSH. We also avoided cannibalization by the mother. P6 hamsters were injected sc with 20 IU eCG in 20 μl 0.5% BSA in saline (vehicle) or vehicle alone at 0800 h, and ovaries were collected at 6, 12, and 24 after the injection. Because 24 h after the injection on P6, ovaries actually became of P7, both mRNA and protein data were compared with ovaries obtained on P7. There were at least three hamsters per time point. One ovary from each hamster was used for immunofluorescence localization of BMPR, whereas the other was used for quantitative PCR quantification of BMPR mRNA.
Immunofluorescence localization of BMPRIA, BMPRIB, and BMPRII in the ovary
Frozen sections at 6 μm were fixed in freshly prepared 4% paraformaldehyde at 4 C in PBS (pH 7.0) and processed for immunofluorescence localization of BMPRIA, BMPRIB, and BMPRII using 1:1000 dilution of the respective antibody in blocking buffer as indicated previously for other antigens (12,20). In brief, the sections were exposed to the antibodies overnight at 4 C followed by 30 min exposure to the second antibody Alexa 488 at room temperature. The nuclei were stained with 4′,6′-diamino-2-phenylindole. After mounting in Fluoromount G medium (Southern Biotechnology Associates, Inc., Birmingham, AL), sections were examined by a Leica DMR epifluorescence microscope equipped with Retiga Fast1394 digital camera (Q-Imaging, Burnaby, British Columbia, Canada), and the images were captured using the Openlab (Improvision, Lexington, MA) image analysis software. The specificity of the antibodies was verified by incubating sections of P8 ovaries in preimmune serum and processed identically as mentioned earlier.
For digital image capturing, the exposure time was adjusted using sections incubated without the primary antibody to subtract any auto- or nonspecific fluorescence recording. The signal obtained after such background correction was considered as antigen-specific signal. The exposure was set so that the lowest level of specific fluorescence signal could be recorded without any nonspecific fluorescence signal. Consequently, increase in fluorescence intensity resulted in images with intense fluorescence signal. All sections for a specific receptor antigen were evaluated under identical exposure settings so that the developmental expression could be compared. Similarly, because the intensity of immunosignal always varied between determinations, we always included no-antibody controls to subtract nonspecific immunosignal and perform the immunolocalization using sections of all groups to be compared. Reproducibility of immunofluorescence localization was verified using ovary sections from three different animals. Digital images of representative sections were captured, and images with BMPR signal were merged with those with nuclear signal using the Openlab software (Improvision) for spatial localization of the immunosignal. For contrast, blue fluorescence of 4′,6′-diamino-2-phenylindole was converted to red color. Photomicrographs were arranged using Adobe Photoshop (San Jose, CA) without any further adjustment to maintain the true nature of the findings. To avoid redundancy, photomicrographs of ovaries reflecting distinct changes in the BMPR expression during perinatal development were presented. Furthermore, fluorescence signal representing critical expression was quantified using the NIH Image J software and presented as mean OD per pixel ± sem.
Real-time RT-PCR quantification BMPR mRNA levels in ovaries during perinatal development, ovaries exposed to the FSH antiserum in vivo, ovaries exposed to FSH in vitro, and ovaries exposed to eCG in vivo
Ovaries were collected in RNAlater solution and RNA was isolated using Trizol (Invitrogen) and RNeasy minikit (QIAGEN Inc., Valencia, CA) sequence as indicated previously (11), quantified using the Ribogreen kit (Molecular Probes) according to the manufacturer's protocol. For real-time quantification, mRNA standards for each BMPR subtype and actin were used in parallel with the sample RNA to obtain true quantitative values. Real-time PCR primers and probes were designed from the hamster BMPRIA (accession no. DQ237888), BMPRIB (accession no. DQ237889), BMPRII (accession no. DQ237890), and actin cDNA (accession no. DQ237887) sequences. The sense strand of mRNA for each BMPR subtype and actin was synthesized using linearized, hamster-specific cDNA, and AmpliScribe T7-Flash transcription kit, quantified using the Ribogreen kit, and its authenticity verified by routine RT-PCR using the forward and reverse primers designed specifically for real-time RT-PCR. A series of eight standards ranging from 0.1 fg to 1 ng (in 10-fold increments) of specific mRNA was used. Duplicates of each standard were reverse transcribed along with ovarian RNA samples. Aliquots of reverse transcribed product of each ovarian sample were used for real-time quantification of BMPRIA, BMPRIB, BMPRII, and actin using the Opticon 2 thermocycler (Bio-Rad, Hercules, CA). PCR was continued for 35 cycles after an initial denaturation at 94 C for 15 min to activate the Hotstart Taq polymerase (QIAGEN). Each cycle of PCR consisted of a 30-sec denaturation at 94 C and a 15-sec annealing at 55 C followed by a plate reading with a final extension at the end of 35 cycles for 10 min at 72 C. The amount of specific mRNA was calculated from the linear portion of the amplification signal. The authenticity of the PCR signal was verified using tubes containing no RNA or RNA without reverse transcription. Besides, the authenticity of the cDNA generated by real-time PCR was also verified by sequencing in preliminary experiments, The values were presented as femtogram mRNA per microgram of total RNA. Because RNA from developing ovaries were used, the amounts of actin mRNA for each day of development were also presented to prove the specificity of BMPR gene expression during development. The sequences for F and R primers and the probes for real-time PCR were: BMPRIA, F, 5′-CTCTGGGAGTGGGTCTGGAC′3′, R, 5′-CTGCCGGACCATCTGAATCT-3′, probe, 6-FAM-5′-ACCTTTATTGGTTCAGCGAACTATTGCCAAA-3′-BLACKHOLE; BMPRIB, F, 5′-TGAAACTTACATTCCTCCTGGAGAGT-3′, R, 5′-TGCTTCACCATCTGAATTTGCT-3′, probe, 6-FAM-5′-TCTCCCTCTGCTGGTCCAAAGGACAAT-3′-BLACKHOLE; BMPRII, F, 5′-TGGAACACTCTCTTAAGCAGTTCAGT-3′, R, 5′-CTTCCACCGCAAGCTTTATGA-3′, probe, 6-FAM-5′-ACCCTTTGAGCAGTACCAGTTCCAGCTTG-3′-BLACKHOLE; and actin, F, 5′-TGACCGAGCGTGGCTACAG-3′, R, 5′-CTTCTCTTTGATGTCACGCACAAT-3′, probe, 6-FAM-5′-TCACCACCACAGCCGAGAGGGA-3′-BLACKHOLE.
Statistical analysis
Immunofluorescence analysis used three different ovaries for each day of the perinatal development, and representative sections were presented. Because primordial follicles are the only follicles on P8, somatic cells associated with this structure were considered granulosa cells. If no primordial follicular structure were present, granulosa cells could not be identified; hence, somatic cells were examined. Because of the reason, the intensity of immunofluorescence signal was quantified and reported in terms of granulosa cells, oocytes, and the interstitial cells. For each ovary in each group, at least 50 cells in each category were examined to obtain the average intensity for specific cell types. ODs for three ovaries were used for calculating the mean and sem. Twelve-bit gray-scale images were used for quantification. Because receptor protein expression was not uniform within cells, we counted the entire surface of cells to obtain the average pixel density for each cell. For fetal ovarian RNA samples, there were three pregnant hamsters for three samples for each indicated day of gestation. For each sample, ovaries from all fetuses from each pregnant females were pooled. There were three RNA samples for each postnatal day. Each sample was prepared from ovaries pooled from three to four postnatal hamsters. All quantitative data were analyzed by one-way ANOVA with Tukey's post hoc test using InStat software (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Results
Nucleic acid and amino acid sequence comparison of hamster BMPRIA, BMPRIB, and BMPRII
Because our preliminary studies indicated that only BMP2 and BMP4 were expressed in the hamster ovary and these ligands act via BMPRIA, BMPRIB, and BMPRII, we focused on these three receptor types. Comparison of partial 359-bp hamster BMPRIA and the 356-bp hamster BMPRIB nucleic acid and the derived amino acid sequences with corresponding available sequences in the GenBank revealed that hamster BMPRIA sequences were 94.4 and 93.8% similar to those of the mouse and rat sequences, respectively, whereas sequences of hamster BMPRIB were 92.1 and 91.6% similar to those of the human and mouse, respectively. Further analysis of BMPRIA and BMPRIB amino acid sequences revealed the presence of a 23-amino acid-long transmembrane domain, five putative serine phosphorylation sites in the cytoplasmic domain similar to those existed for other species, and a SGSG (serine-glycine-serine-glycine) motif that is unique to the type I receptors of the TGFβ superfamily of receptors (21). BMPRIB sequence also contained a putative tyrosine phosphorylation site at the cytoplasmic domain. Because of the partial sequence of the extracellular domain, no cysteine residue could be located. In contrast, a 1342-bp hamster BMPRII cDNA and derived amino acid sequences revealed a 92.2 and 94.1% similarity with that of human and mouse sequence, respectively. The hamster BMPRII also contained a 25-residue-long transmembrane domain, 10 cysteine residues that are the hallmark of BMPR (3,22,23,24,25), three putative serine and two tyrosine phosphorylation sites in the extracellular domain, and 10 serine and seven threonine phosphorylation sites in the cytoplasmic domain similar to the species compared. Moreover, BMPRII sequence contained the cytoplasmic receptor kinase domain that was essential for cross-phosphorylation and activation of type I receptor typical of TGFβ family of receptors (26).
Expression of BMPRIB, BMPRII, and BMPRIA mRNA in perinatal ovaries during in vivo development, FSH withdrawal, and eCG replacement in vivo after FSH exposure in vitro and eCG exposure in vivo
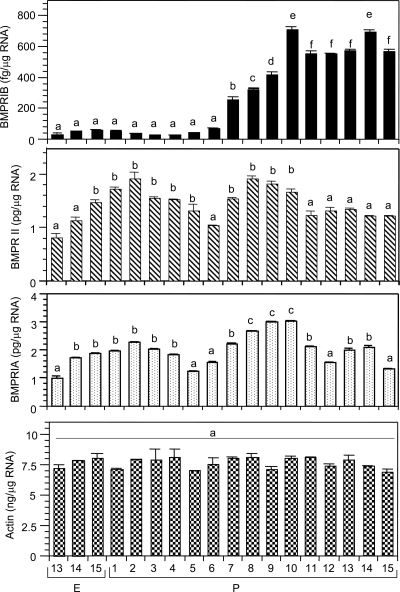
The rationale was to determine whether BMPR genes in the ovary were developmentally expressed and correlated with the formation of primordial follicles. The levels of BMPRII and BMPRIA mRNA were 3 orders of magnitude higher than that of the BMPRIB mRNA throughout perinatal development (Fig. 1). BMPRIB expression occurred from E13 but remained at low baseline until P6 (Fig. 1). BMPRIB mRNA levels increased sharply by P7, the day before the appearance of first cohort of primordial follicles in the hamster ovary (13), and reached the peak on P10 (Fig. 1). BMPRIB mRNA levels declined on P11 and then remained steady (Fig. 1). In contrast, BMPRII mRNA levels increased 2-fold from E13 to P2, and then declined to the fetal levels by P6 (Fig. 1). BMPRII mRNA increased significantly again on P7 and remained high before declining by P11 (Fig. 1). The expression pattern of BMPRIA mRNA was similar to that of BMPRII, except it increased from P6 through P8 and P10 before being stable by P11 (Fig. 1). The levels of actin mRNA remained steady throughout development (Fig. 1), indicating the specificity of BMPR gene expression.
Figure 1.
Quantitative RT-PCR analysis of BMPRIB, BMPRII, BMPRIA, and actin mRNA levels in hamster ovaries during perinatal development. Bars with a different letter; P < 0.05, bars with the same letter, P > 0.05.
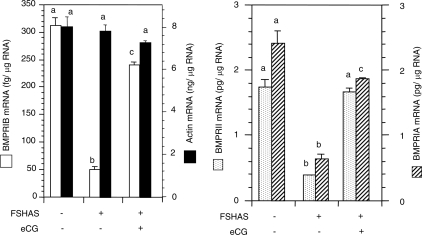
The rationale was to determine whether FSH action, which was shown to be essential for primordial follicle formation in the hamster (13), would also influence BMPR mRNA expression during the formation of primordial follicles in neonatal hamster ovaries. Exposure of E12 pups in utero to preimmune rabbit IgG did not affect BMPR mRNA levels on P8; however, FSH antiserum significantly reduced the receptor mRNA levels (Fig. 2). Treatment with eCG on P1 completely restored BMPRII mRNA and significantly reversed the inhibitory action of the antiserum on BMPRIB and BMPRIA mRNA levels (Fig. 2). Neither the FSH antiserum nor the eCG treatment had any effect on actin mRNA levels (Fig. 2).
Figure 2.
Quantitative RT-PCR analysis of BMPRIB, BMPRII, BMPRIA, and actin mRNA in ovaries of P8 hamsters exposed in utero to preimmune rabbit serum or FSH antiserum (FSHAS) at E12 and received a single injection of eCG or saline on P1. Bars with a different letter, P < 0.05; bars with the same letter, P > 0.05.
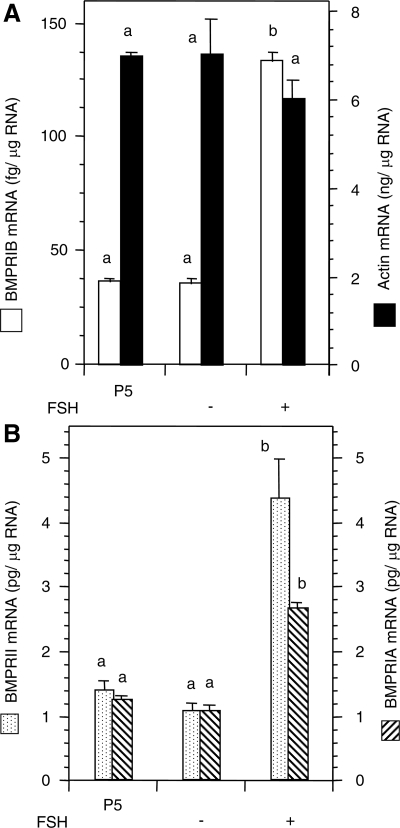
The rationale was to determine whether increases in BMPR mRNA expression in eCG-treated hamsters were indeed due to FSH action on ovarian somatic cells or secondary to eCG induction of primordial follicle formation. We selected ovaries of P4 hamsters to avoid any influence of primordial follicles in BMPR induction and for obtaining adequate amounts of RNA for subsequent analysis. Because P4 ovaries cultured for 24 h would be equal to P5 ovaries, comparison of mRNA levels was done with in vivo developed P5 ovaries. Exposure of P4 ovaries to FSH for 24 h significantly (P < 0.05) up-regulated the levels of BMPRIA, BMPRIB, and BMPRII without affecting the levels of actin mRNA (Fig. 3). Similar to in vivo developed P5 ovaries, the levels of BMPRIA and BMPRII mRNA levels were 3 orders of magnitude higher than that of the BMPRIB (Fig. 3). Nevertheless, 4-fold increase in mRNA levels was noted for BMPRIB and BMPRII, whereas BMPRIA mRNA levels increased approximately 2-fold (Fig. 3, A and B).
Figure 3.
Quantitative RT-PCR analysis of BMPRIB and BMPRII (A), BMPRIA (B), and actin (A and B) mRNA expression in P4 hamster ovaries cultured 24 h in the absence or presence of 1 ng/ml of ovine FSH-20. Because P4 ovaries would be equal to P5 ovaries after 24 h culture, in vitro data were compared with P5 ovaries developed in vivo to examine the effect of FSH. Bars with a different letter, P < 0.05; bars with the same letter, P > 0.05.
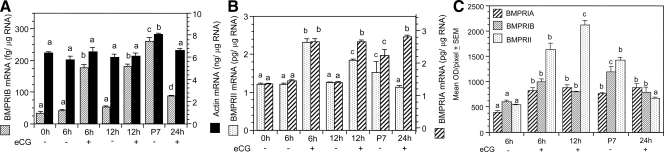
The rational was to determine whether FSH would temporally regulate the levels of BMPR mRNA. We used eCG to avoid repeated injection of pups, which resulted in maternal cannibalism and induced unnecessary pain. Because ovaries exposed to eCG on P6 would become of P7 after 24 h, comparison was done with normal P7 ovaries. Furthermore, the experiments were restricted to a 24-h period to avoid any confounding variable that might be imposed by the development of primordial follicles on P8. This approach did not compromise with the objective of the study. Injection of eCG on P6 resulted in a significant increase in the amount of all BMPR mRNA by 6 h compared with saline-treated controls (Fig. 4, A and B). Whereas the levels of mRNA remained steady for BMPRIA and BMPRIB up to 12 h, BMPRII mRNA started to decrease (Fig. 4, A and B). Thereafter mRNA levels of both BMPRIB and BMPRII decreased markedly, but the levels of BMPRIA remained high (Fig. 4B). The levels of BMPR mRNA increased significantly on P7 compared with P6, but no change was observed on any other time in P6, and no change in actin mRNA levels was visible (Fig. 4, A and B).
Figure 4.
Temporal effects of eCG on BMPRIB, BMPRII, BMPRIA, and actin mRNA levels (A and B), and on BMPR protein expression (C). Six-day-old female hamsters were injected sc with 20 IU eCG or vehicle, and ovaries were collected at 0, 6, 12, and 24 h after the injection. P7 ovaries served as controls for the 24-h-exposure group. Immunofluorescence signal was quantified using the NIH Image J software, and the results were expressed as the mean OD/pixel ± sem. Bars with a different letter, P < 0.05; bars with the same letter, P > 0.05.
Spatiotemporal expression of BMPRIB, BMPRII and BMPRIA protein during perinatal ovary development and FSH withdrawal and eCG replacement in vivo
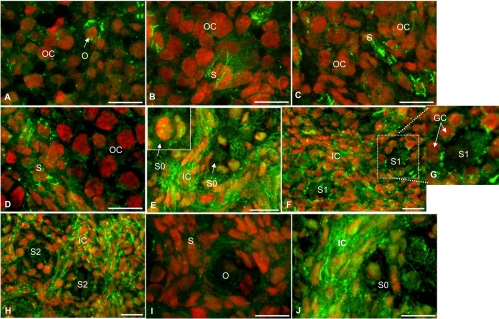
Overall, BMPR protein expression correlated with receptor mRNA expression. Low intensity of BMPRIB expression was observed for somatic cells on E13, but the oocytes had a distinct expression (Fig. 5A). The expression pattern remained stable through P3 with scattered somatic cells showing appreciable expression (Fig. 5, B and C). Whereas BMPRIB expression in somatic cells surrounding the egg nest tended to increase by P6, considerable receptor expression was evident in newly formed granulosa cells of primordial follicles and in large number of interstitial cells by P8 (Figs. 5, D and E, and 7A). By P10-15, BMPRIB expression was evident in all types of cells, including the oocytes, although interstitial cell compartment showed relatively higher expression (Fig. 5, F and H). Notably increased BMPRIB expression in granulosa cells was prominent at the apical side adjacent to the oocyte plasma membrane (Fig. 5G).
Figure 5.
Immunofluorescence localization of BMPRIB protein in hamster ovary sections during perinatal development and after in vivo exposure to FSH antiserum. For the sake of brevity, images of selected days are furnished to highlight the pattern of developmental expression. E13 and E15 (A and B), P3 and P6 (C and D), P8 (E, insert) show receptor expression in the granulosa cells and oocyte of a primordial follicle. P10 (F and G, insert) show increased BMPRIB expression at the oocyte plasma membrane and granulosa cells interface of an S1 follicle on P10. P15 (H) and ovaries of P8 hamsters (I and J) exposed in utero to an FSH antiserum on E12 and received a single injection of saline (I) or eCG (J) on P1. OC, Oocyte cluster; O, oocyte; S, somatic cells; GC, granulosa cell; S0, primordial follicle; S1, primary follicle; S2, preantral follicles at stage 2; IC, interstitial cells.
Figure 7.
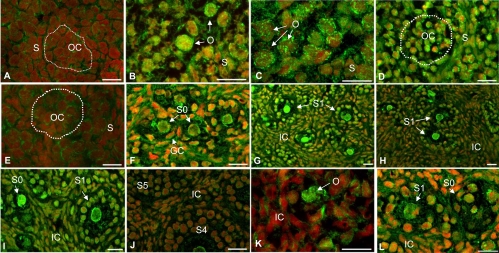
Immunofluorescence localization of BMPRIA protein in hamster ovary sections during perinatal development and after in vivo exposure to FSH antiserum. For the sake of brevity, images of selected days of development are furnished to highlight the pattern of developmental expression. E13 and E15 (A and B), P1, P5, P6, P8, P10, P13, P14, and P15 (C-J), and ovaries of P8 (K and L) hamsters were exposed in utero to an FSH antiserum on E12, and received a single injection of saline (K) or eCG (L) on P1. OC, Oocyte cluster circled by dotted line, O, oocyte; S, somatic cells; GC, granulosa cell; S0, primordial follicle; S1, primary follicle; S4 and S5, large preantral follicles at stage 4 and 5; IC, interstitial cells.
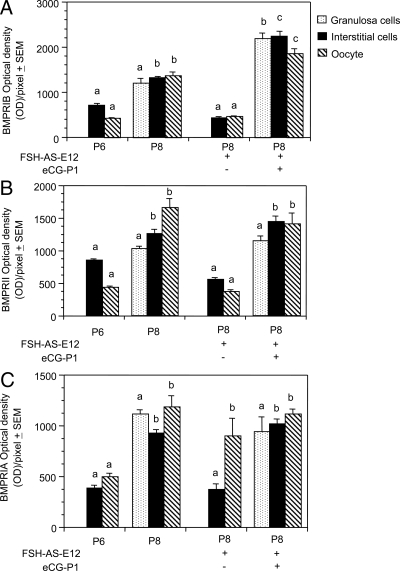
In utero exposure to the FSH antiserum markedly attenuated BMPRIB expression in P8 ovaries, but eCG treatment on P1 not only reversed the inhibition (see Figs. 5, I and J, and 8A) but stimulated the receptor expression in all cell types (see Fig. 5J and 8A).
Figure 8.
Quantification of BMPR immunofluorescence. A, BMPRIB. B, BMPRII. C, BMPRIA. For the sake of brevity, OD/pixel of each receptor before and after normal primordial follicle formation and after FSH antiserum and eCG treatments were presented. Each bar represents a mean of three ovaries ± sem. The intensities for the granulosa cells, interstitial cells, and oocytes of one group were compared with the granulosa cells, interstitial cells, and oocytes, respectively, of other groups. Bars with a different letter, P < 0.05; bars with the same letter, P > 0.05.
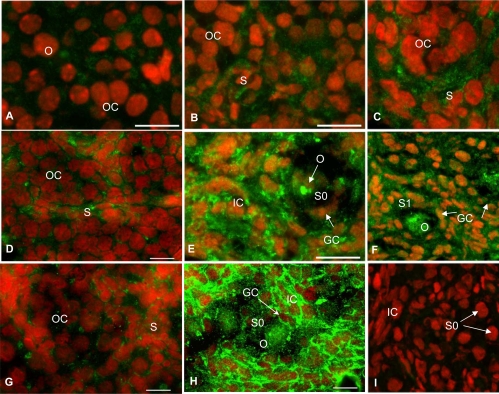
Low-intensity BMPRII expression was evident on E13, and the receptor expression became prominent on E15 (Fig. 6, A and B). More somatic cells and oocytes expressed BMPRII with further ovarian development through P6 (Fig. 6, C and D) and P7 (data not shown). The expression of receptor in the interstitial cells and oocytes increased markedly by P8, whereas the newly formed granulosa cells of primordial follicles showed distinct receptor expression (Figs. 6E and 7B). The intensity of BMPRII expression decreased markedly by P10, but receptor expression was present in all cells in follicular and nonfollicular compartments (Fig. 6F). BMPRII expression remained at P10 levels through subsequent postnatal days (data not shown).
Figure 6.
Immunofluorescence localization of BMPRII protein in hamster ovary sections during perinatal development and after in vivo exposure to FSH antiserum. For the sake of brevity, images of selected days are furnished to highlight the pattern of developmental expression. E13 and E15 (A and B), P1, P6, P8, and P10 (C–F), ovaries of P8 (G and H) hamsters were exposed in utero to an FSH antiserum on E12 and received a single injection of saline (G) or eCG (H) on P1. I, Section of a P8 ovary incubated without the primary antibody. OC, Oocyte cluster; O, oocyte; S, somatic cells; GC, granulosa cell; S0, primordial follicle; S1, primary follicle; S2, preantral follicle at stage 2; IC, interstitial cells.
FSH antiserum treatment on E12 significantly suppressed BMPRII expression in P8 ovaries (Fig. 6G), but eCG treatment on P1 restored the receptor expression to the untreated P8 level (Figs. 6H and 8B). No receptor-specific immunostaining was present without the primary antibody (Fig. 6I) indicating the specificity of the BMPRII immunoreactivity. Similar results were obtained for BMPRIB and BMPRIA without the primary antibodies (data not shown).
A homogeneous but low intensity expression of BMPRIA was evident in E13 ovaries (Fig. 7A), but the expression increased appreciably both in the oocytes and somatic cells on E15 and remained high until P5 (Fig. 7, B–D). BMPRIA expression in both somatic cells and the oocytes decreased momentarily on P6 (Fig. 7E) followed by an attributable increase by P8 (Figs. 7F and 8C). Whereas BMPRIA expression in the granulosa cells and oocytes remained steady until P14, receptor expression in the interstitial cells decreased on P13 followed by a recovery on P14 (Fig. 7, G–I). BMPRIA expression then decreased significantly in all three cell compartments by P15 (Fig. 7J) and remained low thereafter (data not shown).
Treatment with the FSH antiserum on E12 significantly attenuated BMPRIA expression in the somatic cells without affecting the expression in the oocytes on P8 (Figs. 7K and 8C). The inhibition was completely reversed by treating P1 hamsters with a single dose of eCG (Figs. 7L and 8C).
Because there was no visible primordial follicle in P6 or P7 ovaries, immunosignal was recorded only from the somatic cells. Consistent with the increase in mRNA levels by 6 h after the eCG injection, immunosignal corresponding to all three BMPR proteins increased at least 2-fold, although BMPRII immunosignal showed the maximum increase (Fig. 4C); however, the intensity of BMPR immunosignal in vehicle-treated P6 ovaries at 6 or 12 h was identical with that of ovaries at 0 h (data not shown), thus corroborating the results presented in Figs. 5–7. Receptor immunosignal also increased markedly on P7 compared with P6, indicating a shift toward endogenous receptor expression (Fig. 4C). Whereas BMPRIA immunosignal was similar to that of P7, the level of BMPRIB was lower than that of P7, although it remained steady at the 12-h level (Fig. 4C). BMPRII protein level decreased remarkably 24 h after the eCG injection (Fig. 4C), suggesting a differential processing of the receptor proteins.
Discussion
The results provide the first evidence of the ontogeny of BMP receptor expression during perinatal ovary development, especially during the formation of the first cohort of primordial follicles in the ovary, and a possible role of FSH on receptor expression during this critical phase of follicular morphogenesis. The results also suggest that increased expression of all three forms of the BMP receptor correlates also with the formation of primordial follicles. Although the presence of BMPRIA, BMPRIB, and BMPRII in ovarian cells have been reported (1), their potential involvement during follicular development remains unknown. The hormonal regulation of BMPR expression in the ovary also remains largely unknown. Our data indicate that activation of FSH receptor by eCG up-regulates all three forms of BMPR in the primordial granulosa cells, but it up-regulates only BMPRIB and BMPRII, not BMPRIA expression in the oocytes. The significant increases in all three BMPR mRNA after FSH exposure in vitro suggest strongly that eCG-induced up-regulation of BMPR mRNA and proteins in postnatal ovaries is indeed due to FSH effect on ovarian somatic cells rather than secondary to the reversal of primordial follicle formation. This is further supported by the fact that all three forms of BMPR are expressed before the appearance of primordial follicles on P8. Additionally, the precocious temporal expression of receptor mRNA and proteins by exogenous eCG strongly suggests that somatic cells in the postnatal ovary are responsive to FSH stimulus in terms of BMPR expression, and BMPR expression depends on the gradual increase in FSH action during the critical period of primordial follicle formation. Previously we have shown that FSH is present in the plasma of newborn hamsters, and the levels increase gradually from P3 onward (27). Additional studies are in progress to examine the role of FSH in BMPR expression.
The remarkable difference in BMPRII expression between P1 and P8 suggests that translational of the mRNA is developmentally regulated; however, the exact mechanism(s) is not known at this time. The mRNA and protein levels do not correlate for type II receptor of the TGFβ family of ligands (28). Intense TGFBRII immunosignal in the hamster ovary (29), coincides with very low levels of mRNA (Roy, S. K., unpublished observation). The role of μRNA in posttranslational regulation of mRNA levels is emerging for many tissues and cells types (30). Whether such a mechanism is in play for BMPRII mRNA remains to be examined. Previously we have shown that in vivo neutralization of endogenous FSH with an anti-FSH antiserum during fetal ovary development results into significant attenuation of primordial follicle formation, which is reversed by eCG treatment on P1 (13). Using similar experimental paradigms, we provide evidence that neutralization of endogenous FSH results in decreased BMPRIA, BMPRIB, and BMPRII mRNA as well as protein expression in postnatal ovaries. Therefore, we postulate that FSH may modulate BMPR transcription and translation in postnatal ovaries, and BMP receptor signaling may be important for somatic cell differentiation into primordial granulosa cells. Restoration of BMPR mRNA and protein levels by eCG concurrent with primordial follicle formation (13).
Based on the in vitro effect of FSH and the temporal effect of eCG in vivo on ovarian BMPR mRNA, it is logical to postulate that BMPR expression; hence, BMP action may be important for ovarian cell differentiation during primordial follicle formation. Undifferentiated granulosa cells in fetal and neonatal hamster ovaries express a full-length FSH receptor mRNA (Roy, S. K., unpublished observation), and FSH stimulates CYP19 gene expression and estradiol-17β (E2) synthesis in 4-d-old hamster ovaries long before the formation of primordial follicles (19). The temporal expression patterns of BMPR mRNA and proteins after eCG administration suggest that FSH differentially regulates the expression of receptor isoforms in the postnatal ovary. The decrease in the levels of BMPRIB or BMPRII on P7 despite the availability of the FSH stimulus suggests that somatic cells may become refractory to a chronic FSH stimulus when prematurely exposed to a relatively higher amplitude of such stimulus. In contrast, the steady high levels of BMPRIA mRNA despite an attributable decrease in receptor protein levels 24 h after eCG injection indicates that such refractoriness and intracellular processing of the receptor proteins may be specific to BMPR isoforms. Studies are in progress to evaluate this issue further.
The decrease in BMPRIB and BMPRII proteins in the oocytes after FSH neutralization and its reversal by eCG suggest that these two forms of BMP receptor may play an important role in regulating oocyte activities critical to oocyte-somatic cell interaction. Eppig and coworkers (31,32,33,34,35) elegantly demonstrated the importance of oocyte and somatic cell communication in follicle formation. Although BMPRIB is not present in mouse primordial follicles, BMPRIB−/− mice are infertile due to defects in cumulus cell expansion in vivo and the reproductive tract (6). In contrast, BMPRIA but not BMPRIB mRNA has been detected in the gonad primodium of midgestation mouse embryos (25), and deletion of BMPRIA has been shown to be embryo lethal on E7 due to defect in mesodermal differentiation and gastrulation (36). In situ hybridization studies have revealed that rat oocytes express detectable levels of BMPRIA and BMPRIB, and low levels of BMPRII mRNA, whereas the granulosa cells of primordial follicles express modest levels of BMPRIA and BMPRIB but not BMPRII mRNA (1). Similarly, immunohistochemical studies have shown that in the sheep ovary, BMPR expression in the granulosa cells and oocytes occurs from primary follicles onward (37). In contrast, the results of the present study provide clear evidence that BMPR expression occurs not only in the granulosa cells and oocytes of primordial follicles but also in undifferentiated somatic cells before the formation of primordial follicles, thus highlighting a species difference in BMPR expression. The low levels of BMPRII protein before P6 despite mRNA levels similar to P8 suggest that BMPRII expression may be posttranslationally regulated. The significance of a sharp decrease in BMPRIA protein primarily in the interstitial cells on P13 is not clear at present, although the expression correlates well with the decrease in BMPRIA mRNA on P12. Serum levels of LH in the hamster start to increase from P12/P13 and onward (38), and the increase corresponds with the appearance of LH receptor (Roy, S. K., unpublished observation) and CYP11A1 (39) in the interstitial cells. Whether the initiation of LH action in the interstitial cells affects BMPRIA expression remains to be determined.
Despite partial cDNA sequences, similarities between the hamster BMPRIA, BMPRIB, and BMPRII with the corresponding regions of those of other species suggest that BMP receptors are highly conserved in mammals. This is further supported by the presence of 10 cysteine residues in the extracellular domain of hamster BMPRII similar to that of mouse and human (22,24). The presence of the transmembrane domain and phosphorylation sites in hamster BMPRIA and BMPRIB confirms their membrane receptor characteristic and a potential substrate for BMPRII receptor kinase typical of TGFβ family of receptors (40,41). This novel information about the hamster BMPR will be useful for cloning the full-length cDNA, identifying receptor genes from the genomic library and targeting specific sites of the receptors for understanding their biological functions in the hamster. The presence of both type I and type II BMPR in the hamster ovary also suggests that similar to other TGFβ family of ligands (42), BMP action on ovarian cells requires the interaction between the receptor type I and type II receptors. This is further supported by the presence of cytoplasmic kinase domain in BMPRII, which is similar to that present in TGFBRII and is necessary for transphosphorylation of type I receptor on ligand binding (3). The presence of putative serine and threonine phosphorylation sites in hamster BMPRIA and BMPRIB corroborates previous findings (25). Furthermore, BMPRII can interact with either BMPRIA or BMPRIB, or activin receptor ActR, and the interaction is determined by BMP ligands (3).
Although primordial follicles have been suggested to be the target of BMP, the unique BMPR mRNA and protein expression coinciding with the appearance of the first cohort of primordial follicles in the ovary suggests that BMP may also be involved in somatic cell differentiation into granulosa cells and oocyte-somatic cell communication. Perinatal hamster ovarian cells express BMP-2 and BMP-4 mRNA (Roy, S. K., unpublished observation), and BMP-2 and BMP-4 are ligands for BMPRIA and BMPRIB (1). BMP-4 (7) and BMP-7 (1) have been shown to stimulate primordial follicle activation in the rat. Furthermore, GDF-9, which acts via BMPRII (1,43,44) and TGFBRI (45), is produced in the hamster oocytes before the formation of primordial follicles (10) and plays an essential role in primordial follicle formation in the hamster (11). GDF-9 expression in the oocytes of human, sheep, and cattle primordial follicles has also been reported (1). Unlike the rat, mouse, or sheep (1), no BMP-15 mRNA or protein can be detected in the hamster oocytes using RT-PCR or a BMP-15 antibody (generously provided by Dr. S. Shimasaki, University of California, San Diego, CA) irrespective of the stage of follicular development. Although ovarian expression of BMP and BMPR has been reported for many species (1), whether endogenous hormone(s) regulate the expression, especially during neonatal ovary development, is poorly understood. E2 at 100 ng/ml dose level stimulates BMPRII mRNA expression in cultured bovine granulosa cells obtained from large antral follicles, whereas the same dose of E2 or progesterone has no effect on BMPRIA mRNA levels (46). Furthermore, whereas FSH alone at 1, 5, or 10 ng/ml dose level suppresses BMPRII mRNA expression in cultured bovine granulosa cells, the 5 or 10 ng/ml dose can stimulate BMPRII mRNA levels to the same degree in the presence of 1 ng/ml E2, which by itself has no effect on BMPRII mRNA expression (46).
The explanation of such a paradoxical effect remains unclear. However, the increase in BMPR expression in P8 hamster ovaries coincides with a serum level of 10 pg/ml FSH (13) and increasing levels of E2 (19). Furthermore, present results show that FSH stimulates BMPR mRNA expression in vitro in 4-d-old hamster ovaries, and eCG temporally up-regulates BMPR mRNA and protein expression in P6 ovaries before the scheduled endogenous increases. Both E2 and FSH stimulate primordial follicle formation in the hamster (13,19). FSH also up-regulates GDF-9 expression in hamster primordial oocytes (10). The presence of GDF-9 in the oocytes (10) and BMPR in the oocytes as well as in surrounding somatic cells lends credence to the hypothesis that BMP system serves as a mechanism of oocyte-somatic cell communication during primordial follicle formation.
In summary, the results of the present study provide the first evidence for a unique BMPR expression during perinatal ovary development, especially during the formation of primordial follicles, and a positive relationship between FSH action and ovarian BMPR expression. The attenuation of BMPR mRNA and protein expression concurrent with a decrease in primordial follicle formation (13) after FSH withdrawal and its reversal after eCG treatment provide strong evidence that BMPR action may play an important for somatic cell differentiation and perhaps oocyte-somatic cell communication, leading to the formation of primordial follicles in the hamster. The study also provides the first hamster-specific nucleic acid and amino acid information of BMPR that can be useful to investigators using this species in research.
Acknowledgments
We thank Dr. Carl Heldin for generously providing the BMP-receptor antibodies.
Footnotes
This work was supported by Grant R01-HD38468 from the National Institute of Child Health and Human Development and Olson Foundation, Obstetrics and Gynecology, University of Nebraska Medical Center (to S.K.R.). C.W. is a recipient of the Lalor Foundation postdoctoral fellowship.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 12, 2008
Abbreviations: BMP, Bone morphogenetic protein; BMPR, BMP receptor; E, embryonic; E2, estradiol-17β; eCG, equine chorionic gonadotropin; F, forward; GDF, growth differentiation factor; P, postnatal day; R, reverse.
References
- Shimasaki S, Moore RK, Otsuka F, Erickson GF 2004 The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101 [DOI] [PubMed] [Google Scholar]
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K 2001 Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci 114:1483–1489 [DOI] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J 1995 Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 15:3479–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Celeste AJ, Thies RS, Song JJ, Bernier SM, Goltzman D, Lyons KM, Nove J, Rosen V, Wozney JM 1994 A mammalian serine/threonine kinase receptor specifically binds BMP-2 and BMP-4. Biochem Biophys Res Commun 205:1944–1951 [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T 1999 Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci 112 (Pt 20):3519–3527 [DOI] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM 2001 The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci USA 98:7994–7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK 2003 Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 69:1265–1272 [DOI] [PubMed] [Google Scholar]
- Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL 2008 The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology 149:1026–1030 [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM 1996 Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- Wang J, Roy SK 2004 Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod 70:577–585 [DOI] [PubMed] [Google Scholar]
- Wang C, Roy SK 2006 Expression of growth differentiation factor 9 in the oocytes is essential for the development of primordial follicles in the hamster ovary. Endocrinology 147:1725–1734 [DOI] [PubMed] [Google Scholar]
- Yang P, Wang J, Shen Y, Roy SK 2004 Developmental expression of estrogen receptor (ER)α and ERβ in the hamster ovary: regulation by follicle-stimulating hormone. Endocrinology 145:5757–5766 [DOI] [PubMed] [Google Scholar]
- Roy SK, Albee L 2000 Requirement for follicle-stimulating hormone action in the formation of primordial follicles during perinatal ovarian development in the hamster. Endocrinology 141:4449–4456 [DOI] [PubMed] [Google Scholar]
- Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S 2001 Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J 20:4132–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SK 2000 Regulation of transforming growth factor-β-receptor type I and type II messenger ribonucleic acid expression in the hamster ovary by gonadotropin and steroid hormone. Biol Reprod 62:1858–1865 [DOI] [PubMed] [Google Scholar]
- Hubbard GM, Rojas FJ 1994 Stimulation of ovarian adenylyl cyclase activity by gonadotrophins during the natural and gonadotrophin-induced cycles in the hamster. Hum Reprod 9:2247–2254 [DOI] [PubMed] [Google Scholar]
- Chiras DD, Greenwald GS 1978 Ovarian follicular development in cyclic hamsters treated with a superovulatory dose of pregnant mare's serum. Biol Reprod 19:895–901 [DOI] [PubMed] [Google Scholar]
- Greenwald GS 1974 Quantitative aspects of follicular development in the untreated and PMS-treated cyclic hamster. Anat Rec 178:139–143 [DOI] [PubMed] [Google Scholar]
- Wang C, Roy SK 2007 Development of primordial follicles in the hamster: role of estradiol-17β. Endocrinology 148:1707–1716 [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK 2007 Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology 148:4853–4864 [DOI] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, Chen GX, Wrana JL, Massague J, Rosenbaum JS 1994 Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol 14:5961–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu H, Minowa O, Miyazono K, Kawabata M 1997 cDNA cloning and genomic organization of the mouse BMP type II receptor. Biochem Biophys Res Commun 235:499–504 [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, Heldin CH 1994 Characterization of type I receptors for transforming growth factor-b and activin. Science 264:101–104 [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K 1995 Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 92:7632–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P 1995 Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology 136:2652–2663 [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J 1994 Mechanism of activation of the TGF-β receptor. Nature 370:341–347 [DOI] [PubMed] [Google Scholar]
- Roy SK, Hughes J 1994 Ontogeny of granulosa cells in the ovary: lineage-specific expression of transforming growth factor β2 and transforming growth factor β1. Biol Reprod 51:821–830 [DOI] [PubMed] [Google Scholar]
- Massague J, Attisano L, Wrana JL 1994 The TGF-β family and its composite receptors. Trends Cell 4:172–178 [DOI] [PubMed] [Google Scholar]
- Roy SK, Kole AR 1995 Transforming growth factor-β receptor type II expression in the hamster ovary: cellular site(s), biochemical properties, and hormonal regulation. Endocrinology 136:4610–4620 [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Maniatis T 2008 Multilevel regulation of gene expression by microRNAs. Science 319:1789–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccione R, Schroeder AC, Eppig JJ 1990 Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod 43:543–547 [DOI] [PubMed] [Google Scholar]
- Eppig JJ 2001 Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ 2002 Intracellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178–2180 [DOI] [PubMed] [Google Scholar]
- Eppig JJ 1991 Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 13:569–574 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL 2002 The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 99:2890–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR 1995 Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9:3027–3037 [DOI] [PubMed] [Google Scholar]
- Souza CJ, Campbell BK, McNeilly AS, Baird DT 2002 Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction 123:363–369 [PubMed] [Google Scholar]
- Vomachka AJ, Greenwald GS 1979 The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology 105:960–966 [DOI] [PubMed] [Google Scholar]
- Schwartz JR, Roy SK 2000 Developmental expression of cytochrome P450 side-chain cleavage and cytochrome P450 17α-hydroxylase messenger ribonucleic acid and protein in the neonatal hamster ovary. Biol Reprod 63:1586–1593 [DOI] [PubMed] [Google Scholar]
- Attisano L, Cárcamo J, Ventura F, Weis FMB, Massagué J, Wrana JL 1993 Identification of human activin and TGFβ type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75:671–680 [DOI] [PubMed] [Google Scholar]
- Vivien D, Attisano L, Wrana JL, Massagué J 1995 Signaling activity of homologous and heterologous transforming growth factor-β receptor kinase complexes. J Biol Chem 270:7134–7141 [DOI] [PubMed] [Google Scholar]
- Derynck R, Feng XH 1997 TGF-β receptor signaling. Biochim Biophys Acta 1333:F105–F150 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Sangkuhl K, Luo CW, Sudo S, Klein C, Hsueh AJ 2005 Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. J Biol Chem 280:32122–32132 [DOI] [PubMed] [Google Scholar]
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJ 2002 Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod 67:473–480 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJ 2004 Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol 18:653–665 [DOI] [PubMed] [Google Scholar]
- Jayawardana BC, Shimizu T, Nishimoto H, Kaneko E, Tetsuka M, Miyamoto A 2006 Hormonal regulation of expression of growth differentiation factor-9 receptor type I and II genes in the bovine ovarian follicle. Reproduction 131:545–553 [DOI] [PubMed] [Google Scholar]