Abstract
Insufficient angiogenesis is one of the causes leading to tissue ischemia and dysfunction. In heart failure, there is increasing evidence showing decreased capillary density in the left ventricle (LV) myocardium, although the detailed mechanisms contributing to it are not clear. The goal of this study was to investigate the role of thyroid hormone receptors (TRs) in the coronary microvascular rarefaction under pathological cardiac hypertrophy. The LV from hypertrophied/failing hearts induced by ascending aortic constriction (AAC) exhibited severe microvascular rarefaction, and this phenomenon was restored by chronic T3 administration. Coronary endothelial cells (ECs) isolated from AAC hearts expressed lower TRβ mRNA than control ECs, and chronic T3 administration restored TRβ mRNA expression level in AAC hearts to the control level. Among different TR subtype-specific knockout mice, TRβ knockout and TRα/TRβ double-knockout mice both exhibited significantly less capillary density in LV compared with wild-type mice. In vitro, coronary ECs isolated from TRβ knockout mice lacked the ability to form capillary networks. In addition, we identified that kinase insert domain protein receptor/fetal liver kinase-1 (vascular endothelial growth factor-2 receptor) was one of the angiogenic mediators controlled by T3 administration in the AAC heart. These data suggest that TRβ in the coronary ECs regulates capillary density during cardiac development, and down-regulation of TRβ results in coronary microvascular rarefaction during pathological hypertrophy.
The loss of small blood vessels in the heart with thickened wall is caused by a reduced number of thyroid receptor.
Adequate capillary supply is important for tissue survival and proper function. Abnormalities of the microvasculature contribute to the pathogenesis of some forms of heart disease. In heart failure, myocardial hypertrophy and accelerated heart rhythm enhance oxygen demand, whereas supply is hampered by endothelial dysfunction (e.g. vascular rarefaction caused by capillary endothelium apoptosis and deficient growth of the microvasculature) and a thickened diffusion barrier (e.g. fibrosis, arterial hyperplasia, and myocyte hypertrophy). Increasing capillary density in the heart is therefore one of the potential therapeutic strategies to restore the impaired oxygen supply to the failing heart (1,2,3).
Thyroid hormone (TH) treatment exerts beneficial effects in the cardiovascular system, such as lowering cholesterol levels and low-density lipoprotein levels and enhancing cardiac contractile function (4,5). Recently developed novel TH analogs lead to lipid lowering and improvement in cardiac function (6,7). It also needs to be noted that TH results in an increased heart rate and arrhythmia (5,8,9). The actions of TH occur largely through its binding to the thyroid hormone receptor (TR), although rapid nongenomic effects have been described (10). Two genes, TRα and TRβ (11), encode TRs and at least two TRα isoforms and three TRβ isoforms have been identified. TRα1, TRβ1, TRβ2, and TRβ3 isoforms bind to T3, whereas TRα2 does not bind to T3 and functions, at least in vitro, as a TRα1 and TRβ1 antagonist (12). Consistent evidence has shown that TRα1 and TRβ1 expression in the heart is down-regulated in pathological hypertrophy (13,14,15); however, it is unclear whether TR regulates capillary density in pathological hypertrophy and which TR subtype controls angiogenesis.
Vascular endothelial cells (ECs) express TRs and according to some reports TRα is the predominant TR isoform (16,17). The effects of TH on microvascular formation, however, are still controversial. It has been demonstrated in some studies that TH or TH analog have the capability to induce angiogenesis (18,19,20), whereas others showed that TH has no effect on angiogenesis (21,22). It is also unclear whether TH affects microvascular formation through its direct effect on ECs or indirect effect on surrounding cells (e.g. cardiac myocytes) to release proangiogenic factors. In this study, we focused on TR subtypes in ECs and investigated whether TRs regulate coronary microvascular formation during cardiac hypertrophy.
Materials and Methods
Antibodies and reagents
M199, antibiotic reagents (Invitrogen Corp., Carlsbad, CA), anti-TRβ, antikinase insert domain protein receptor (KDR)/fetal liver kinase-1 (Flk1), antiplatelet/endothelial cell adhesion molecule 1 (PECAM), antiactin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), EC growth supplement, Matrigel (BD Biosciences, San Jose, CA), collagenase II (Worthington Biochemical Corp., Lakewood, NJ), dispase II (Roche Diagnostics North America, Indianapolis, IN) were used in this study. All other chemicals were from Sigma-Aldrich, Inc. (St. Louis, MO).
Animal preparation
Pressure overload was created in male mice (NIH Swiss, 6 wk old; Harlan Sprague Dawley, Inc. Indianapolis, IN) by ascending aortic constriction (AAC) as previously described (15,23). Mice were anesthetized with a mixture of ketamine (100 mg/kg, ip) and xylazine (5 mg/kg, ip) before surgery. All data were obtained from mice at 10 wk after AAC. T3 administration was started at 8 wk after AAC and continued for 2 wk (3.5 ng/g body weight, ip daily). Plasma T3 levels were 85.2 ± 4 ng/dl in control, 57.4 ± 4.9 ng/dl in AAC (P < 0.05 vs. control), and 68.7 ± 3.3 ng/dl in T3 + AAC group. This study was conducted in accordance with the guidelines established by the institutional Animal Care and Use Committee at the University of California, San Diego.
TR knockout (KO) mice
TRα1 KO animals were generated in the laboratory of Dr. B. Vennstrom (Karolinska Institute, Stockholm, Sweden) as described previously (24). TRβ KO animals were generated in the laboratory of Dr. J. Samarut (Ecole Normale Superieure de Lyon, Lyon, France), by deletion of exons 4 and 5 of the TRβ gene (25). TRα/TRβ double-knockout mice were generated by crossing the TRα1 KO and TRβ KO lines until homozygous for both deletions were obtained. Inducible cardiac myocyte-specific TRβ deletion was achieved by crossing tamoxifen-inducible myosin heavy chain α-Cre mice (26) with mice in which exon 5 of the TRβ gene is flanked by loxP sites (27). Cardiac myocyte-specific TRβ KO mice and littermate controls were treated with tamoxifen at a dosage of 20 mg/kg body weight once a day for 3 consecutive days. Mice were used 4 wk after last tamoxifen injection.
Normalizing T3 levels in TRβ KO mice
TRβ KO mice and wild-type mice were fed for 5 wk with iodine-deficient, 0.15% 6-propyl-2-thiouracil food pellets (Harlan-Teklad, Madison, WI). The first week, the animals were fed 6-propyl-2-thiouracil diet to decrease their endogenous hyperthyroidism. During the remaining 4 wk, daily ip injections of T3 were given in saline at a dosage of 3.5 ng/g body weight. After 4 wk of this treatment, animals were used for experiments.
Analysis of capillary densities in left ventricle (LV) myocardium
After 10 wk of AAC, capillary density was determined in mouse hearts using previously described methods (28). The ventricle was dissected, embedded in optimal cutting temperature compound (Sakura Finetek USA, Inc. Torrance, CA), frozen in 2-methylbutane precooled with liquid nitrogen, and then kept at −80 C until sectioned. Sections (6 μm) were fixed in 4% formaldehyde for 5 min, blocked with 5% BSA for 30 min, and incubated with Bandeiraea Simplicifolia lectin (BS-l)-fluorescein isothiocyanate for 30 min. Subepicardial regions of the LV free wall on the section were photographed in sequence by a charge-coupled device camera connected to a fluorescence microscope with a ×20 objective lens. For every experimental condition, at least two sections from each sample were examined, and at least eight microscopic fields were investigated. Capillary count was analyzed with ImageJ 1.33 (National Institutes of Health, Bethesda, MD) and the average capillary numerical density [number of capillaries (NA) per square millimeter] was calculated for each heart.
Isolation of coronary vascular endothelial cells
Mouse coronary ECs were isolated using a modification of previously described methods (28). Briefly, dissected heart tissues were minced and incubated with M199 containing 1 mg/ml collagenase II and 0.6 U/ml dispase II, for 1 h at 37 C. The digested material was filtered through sterile 40 μm nylon mesh and washed in 2% fetal calf serum-M199. Subsequently the cells were incubated with Dynabeads (Invitrogen), which were prepared as follows: M-450 Epoxy dynabeads were incubated with BS-l (1 mg/ml) at 4 C for 2 d, washed with PBS containing 0.1% BSA, and incubated overnight at 4 C. The cell suspension was incubated with beads for 30 min at 4 C and then the beads attached to ECs were captured by Dynal magnet (Invitrogen).
Assay of TR mRNA
cDNA templates for PCR were generated using oligo (dT)12–18 (Invitrogen) and Superscript III (Invitrogen) following the manufacturer's instructions. Gene expression data for TRα (assay ID Mm00617505_m1), TRβ (Mm00437044_m1), KDR/Flk1 (Mm01222419_m1), PECAM1 (Mm00476702_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mm99999915_g1) were obtained using TaqMan Assays-on-Demand (Applied Biosystems Foster City, CA). Ten nanograms of cDNA were used in each 25-μl reaction. All samples were assayed in duplicate on the ABI Prism 7300 real-time PCR system using standard run cycle conditions. Threshold cycle values of the target genes were normalized to the endogenous control gene (GAPDH). Differential expression in AAC samples or T3-treated samples relative to control samples was calculated using the comparative threshold cycle method.
Assay for in vitro capillary network formation
Isolated coronary ECs or a rat vascular EC line (Cell Applications, Inc., San Diego, CA) were used for this study. Coronary ECs from two to four hearts were pooled to collect a sufficient quantity of cells for one experiment of in vitro capillary network formation. Isolated ECs were cultured in M199 supplemented with 20% fetal calf serum, 100 μg/ml EC growth supplement, 100 IU/ml penicillin, 100 μg/ml streptomycin, 50 mg/liter d-valine, and 16 U/ml heparin. Cells were plated on the coverslips coated with 1% gelatin, cultured for 3 d, and then used for the capillary network formation experiment. The 8-well permanox cell culture slides were coated with Matrigel (50 μl/well) and allowed to solidify for 1 h at 37 C. ECs (104 cells/well) were seeded on Matrigel-coated slides. After 24 h, four microscopic fields selected at random were photographed. Each cell was outlined manually, and the total tube length (L, millimeter per square millimeter) and density of capillary network, D per square millimeter (D = number of cross-section of cells and grid; grid width = 23.8 μm in 1219 μm × 1219 μm image), were analyzed by MATLAB software (The Math Works, Inc., Natick, MA).
Western blot analysis
ECs were lysed and centrifuged at 16,000 × g for 10 min at 4 C. Supernatants were used as a sample protein. Samples were separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were then incubated with a primary antibody [anti-TRβ antibody (1:1000), anti-KDR/Flk1 antibody (1:500), anti-PECAM antibody (1:1000), or anti-actin antibody (1:4000)] followed by incubation with an horseradish peroxidase-conjugated secondary antibody. The immunoblots were detected with Western lightning enhanced chemiluminescence detection reagent (PerkinElmer LAS, Inc., Norton, OH). Band intensity was normalized to actin controls and expressed in arbitrary units.
For positive control of TRβ protein expression, human coronary ECs (HCECs) were transfected with TRβ adenovirus (TRβ-Adv) or control vector (SR−-Adv) (10 pfu/cell) for 1 d and then used for Western blot analysis.
Coronary vascular conductance (CVC) measurement
Data were obtained from mice at 8 wk after AAC. T3 administration was started at 5 wk after AAC and continued for 3 wk (3.5 ng/g body weight, ip daily). Hearts were isolated and the aorta was cannulated for Langendorff perfusion with Kreb's-Henseleit buffer at a perfusion pressure of 60 mm Hg and paced at a rate of 400 beats/min. Timed collection of coronary effluent was used to establish coronary flow and heart mass was determined for calculation of CVC in terms of milliliters per minute per gram per millimeter of mercury.
Statistical analysis
Values are expressed as mean ± se. Bonferroni tests for multiple statistical comparisons and Student's t test for unpaired samples were carried out to identify significant differences. Differences were considered to be statistically significant when P < 0.05.
Results
Effect of pressure overload on capillary density in LV myocardium
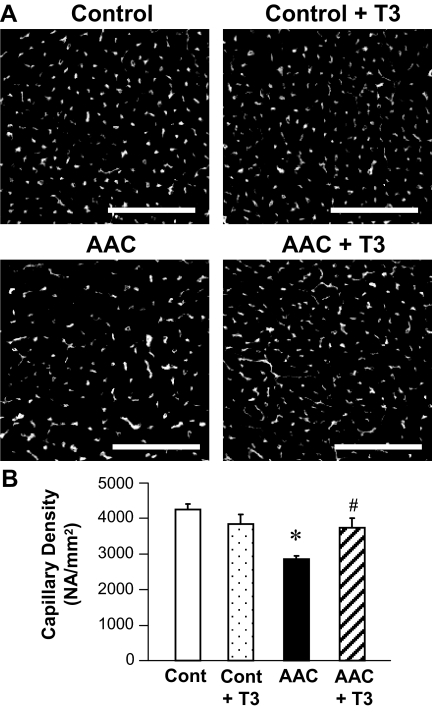
To determine capillary density, heart sections of subepicardial regions in LV were photographed (Fig. 1A), and at least two sections from each sample with eight microscopic fields were examined to calculate the average capillary numerical density (see Materials and Methods). Pressure overload by AAC significantly decreased the capillary density in LV myocardium, whereas administration of T3 for 2 wk restored the capillary density in AAC mice but had no effect on the capillary density in control mice (Fig. 1B).
Figure 1.
Sustained pressure overload reduces capillary density in LV myocardium and T3 administration restores the level of capillary density. Pressure overload was mediated by AAC. A, Representative photographs showing capillary images in LV of control and AAC heart with T3 administration (+ T3) or without T3 administration. Bar, 100 μm. B, Averaged data (mean ± se) showing the capillary density in control heart (cont; n = 5), control with T3 administration (cont + T3; n = 3), AAC heart (AAC; n = 4), and AAC with T3 administration (AAC + T3; n = 4). *, P < 0.05 vs. control; #, P < 0.05 vs. AAC.
Effect of pressure overload on TR mRNA level in coronary ECs
Real-time PCR data using coronary ECs isolated from mice of each group demonstrated that TRα mRNA level was unexpectedly increased by AAC, whereas TRβ mRNA level was significantly decreased by AAC. T3 administration in AAC mice restored the TRβ mRNA expression to the control level (Table 1). These data directed us to speculate that TRβ could indeed control coronary microcvascular formation in AAC mice.
Table 1.
TR mRNA levels in ECs isolated from control, AAC, and AAC + T3-treated hearts
mRNA levels were determined by real time-PCR. Each group has n = 3–4. Data are mean ± se.
P < 0.05 vs. control.
Effect of pressure overload on TRβ protein expression level in coronary ECs
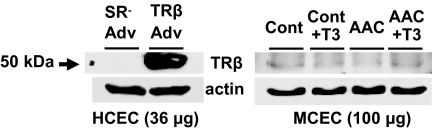
To collect sufficient amount of protein for detection of TRβ expression level, at least four hearts as one sample are used to isolate coronary ECs. Because TRβ protein level in coronary ECs is very low to obtain clear band with Western blot, we confirmed the molecular weight with use of TRβ-Adv trasnsfected HCECs as a positive control. As shown in Fig. 2, TRβ protein level was markedly decreased by AAC and was increased by T3 administration in AAC.
Figure 2.
Chronic T3 administration in AAC mice restores TRβ protein expression level. Left panel, Western blots were obtained from HCECs transfected with TRβ-Adv or control Adv (SR-Adv) to show the molecular weight of TRβ (36 μg protein per each well). In coronary ECs, TRβ protein is very low expression, and to collect sufficient amount of protein for detection of TRβ expression level, at least four hearts as one sample are used to isolate mouse coronary ECs (MCECs). Right panel, Western blots demonstrate that TRβ protein level is markedly decreased by AAC and is markedly increased by T3 administration (100 μg protein per each well).
TR contribution to angiogenesis in vivo
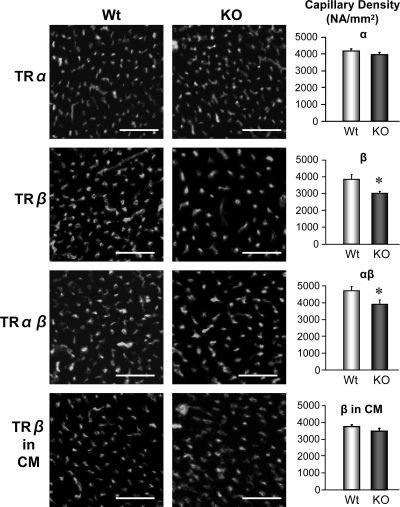
To explore the potential role of TRα and TRβ in influencing capillary density, we investigated the capillary density in vivo using mice with ubiquity deletion of TRα and TRβ and cardiac myocyte-specific deletion of TRβ. As shown in Fig. 3, TRβ KO mice and TRα/TRβ-double KO mice exhibited a significant decrease in capillary density compared with wild-type mice, whereas there was no change in capillary density in TRα KO mice and cardiac myocyte-specific TRβ KO mice. These data indicate that TRβ expression in ECs plays a crucial role in angiogenesis during cardiac development.
Figure 3.
TRβ plays a crucial role in angiogenesis during cardiac development. Representative photographs (left panels) showing capillary images in LV of ubiquitous TRα KO mouse, TRβ KO mouse, TRα/TRβ double KO mouse, and cardiac myocyte-specific TRβ KO mouse as well as their age-matched wild-type (Wt) mice. Bar, 100 μm. Averaged data (right panels) showing the capillary density in Wt (n = 6) and KO mice (n = 5) for TRα, Wt and KO mice for TRβ (each group, n = 3), Wt, and KO mice for TRα/TRβ (each group, n = 3), and Wt and KO mice for cardiac myocyte-specific TRβ (each group, n = 3). Data are mean ± se. *, P < 0.05 vs. Wt. In contrast to the ubiquitous TRβ KO with deletion in vascular ECs and cardiac myocytes, the cardiac myocyte-specific deletion of TRβ did not significantly decrease capillary density.
Effect of T3 treatment on capillary network formation in vitro
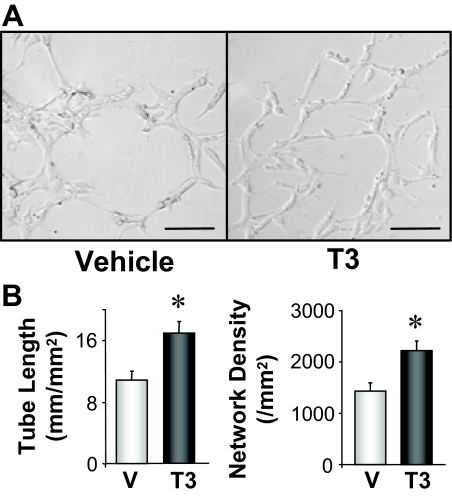
To examine the effect of T3 on angiogenesis in vitro, we measured capillary network formation by ECs on Matrigel-coated chamber. As shown in Fig. 4, treatment with 1 nmol/liter T3 caused significant enhancement of capillary network formation in vitro. These data from Figs. 1 and 4 suggest that T3 may directly affect on ECs to stimulate microvascular network formation and result in the restoration of capillary density in AAC heart.
Figure 4.
Treatment with 1 nmol/liter T3 enhances rat vascular EC capillary network formation. A, Representative images showing capillary network formation in vehicle (V; 10 nmol/liter NaOH)-treated ECs and T3-treated ECs. Bar, 100 μm. B, Summarized data showing total tube length (left panel) and network density (right panel) in vehicle (n = 6) and T3-treated (n = 4) ECs.
TR contribution to capillary network formation in vitro
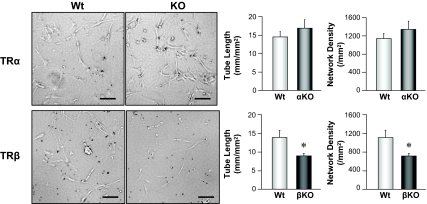
We investigated capillary network formation by isolated coronary ECs in vitro, using mice with ubiquity deletion of TRα and TRβ. Coronary ECs from TRβ KO mice had significantly less ability to form capillary networks than ECs from wild-type mice, whereas ECs from TRα KO mice exhibited a slightly enhanced ability to form capillary networks in comparison with ECs from wild-type mice (Fig. 5). These results imply that decreased capillary density in LV myocardium of TRβ KO mice may be due to an attenuated ability to form capillary networks by coronary ECs.
Figure 5.
Deletion of TRβ attenuates EC capillary network formation. Representative images (left panels) showing capillary network formation in coronary ECs isolated from wild-type (Wt), TRα KO, and TRβ KO mice. Bar, 100 μm. Right panels, Summarized data of total tube length and network density in coronary ECs from Wt (n = 6) and TRα KO (αKO, n = 6) and Wt (n = 4) and TRβ KO (βKO, n = 5) mice. *, P < 0.05 vs. ECs isolated from Wt. Data are mean ± se.
T3-mediated restoration of vascular rarefaction in AAC myocardium
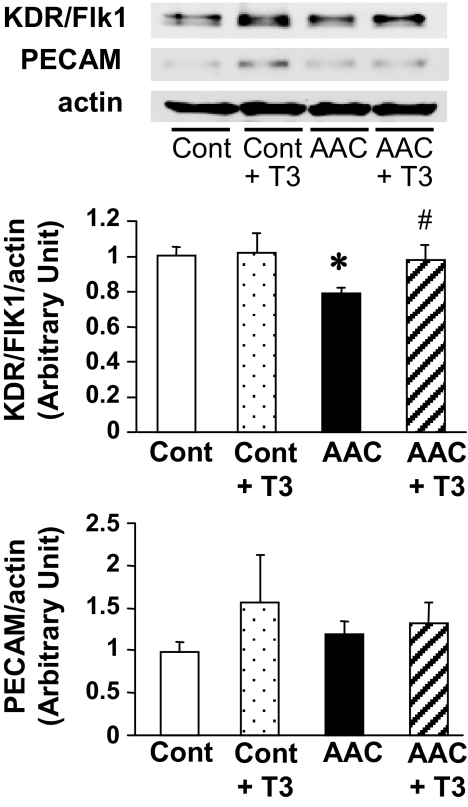
Because T3 administration shows an angiogenic effect in the AAC heart (Fig. 1), we screened the potential proteins that are involved in this beneficial effect of T3 by use of an angiogenic peptide oligonucleotide microarray (29). Whole heart was used to isolate mRNA. We found three proangiogenic mRNAs (epidermal growth factor, KDR/Flk1, and Pecam1) whose levels were decreased by AAC and restored by T3 administration, and one antiangiogenic mRNA (tissue inhibitor of metalloproteinase 3), which was increased by AAC and returned to the basal level by T3 administration. Based on this information, we measured those proangiogenic mRNAs and proteins in coronary endothelial cells isolated from control, AAC and AAC + T3 mice. RNA levels of KDR/Flk1 and PECAM were measured by real-time PCR. Although PECAM1 and KDR/Flk1 mRNA level does not have any change between groups (PECAM1: control, 1.00 ± 0.01; AAC, 1.11 ± 0.04; AAC + T3, 1.20 ± 0.03, KDR/Flk1: control, 1.00 ± 0.06; AAC, 1.17 ± 0.09; AAC + T3, 1.07 ± 0.02, each group, n = 3), Fig. 6 demonstrates that KDR/Flk1 protein level was significantly decreased by AAC and restored to the control level by T3 administration, whereas PECAM protein level was not affected by either AAC or T3 administration.
Figure 6.
Protein expression level of KDR/Flk1, but not PECAM, is decreased in coronary ECs isolated from AAC heart and T3 administration restored KDR/Flk1 protein level in AAC to the control level. Western blot (upper panel) showing KDR/Flk1, PECAM, and actin protein expression in coronary ECs from control (Cont) and AAC heart with T3 administration (+ T3) or without T3 administration. Summarized data (lower panels) showing protein levels of KDR/Flk1 and PECAM (normalized by actin level) in ECs isolated from different groups of hearts. Data are mean ± se from five experiments in each group. *, P < 0.05 vs. control; #, P < 0.05 vs. AAC.
Effect of T3 treatment on KDR/Flk1 protein expression in vitro
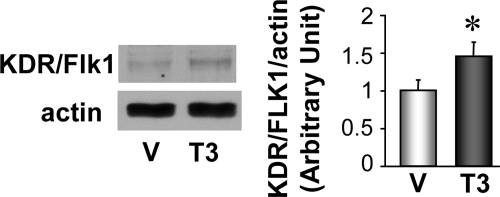
ECs were treated with 1 nmol/liter T3 during capillary network formation and cells were collected after 24 h. KDR/Flk1 protein level was significantly increased during capillary network formation under T3 treatment (Fig. 7). Figures 6 and 7 suggest that T3-induced angiogenic effect on AAC heart might be mediated by an increase in KDR/Flk1 protein level.
Figure 7.
T3 treatment during tube formation increased KDR/Flk1 protein expression in rat vascular ECs. Western blot (left panels) showing KDR/Flk1 and actin protein expression in ECs with 1 nmol/liter T3 treatment or vehicle treatment (V; 10 nmol/liter NaOH). The summarized data (mean ± se) shown in the right panel are from four experiments in each group. *, P < 0.05 vs. vehicle-treated ECs.
Effect of T3 treatment on CVC in AAC hearts
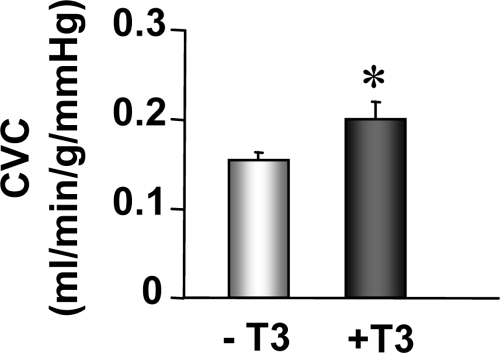
The reduction in myocardial capillary density, which accompanies pressure overload hypertrophy, correlates well with the observed reduction in coronary blood flow (30,31,32). To test whether T3 treatment improves the coronary function, we measured CVC in AAC hearts with or without T3 treatment. As shown in Fig. 8, T3 administration significantly increased CVC in AAC hearts as a consequence of increased capillary density in the LV myocardium.
Figure 8.
T3 administration in AAC mice significantly increases CVC. CVC was measured in Langendorff-perfused mouse hearts at a constant pressure of 60 mm Hg and paced at 400 beats/min. Groups included mice subjected to AAC for 8 wk to induce hypertrophy and treated with T3 (3.5 ng/g) injections (+T3, n = 9) or saline (−T3, n = 11) for 3 wk. *, P < 0.05 vs. −T3.
Discussion
Using subtype-specific TR KO mice, we were able to evaluate the role of TRβ in angiogenesis during cardiac development and during pathological cardiac hypertrophy. In addition, we demonstrated that TH administration restored the capillary density in the pathological hypertrophied heart by increasing TRβ action. Thus, TRβ activation by TH in ECs may be a therapeutic strategy for cardiac dysfunction in pathological hypertrophy and heart failure via the restoration of cardiac myocyte function (14,15,33) and/or the improvement of the capacity to transport oxygen to cardiac tissue by increasing capillary density.
In the normal heart, coronary blood flow and myocardial oxygen consumption are closely matched through changes in coronary vascular resistance in response to metabolic demands of the myocardium (34). Vascular resistance is determined by vascular tone and vascular density (30,31,35,36). Therefore, insufficient growth and rarefaction of capillaries, followed by endothelial dysfunction, represent a critical mechanism involved in heart damage. As shown in Fig. 1, capillary density in LV myocardium was significantly decreased in AAC heart. These data are in line with results from other researchers showing that sustained pressure overload led to suppression of angiogenesis (37,38), although the mechanisms described varied between researchers, and no reports showed the contribution of TR action in ECs.
Interestingly, TRα mRNA level in ECs was increased in AAC compared with control, whereas TRβ mRNA level was significantly decreased in AAC heart and restored by T3 administration to the control level (Table 1). An important question is whether this change in TRβ mRNA level in the AAC heart is significant to vascular formation. ECs express both TRα and TRβ (16,17). Several groups reported that the level of TRβ protein is almost similar to the levels of TRα1 and TRα2 protein, although TRβ mRNA level is much lower than TRα mRNA because of the short half-life of the TRβ mRNA (17,39). Although it is very hard to get clear band of TRβ protein by Western blot, we could successfully detect the band from isolated coronary ECs, which is the same molecular weight of the band in TRβ-overexpressed HCECs. Figure 2 demonstrates that TRβ protein expression in coronary ECs was markedly decreased in AAC and was increased by T3 administration in AAC. To support these data, we first tested the role of TRs in angiogenesis during cardiac development. Figure 3 shows that a systemic lack of TRβ resulted in a significant decrease of capillary density in comparison with the wild-type littermate, whereas deletion of either ubiquitous TRα or cardiac myocyte-specific-TRβ had no effect on capillary density. These data suggest that TRβ in ECs (but not TRα) is required for capillary development during cardiac development. In addition, we demonstrated that coronary ECs isolated from TRβ KO mice exhibited attenuated ability to form capillary networks in vitro in comparison with ECs from wild-type mice (Fig. 5). Taken together, these observations indicate that TRβ in coronary ECs serves as a key receptor in capillary formation during cardiac hypertrophy.
For more than a decade, therapeutic angiogenesis, including injection of growth factors, has been investigated in myocardial ischemia, heart failure, and graft failure (1,2,3). The main concern in regard to growth factor-mediated angiogenesis is pathological angiogenesis, which is thought to play a role in several diseases including tumor growth, diabetic proliferative retinopathy, and progression of atherosclerosis (40,41). Several studies have shown that exogenous TH leads to coronary angiogenesis (18,19,20). Wang et al. (18) reported that diiodothyropropionic acid induces formation of new coronary arteries by increasing the protein expression of fibroblast growth factor, vascular endothelial growth factor (VEGF), angiopoietin-1 and endothelium-specific receptor tyrosine kinase 2 in the heart. Dedecjus et al. (42) demonstrated that T4 administration increased plasma VEGF levels. Our results from the angiogenesis-focused array show that VEGF mRNA was not changed by administration of T3 in AAC heart, and VEGF protein expression level in coronary ECs was not affected by T3 administration in control mice (control, 1.01 ± 0.12; control + T3, 1.01 ± 0.03 arbitrary unit) or AAC mice (AAC, 0.86 ± 0.13; AAC + T3, 0.81 ± 0.01 arbitrary unit, determined by Western blot). This could be because the concentration of T3 used in this study was 1000 times lower than in other studies and closer to physiological concentration to avoid inducing hyperthyroidism. At this concentration, however, we were able to see the change in the protein expression level of angiogenesis-related peptide in the AAC heart by T3 administration. Figure 6 demonstrates that KDR/Flk1 (VEGF receptor-2) protein expression in coronary ECs was decreased in the AAC heart and restored by T3 administration. If this effect is specific to coronary ECs but not other cell types, it would ultimately prove useful as a therapy for angiogenesis in pathological cardiac hypertrophy. In line with in vivo data, in vitro T3 treatment augmented the ability of ECs to form capillary networks with an increase in KDR/Flk1 protein level (Figs. 4 and 7). These data suggest that restoration of capillary density in the pathological hypertrophic heart by T3 administration is mediated by increasing VEGF receptor-2 expression in coronary ECs.
T3 can regulate TR mRNA expression in various tissue and cell types with different pattern (43,44,45,46). We found that T3 administration in AAC mice increased TRβ mRNA in coronary ECs and increased capillary density. The up-regulated TRβ mRNA expression by T3 seemed to be crucial for improvement of capillary density in the AAC heart because administration of TRβ selective agonist, GC-1, was unable to restore capillary density in the AAC heart (control, 3746 ± 263/mm2; AAC, 2910 ± 233/mm2*; AAC + GC-1, 3041 ± 71/mm2*. *, P < 0.05 vs. control; each group, n = 4). The possible reasons for the inability of GC-1 to restore capillary density in the AAC heart include that it may be necessary to induce sufficient new TRβ by T3 to induce angiogenesis in AAC heart and/or that the concentration of GC-1 (1.75 ng/g body weight, equimolar to T3 concentration) used for this study was not enough to affect coronary EC function. Further experiments are required to clarify these mechanisms.
In conclusion, TRβ is required for angiogenesis during cardiac development, and lack of TRβ is one of the causes for microvascular rarefaction under pathological hypertrophy. In addition, administration of the physiological concentration of T3 would be a beneficial therapeutic approach to restore the microvascular density by up-regulating mRNA and protein expression of TRβ and VEGF receptor-2.
Footnotes
This work was supported by Grant HL89938 from the National Institutes of Health (to W.H.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 12, 2008
Abbreviations: AAC, Ascending aortic constriction; CVC, coronary vascular conductance; EC, endothelial cell; Flk1, fetal liver kinase-1; HCEC, human coronary EC; KDR, kinase insert domain protein receptor; KO, knockout; LV, left ventricle; PECAM, platelet/endothelial cell adhesion molecule 1; TH, thyroid hormone; TR, thyroid hormone receptor; TRβ-Adv, TRβ adenovirus; VEGF, vascular endothelial growth factor.
References
- Yla-Herttuala S, Martin JF 2000 Cardiovascular gene therapy. Lancet 355:213–222 [DOI] [PubMed] [Google Scholar]
- Tamirisa KP, Mukherjee D 2002 Gene therapy in cardiovascular diseases. Curr Gene Ther 2:427–435 [DOI] [PubMed] [Google Scholar]
- Mukherjee D 2004 Current clinical perspectives on myocardial angiogenesis. Mol Cell Biochem 264:157–167 [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K 2001 Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- Motomura K, Brent GA 1998 Mechanisms of thyroid hormone action. Implications for the clinical manifestation of thyrotoxicosis. Endocrinol Metab Clin North Am 27:1–23 [DOI] [PubMed] [Google Scholar]
- Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A 1998 Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol 81:443–447 [DOI] [PubMed] [Google Scholar]
- Morkin E, Pennock GD, Spooner PH, Bahl JJ, Goldman S 2002 Clinical and experimental studies on the use of 3,5-diiodothyropropionic acid, a thyroid hormone analogue, in heart failure. Thyroid 12:527–533 [DOI] [PubMed] [Google Scholar]
- Degens H, Gilde AJ, Lindhout M, Willemsen PH, Van Der Vusse GJ, Van Bilsen M 2003 Functional and metabolic adaptation of the heart to prolonged thyroid hormone treatment. Am J Physiol Heart Circ Physiol 284:H108–H115 [DOI] [PubMed] [Google Scholar]
- Ladenson PW 1990 Recognition and management of cardiovascular disease related to thyroid dysfunction. Am J Med 88:638–641 [DOI] [PubMed] [Google Scholar]
- Harvey CB, Williams GR 2002 Mechanism of thyroid hormone action. Thyroid 12:441–446 [DOI] [PubMed] [Google Scholar]
- Freedman LP 1992 Anatomy of the steroid receptor zinc finger region. Endocr Rev 13:129–145 [DOI] [PubMed] [Google Scholar]
- Katz D, Lazar MA 1993 Dominant negative activity of an endogenous thyroid hormone receptor variant (α2) is due to competition for binding sites on target genes. J Biol Chem 268:20904–20910 [PubMed] [Google Scholar]
- Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR 2001 Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation 103:1089–1094 [DOI] [PubMed] [Google Scholar]
- Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, Camacho SA, Bristow MR, Long CS, Simpson PC 2001 Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res 89:591–598 [DOI] [PubMed] [Google Scholar]
- Belke DD, Gloss B, Swanson EA, Dillmann WH 2007 Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-α1 and -β1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology 148:2870–2877 [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK 2006 Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA 103:14104–14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman MJ, Zandieh Doulabi B, Platvoet-Ter Schiphorst M, Fliers E, Bakker O, Wiersinga WM 2001 The biological relevance of thyroid hormone receptors in immortalized human umbilical vein endothelial cells. J Endocrinol 168:427–433 [DOI] [PubMed] [Google Scholar]
- Wang X, Zheng W, Christensen LP, Tomanek RJ 2003 DITPA stimulates bFGF, VEGF, angiopoietin, and Tie-2 and facilitates coronary arteriolar growth. Am J Physiol Heart Circ Physiol 284:H613–H618 [DOI] [PubMed] [Google Scholar]
- Heron MI, Kolar F, Papousek F, Rakusan K 1997 Early and late effect of neonatal hypo and hyperthyroidism on coronary capillary geometry and long-term heart function in rat. Cardiovasc Res 33:230–240 [DOI] [PubMed] [Google Scholar]
- Mousa SA, O'Connor LJ, Bergh JJ, Davis FB, Scanlan TS, Davis PJ 2005 The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J Cardiovasc Pharmacol 46:356–360 [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Connell PM, Butters CA, Torry RJ 1995 Compensated coronary microvascular growth in senescent rats with thyroxine-induced cardiac hypertrophy. Am J Physiol 268:H419–H245 [DOI] [PubMed] [Google Scholar]
- Anjos-Ramos L, Carneiro-Ramos MS, Diniz GP, Martins-Silva J, Barreto-Chaves ML 2006 Early cardiac hypertrophy induced by thyroxine is accompanied by an increase in VEGF-A expression but not by an increase in capillary density. Virchows Arch 448:472–479 [DOI] [PubMed] [Google Scholar]
- Suarez J, Gloss B, Belke DD, Hu Y, Scott B, Dieterle T, Kim YK, Valencik ML, McDonald JA, Dillmann WH 2004 Doxycycline inducible expression of SERCA2a improves calcium handling and reverts cardiac dysfunction in pressure overload-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol 287:H2164–H2172 [DOI] [PubMed] [Google Scholar]
- Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B 1998 Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J 1999 Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J 18:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal DS, Nghiem M, Crackower MA, MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD 2001 Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89:20–25 [DOI] [PubMed] [Google Scholar]
- Flamant F, Samarut J 2003 Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab 14:85–90 [DOI] [PubMed] [Google Scholar]
- Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH 2008 Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295:C221–C230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Weichert A, Zakrzewicz A, Da Silva-Azevedo L, Pries AR, Baum O, Egginton S 2006 Differential gene and protein expression in abluminal sprouting and intraluminal splitting forms of angiogenesis. Clin Sci (Lond) 110:587–595 [DOI] [PubMed] [Google Scholar]
- Breisch EA, White FC, Nimmo LE, Bloor CM 1986 Cardiac vasculature and flow during pressure-overload hypertrophy. Am J Physiol 251:H1031–H1037 [DOI] [PubMed] [Google Scholar]
- Breisch EA, White FC, Bloor CM 1984 Myocardial characteristics of pressure overload hypertrophy. A structural and functional study. Lab Invest 51:333–342 [PubMed] [Google Scholar]
- Mueller TM, Marcus ML, Kerber RE, Young JA, Barnes RW, Abboud FM 1978 Effect of renal hypertension and left ventricular hypertrophy on the coronary circulation in dogs. Circ Res 42:543–549 [DOI] [PubMed] [Google Scholar]
- Villicev CM, Freitas FR, Aoki MS, Taffarel C, Scanlan TS, Moriscot AS, Ribeiro MO, Bianco AC, Gouveia CH 2007 Thyroid hormone receptor β-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol 193:21–29 [DOI] [PubMed] [Google Scholar]
- Berne RM 1964 Regulation of coronary blood flow. Physiol Rev 44:1–29 [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Palmer PJ, Peiffer GL, Schreiber KL, Eastham CL, Marcus ML 1986 Morphometry of canine coronary arteries, arterioles, and capillaries during hypertension and left ventricular hypertrophy. Circ Res 58:38–46 [DOI] [PubMed] [Google Scholar]
- Flanagan MF, Fujii AM, Colan SD, Flanagan RG, Lock JE 1991 Myocardial angiogenesis and coronary perfusion in left ventricular pressure-overload hypertrophy in the young lamb. Evidence for inhibition with chronic protamine administration. Circ Res 68:1458–1470 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ 2007 Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 204:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I 2007 p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446:444–448 [DOI] [PubMed] [Google Scholar]
- Ercan-Fang S, Schwartz HL, Oppenheimer JH 1996 Isoform-specific 3,5,3′-triiodothyronine receptor binding capacity and messenger ribonucleic acid content in rat adenohypophysis: effect of thyroidal state and comparison with extrapituitary tissues. Endocrinology 137:3228–3233 [DOI] [PubMed] [Google Scholar]
- Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun TH, Masatsugu K, Becker AE, Nakao K 1998 Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation 98:2108–2116 [DOI] [PubMed] [Google Scholar]
- Walsh DA 2007 Pathophysiological mechanisms of angiogenesis. Adv Clin Chem 44:187–221 [DOI] [PubMed] [Google Scholar]
- Dedecjus M, Kolomecki K, Brzezinski J, Adamczewski Z, Tazbir J, Lewinski A 2007 Influence of l-thyroxine administration on poor-platelet plasma VEGF concentrations in patients with induced short-term hypothyroidism, monitored for thyroid carcinoma. Endocr J 54:63–69 [DOI] [PubMed] [Google Scholar]
- Monden T, Nakajima Y, Hashida T, Ishii S, Tomaru T, Shibusawa N, Hashimoto K, Satoh T, Yamada M, Mori M, Kasai K 2006 Expression of thyroid hormone receptor isoforms down-regulated by thyroid hormone in human medulloblastoma cells. Endocr J 53:181–187 [DOI] [PubMed] [Google Scholar]
- Opitz R, Lutz I, Nguyen NH, Scanlan TS, Kloas W 2006 Analysis of thyroid hormone receptor betaA mRNA expression in Xenopus laevis tadpoles as a means to detect agonism and antagonism of thyroid hormone action. Toxicol Appl Pharmacol 212:1–13 [DOI] [PubMed] [Google Scholar]
- Shi YB, Ritchie JW, Taylor PM 2002 Complex regulation of thyroid hormone action: multiple opportunities for pharmacological intervention. Pharmacol Ther 94:235–251 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Mangoura D, Refetoff S 1996 A mouse model of resistance to thyroid hormone produced by somatic gene transfer of a mutant thyroid hormone receptor. Mol Endocrinol 10:100–106 [DOI] [PubMed] [Google Scholar]