Abstract
A gene termed EAP1 (enhanced at puberty 1) was recently identified as a transcriptional regulator of female neuroendocrine reproductive function. We have now used in vivo and in vitro assays, and the female rat as an animal model, to determine whether Eap1 gene expression is regulated by ovarian steroids. Eap1 mRNA abundance decreases in both the hypothalamus and cerebral cortex during the infantile-juvenile phases of development, but it increases selectively in the hypothalamus at puberty, suggesting that in contrast to the general decline in expression observed in immature animals, the region-specific increase in Eap1 mRNA levels that occurs at puberty might be elicited by ovarian steroids. This is, however, not the case, because hypothalamic Eap1 mRNA levels increase at the expected time of puberty in rats ovariectomized at the beginning of the juvenile period. Although a subpopulation of EAP1-containing cells in the medial basal hypothalamus (MBH) and preoptic area express estrogen receptor-α (ERα), the 5′-flanking region of the rat Eap1 (rEap1) gene does not contain a complete estrogen-responsive element, and no such estrogen-responsive element is detected within 100 kb of the rEap1 locus. Functional promoter assays showed that neither estradiol (E2) alone nor a combination of E2 plus progesterone increases rEap1 gene transcription. Likewise, E2 administered to ovariectomized immature rats elicited a robust surge of LH but increased neither preoptic area nor MBH Eap1 mRNA levels. E2/progesterone-treated rats showed a massive elevation in plasma LH but only a modest increase in Eap1 mRNA levels, limited to the MBH. These results indicate that hypothalamic Eap1 expression is not directly controlled by ovarian steroids and suggest that Eap1 expression increases at puberty driven by ovary-independent, centrally initiated events.
The expression of Eap1, a transcriptional regulator of reproductive function, increases in the hypothalamus at puberty in an ovary-independent manner, suggesting that Eap1 contributes to the centrally originated events that initiate puberty.
When examined superficially, the process governing the secretory activity of GnRH neurons appears to be simple: variations in GnRH output are determined by synchronized changes in transsynaptic and glial inputs to GnRH neurons. In turn, these changes are influenced by signals of hormonal, metabolic, and circadian nature. The system, however, not only is composed of different subpopulations of neurons and glial cells of diverse chemical phenotypes but also requires a myriad of ligands, receptors, synaptic specifiers, cell-cell adhesion molecules, intracellular signaling complexes, and transcriptional regulators. Needless to say, for this complex machinery to generate a physiologically meaningful change in GnRH output, it needs to function in a highly interactive and coordinated fashion.
In an initial attempt to address this issue, it has been proposed that the neuroendocrine regulation of reproductive development requires a level of control provided by gene networks that, operating within the neuroendocrine brain, coordinate neuronal-glial interactions in a developmentally regulated manner (1). Although there is no doubt that regulatory control systems such as this one have the potential for thousands of interactions, it is also clear that the heart of such complex systems always consists of a handful of genes encoding transcription factors. Those involved in the central control of reproductive function would be expected to establish the conditions required for the productive engagement of neuron-to-neuron, neuron-to-glia, and glia-to-neuron circuitries controlling GnRH secretion, regardless of the transcriptional process they control.
Our knowledge of the number and composition of regulatory gene networks that may operate in the hypothalamus to control sexual development is rudimentary at best. However, at least one of these presumptive networks and its major hubs has been identified (2), and evidence has been presented implicating three genes as potential upstream transcriptional regulators of the pubertal process: Oct2 (3), Ttf1 (4), and Eap1 (5). The latter is an intronless gene, earlier known as chromosome 14 open reading frame 4 (C14ORF4) (6). Eap1 encodes a nuclear protein expressed in neurons involved in the stimulatory and inhibitory control of GnRH secretion, including GnRH neurons themselves, as well as glutamatergic, GABAergic, and proenkephalinergic neurons. Eap1 also has dual transregulatory activity; it activates the GnRH promoter but represses the preproenkephalin gene, which is inhibitory to the pubertal process. Reducing hypothalamic EAP1 expression in vivo using small interfering RNAs delayed puberty and disrupted estrous cyclicity, indicating that enhanced at puberty 1 (EAP1) is necessary for the timely activation of GnRH secretion at puberty and for the periodic fluctuations in GnRH release associated with the estrous cycle (5).
Because puberty is initiated by events that occur centrally (7,8,9), a consideration that needs to be made when implicating genes in the process that sets puberty in motion is whether the pubertal expression of such genes requires the stimulatory effect of gonadal steroids. If it does, one may not assume that the gene in question is a bona fide component of the primum movens of puberty, even if the gene is still important (or even critical) for puberty to take place. Ttf1 and Oct2 may be early components of the initiating process, because their expression increases before puberty without the need of a change in gonadal steroid output (3,4). Because no such information exists in the case of Eap1, the present study addresses this issue.
Materials and Methods
Animals
Upon arrival, timed-pregnant rats and 21-d-old female Sprague Dawley rats (Harlan, Indianapolis, IN) were housed under controlled conditions of temperature (23–25 C) and light (14-h light, 10-h dark; lights on from 0500–1900 h). Food (Purina laboratory chow; Ralston-Purina, St. Louis, MO) and water were provided ad libitum. Animal use was duly approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center.
In vivo procedures
Developmental changes in rat Eap1 (rEap1) mRNA expression
To determine the changes in Eap1 mRNA abundance that may occur in the medial basal hypothalamus (MBH) during female sexual development, the MBH was collected from female rats euthanized at different postnatal (PN) ages (PN d 0, 6, 12, 18, and 24) and at different stages of puberty. Samples of cerebral cortex (CTX) were also collected as a control tissue. When collecting the MBH from peripubertal rats, the tissue was dissected away from the median eminence (ME), which was used separately for Eap1 mRNA measurement. The animals were classified into five different stages of puberty following criteria previously established (10): late juvenile (LJ), early proestrus (EP), late proestrus (LP), estrus, and diestrus. Briefly, LJ animals (28–30 d old) have a closed vagina and a uterine weight of 60 mg or less. EP animals show enlarged uteri containing fluid and weighing from 60–195 mg. LP animals have heavier uteri (200 mg or more) filled with fluid (ballooned). Animals at this latter stage are considered as being in midpuberty, because they have not yet ovulated. The next day is the day of first estrus (E) when ovulation takes place. The vagina is open and the ovaries contain fresh corpora lutea. The estrous stage is followed by the first diestrus (D1). After dissection, the tissues were frozen on dry ice and stored at −85 C until RNA extraction.
Ovariectomy (OVX) and steroid treatments
Two studies were performed. In both of them, the rats were OVX on PN d 23, under tribromoethanol anesthesia (Sigma, St. Louis, MO; 0.5 g/kg body weight, ip). After surgery, the rats received a sc injection (0.1 ml) of the analgesic Kebuprofen (Butler Animal Health Supply, Dublin, OH; 1 mg/ml). In study 1, the rats were given a sc injection of estradiol benzoate (EB) (Sigma; 100 μg/kg body weight, sc) or vehicle alone (100 μl corn oil) 5 d after OVX. Two days later, they received progesterone (P) (Sigma; 1 mg in 50 μl corn oil, sc) or oil alone. Animals were killed 12, 24, or 54 h after the first injection, and different areas of the brain [CTX, preoptic area (POA), and MBH] were dissected, snap frozen on dry ice, and stored at −85 C until RNA extraction.
In the second study, the animals were OVX and housed with intact animals (two OVX and two intact rats per cage). Starting on d 28, the intact animals were inspected daily for signs of impending vaginal opening. Swelling and hyperemia of the genital area was observed in most rats of this group between 32 and 34 d of age. When these changes were detected, both the intact rats entering puberty and the OVX animals housed in the same cage were euthanized, and the brain areas described above were collected for RNA extraction.
In silico analysis of the rEap1 gene 5′-flanking region
The rEap1 sequence was identified online by the Basic Local Alignment Search Tool (BLAST). The identified rat sequence (GenBank accession no. NM_001012470, derived from AY879229) is located on chromosome 6 (GenBank accession no. NC_005105.2).
The position of the translation start site was predicted by the ATG predictor online tool (www.hri.co.jp/atgpr/; no longer available) (11). The region upstream from the ATG site was searched for potential transcription start sites (TSSs). The position of a TSS was predicted using several search tools including Promoter 2.0 Prediction server (http://www.cbs.dtu.dk/services/Promoter/), Dragon Promoter Finder version 1.5 (http://research.i2r.a-star.edu.sg/promoter/promoter1_5/DPF.htm), and Softberry TSSG (http://www.softberry.com). For sequence analysis and alignments, we used the Lasergene software version 5.08 (DNASTAR, Madison, WI). NSITE (http://www.softberry.com), TESS (http://www.cbil.upenn.edu/cgi-bin/tess/tess), and Dragon ERE finder 2.0 (http://research.i2r.a-star.edu.sg/DRAGON/) were used to predict the position of potential transcription factor binding sites, including that of estrogen-responsive elements (EREs).
Experimental identification of rEap1 TSS
The position of rEap1 TSS was determined in vitro using the GeneRacer kit (Invitrogen, Carlsbad, CA). Four micrograms of total RNA from rat brain were dephosphorylated, decapped, and ligated to a 44-mer 5′ GeneRace oligodeoxynucleotide provided in the kit, before being reverse-transcribed using Superscript III reverse transcriptase and random hexamers. The resulting cDNAs were amplified using Platinum Pfx DNA polymerase in a 50-μl reaction containing 4.5 μl GeneRacer 5′ primer (10 μm) and 1.5 μl of a gene-specific 3′ primer (50 μm, 5′-AGTCCCACGCCGAGAAGCCACCGATGTT-3′) corresponding to nucleotides 630–657 in the rEap1 mRNA sequence (NM_001012470). The PCR program employed consisted of an initial step of denaturation at 94 C for 2 min, followed by five cycles of 30 sec at 94 C and 2.5 min at 72 C, five cycles of 30 sec at 94 C and 2.5 min at 70 C, and 25 cycles of 30 sec at 94 C, 30 sec at 68 C, and 2.5 min at 68 C. The program ended with a final extension of 10 min at 68 C. The PCR products were then cloned into the plasmid pGEM-T (Promega, Madison, WI), and 14 positive clones were sequenced from both ends to determine the initiation of Eap1 mRNA transcription.
RNA extraction, RT-PCR, and semiquantitative PCR
Total RNA was prepared by the acid phenol-extraction method, as described previously (5). To remove DNA contamination, the RNA samples were treated with the DNA-free DNase I kit (Ambion, Austin, TX) according to the manufacturer's protocol. RNA concentrations were determined spectrophotometrically, and RNA integrity was verified on 1% denaturing agarose gels.
Five hundred nanograms of total RNA were reverse transcribed using the Omni RT kit (QIAGEN, Valencia, CA) following the manufacturer's recommendations. To quantify rEap1 mRNA, we used a semiquantitative PCR procedure described elsewhere (12). Additional details are given in supplemental note 1 (published as supplemental data on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Real-time PCR
Real-time PCR was performed essentially as described (5,12). Other details are provided in supplemental note 2.
To clone the rEap1 cDNA fragment that served as a template for the preparation of rEap1 sense mRNA standards, we used the PCR products obtained with the primers described in supplemental note 1. This procedure resulted in a single PCR product that was cloned into the plasmid pGEM-T (Promega) and sequenced from both ends to verify its identity.
Plasmid constructs
To investigate the effects of sex steroids on rEap1 promoter activity, we subcloned a fragment of the rEap1 5′-flanking region (−2100 to +427 into the luciferase reporter plasmid pGL-3-Basic (Promega). The position of the TTS1 detected by 5′-rapid amplification of cDNA ends (5′-RACE) PCR (see Results) was used as +1. Details of the cloning procedure are provided in supplemental note 3.
Cell culture and promoter assays
Promoter assays were carried out in C6 cells expressing the ERα or ERβ subtype (C6α and C6β), as reported previously (13). Additional details are provided in supplemental note 4.
To examine the effect of estradiol (E2) on rEap1 promoter activity C6, C6α, and C6β cells were transiently transfected with the control plasmid (pGL3, 250 ng/ml), 250 ng rEap1-pGL3, or 750 ng of the tkERE-luc plasmid used as a positive control (a kind gift from Dr. Peter Burbach, Rudolf Magnus Institute, University of Utrecht, Utrecht, The Netherlands). Twenty four hours after transfection, the medium was replaced with fresh medium containing the E2 vehicle (ethanol, 0.1 μl/ml medium) and either 1 or 10 nm E2 (Sigma). The cells were harvested 24 and 48 h later for luciferase and β-galactosidase assays. Luciferase activity was expressed as percent of the activity detected in control cells transfected with rEap1-pGL3 only.
To examine the effect of P on rEap1 promoter activity, the cells were transfected with 250 ng rEap1-pGL3 and 500 ng of the PR-A-expressing plasmid PRA-pCMV5 (kindly provided by Benita Katzenellenbogen, University of Illinois, Urbana, IL). Control cells received the pCMV5 empty plasmid instead. The day after transfection, the medium was replaced with fresh medium containing 10 nm E2 (Sigma), whereas control wells received medium with vehicle only (ethanol). Twenty four hours later, the cells either received P (Sigma, 250 nm) or dimethylsulfoxide (Sigma, 0.2 μl/ml medium), the P vehicle. The next day, the cells were harvested for luciferase and β-galactosidase assays. Every experiment was repeated three times (using at least three wells per condition each time). Luciferase activity was expressed as percent of the activity detected in cells transfected with rEap1-pGL3 and treated with E2 only.
Immunohistochemistry
To determine whether the cells that contain EAP1 protein in the rat hypothalamus also express ERα immunoreactive material, we used rabbit polyclonal antibodies against EAP1 (5) and a monoclonal antibody (clone 1D5; LabVision Neomarkers, Fremont, CA) against ERα. Frozen sections (30 μm) obtained from the brain of immature 28-d-old rats (perfusion fixed with 4% paraformaldehyde-PBS, pH 7.4) were mounted on Superfrost glass slides, dried for 2 h under an air stream, and subjected to an antigen retrieval protocol (14). Thereafter, the sections were incubated for 48 h at 4 C with EAP1 antibodies diluted 1:5000 and anti-ERα antibody (1:100). At the end of this period, the reactions were developed and visualized as indicated in supplemental note 5.
LH assay
Trunk blood was collected at the time of killing. Serum levels of LH were measured by RIA as reported (5).
Statistics
Data passing a normality test were analyzed using the two-tailed Student's t test to compare two groups or a one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test to compare several groups. When the data failed the normality test, they were analyzed using an ANOVA test on ranks followed by the Kruskal-Wallis one-way ANOVA ranks test (SigmaStat version 3.11; Systat Software Inc., San Jose, CA). A P value of <0.05 was considered statistically significant.
Results
Eap1 gene expression decreases in the female rat hypothalamus during neonatal-juvenile development to increase again at the time of puberty
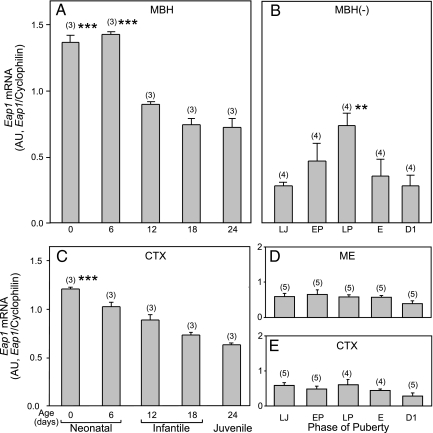
We used semiquantitative PCR to determine whether expression of the Eap1 gene changes throughout development in the female rat hypothalamus. Eap1 mRNA levels were high in the MBH during the first 6 d after birth but declined thereafter throughout infantile-early juvenile development (PN d 12–24; Fig. 1A). A slightly less pronounced but significant decrease was also observed in the CTX (Fig. 1C). Consistent with a previous report (5), Eap1 mRNA abundance increased again in the MBH (isolated from the ME) at the time of puberty, with the highest levels found on the day of the first preovulatory surge of gonadotropins (LP phase of puberty, Fig. 1B). After ovulation (E and D1 phases of puberty), Eap1 mRNA levels declined toward late juvenile values. No such puberty-related changes were observed in either the ME (Fig. 1D), a region of the hypothalamus devoid of neuronal cell bodies, or the CTX (Fig. 1E), a brain region not directly implicated in the control of puberty.
Figure 1.
Eap1 mRNA abundance changes in the rat hypothalamus during female sexual development, as assessed by semiquantitative PCR. A, Eap1 mRNA content decreases in the MBH between the end of the infantile period (PN d 6) and the first half of juvenile development (PN d 24). B, Eap1 expression in the MBH separated from the ME (MBH−) increases at the time of puberty reaching maximum values on the day of first proestrus (LP). C, Eap1 mRNA content in the CTX decreases steadily from the time of birth to the first half of the juvenile period. D, Eap1 mRNA content does not change in the ME at the time of puberty. E, Eap1 mRNA content remains at low levels in the CTX throughout puberty. The results are expressed as ratios between Eap1 and cyclophilin mRNA. AU, Arbitrary units; E, first estrus. Numbers in parentheses above bars indicate the number of animals per group, and vertical bars represent sem. ***, P < 0.01 vs. all older ages (A and C); **, P < 0.02 vs. all other groups, except EP (ANOVA followed by Student-Newman-Keuls test) (B).
Some EAP1-containing cells of the MBH and POA also express ERα
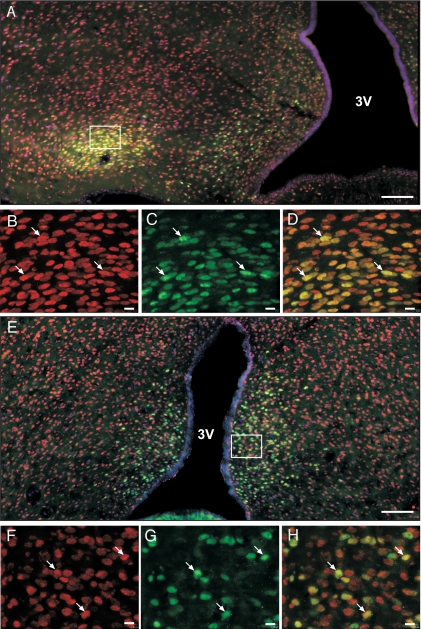
Because Eap1 mRNA abundance increases in the MBH at the stages of puberty during which E2 levels are also increasing (EP and LP), we performed experiments to determine whether this peripubertal change in Eap1 gene expression is ovarian steroid dependent. We first carried out immunohistofluorescence studies to determine whether EAP1-containing cells are ERα or ERβ immunopositive. It is well established that ERα and ERβ are expressed in discrete cell populations of the MBH and POA (15). We were unable to reliably localize cells containing immunoreactive ERβ, because of the intense background shown by two antibodies tested (Mab EMR02 from Novocastra, Newcastle, UK; Mab PPG5/10 from Serotec, Oxford, UK). However, we found that a subpopulation of EAP1-containing cells in the ventromedial nucleus (VMH) and arcuate nucleus (ARC) of the MBH (Fig. 2, A–D), and in the POA (Fig. 2, E–H) are also ERα positive. This colocalization suggests the possibility of a direct effect of E2 on hypothalamic Eap1 expression mediated by ERα.
Figure 2.
Subpopulations of EAP1-containing cells of the MBH and POA also express ERα. A, Montage image of the female rat hypothalamus (LJ, 30-d-old rat) showing that EAP1 immunoreactive protein (red) is abundant in cells of the ARC and VMH and that a subpopulation of cells in both of these nuclei also contain ERα immunoreactive material (green); B, high-magnification view of the VMH showing EAP1 immunoreactive cells (in red); C, same as in B, but showing only ERα-positive cells (green); D, merged images B and C, with arrows pointing to examples of EAP1-positive cells that also contain ERα; E, montage image of the female rat POA showing that many ERα-positive cells (green) also contain immunoreactive EAP1 (red); F, high-magnification view of the POA showing EAP1 immunoreactive cells (in red); G, same as in E, but showing only ERα-positive cells (green); H, merged images F and G, with arrows pointing to examples of EAP1-positive cells that also contain ERα. Bars, 100 μm (A and E) and 10 μm (B–D and F–H). 3V, Third ventricle.
The rEap1 5′-flanking region
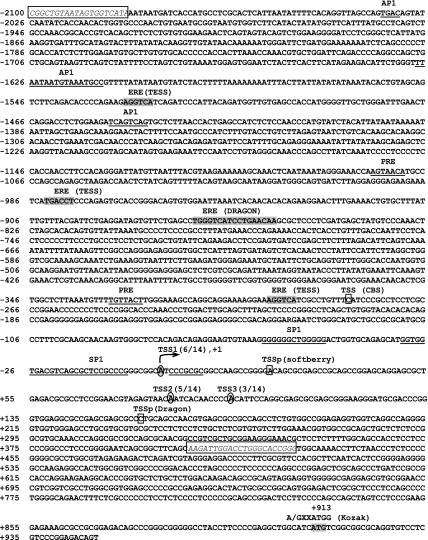
The direct effects of E2 on rEap1 transcription were investigated using promoter assays. Because no information on the rEap1 promoter region was available, we first sought to identify the position of the TSS. In silico analysis predicted that the TSS is located at about 900 bp upstream of the translational initiation site. Multiple RACE PCR products showed the presence of more than one TSS, which is a characteristic of genes lacking a TATA box (16), such as Eap1. All TSSs were found in the predicted area and are located 911 (TSS1), 828 (TSS2), and 816 (TSS3) bp upstream from the ATG translation initiation site, respectively (Fig. 3). Because six of 14 clones showed initiation of transcription 911 bp upstream of the ATG codon, we named this site TSS1 and assigned it the position +1. It is likely, however, that TSS2 is also used as a major TSS, because it was found in five of 14 clones. A 2-kb region upstream of TSS1 was then searched for potential EREs and progesterone response elements (PREs), known to mediate the effects of E2 and/or P. Web-based tool analysis showed the presence of three regions (Fig. 3, labeled ERE and highlighted in gray) containing one of the arms of the ERE palindromic inverted repeat. One additional region (−872 to −856, also labeled ERE and highlighted in gray) contains an imperfect palindrome due to substitutions in positions +5 (A for C), +7 (A for T), and +8 (A for G) of the downstream arm. Because EREs may be present at distances greater than 5 kb from the TSS of genes (17), we inspected both visually and using TESS software 50 kb upstream and 50 kb downstream from TSS1 and found no complete EREs in this large DNA fragment.
Figure 3.
The 5′-flanking region of the rEap1 gene. Based on the human EAP1 gene sequence, the homologous rat sequence was identified, and a 2-kb upstream region was analyzed in silico for potential TSSs (denoted by squares; the online tools used to predict TSSs are shown in parentheses) and transcription factor binding sites (underlined sequences). A search for EREs was performed using the Dragon Promoter Finder and TESS search webtools (gray highlights, names of web tools in parentheses). Experimental identification of rEap1 TSSs by RACE PCR identified three TSSs (shown by letters inside ovals). All three sites are located within a 100-bp range. The most frequent site, detected in six of 14 clones, was termed TSS1 and was given the position +1. The second most frequently used TSS (five of 14 clones) was termed TSS2, and the third most frequently used TSS (three of 14 clones) was termed TSS3. A fragment containing 2100 bp of DNA upstream from TSS1 and 429 bp of 5′-untranslated mRNA sequence was then PCR amplified using specific primers (framed sequences in italics) and subcloned into the pGL3-Basic luciferase vector.
Two incomplete PRE half-sites were detected in the cloned 5′ flanking region of rEap1 (Fig. 3, labeled PRE and underlined). Putative binding motifs for SP1 and AP1, known to mediate some transcriptional effects of E2 (18,19,20), were also detected. This genomic fragment was then subcloned into the luciferase reporter vector pGL3-Basic. The construct, named EAP1-pGL3, showed a dose-dependent activity when transiently transfected into C6 cells (data not shown).
Estrogen effects on rEap1 transcriptional activity
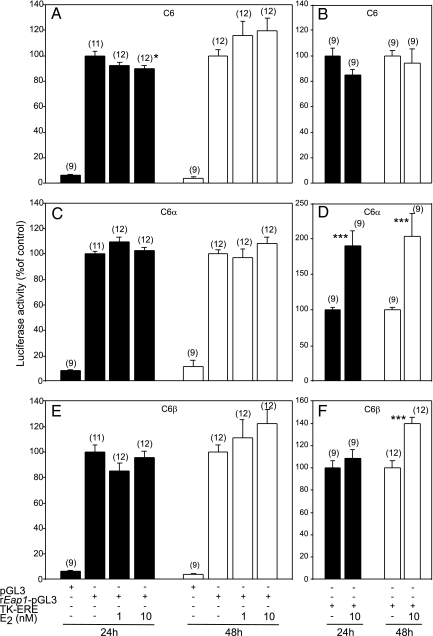
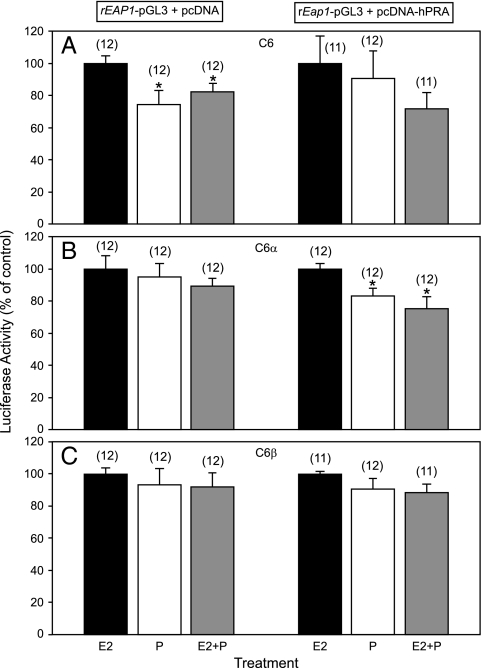
To test the effect of E2 on the rEap1 promoter, we used C6, C6α, and C6β cells in transient transfection assays. Twenty-four hours after transfection with EAP1-pGL3, C6 cells were treated with 1 and 10 nm estradiol or the vehicle (ethanol) as a control. Other cells were transfected with a luciferase construct containing a tandem of EREs linked to the thymidine kinase promoter (tkERE-luc) as a positive control. Although in native C6 cells estrogen at 10 nm resulted in a small (∼10%) reduction in rEap1 promoter activity after 24 h exposure (Fig. 4A), this effect was no longer evident at 48 h (Fig. 4A) and was not seen in either C6α (Fig. 4C) or C6β (Fig. 4E) cells. As shown earlier (13), E2 failed to increase tkERE-luc transcriptional activity in native C6 cells. Instead, these cells showed after 24 h treatment a spurious decline (Fig. 4B) similar to that seen in native C6 cells treated with E2 for 48 h (Fig. 1A). Estradiol did, however, increase the activity of the tkERE promoter at both 24 and 48 h in C6α cells (Fig. 4D) and at 48 h in C6β cells (Fig. 4F).
Figure 4.
E2 does not affect in vitro rEap1 promoter activity in cells stably expressing ERα (C6α) or ERβ (C6β). A, C6 cells transfected with rEap1-pGL3 and challenged with E2 (1 or 10 nm); B, C6 cells transfected with an estrogen-responsive reporter construct (TK-ERE) and challenged with E2 (10 nm); C, C6α cells transfected with rEap1-pGL3 and treated with E2; D, C6α cells transfected with TK-ERE and treated with E2; E, C6β cells transfected with rEap1-pGL3 and treated with E2, F, C6β cells transfected with TK-ERE and treated with E2. Numbers in parentheses above bars represent number of wells per group; vertical lines are sem. *, P < 0.05; ***, P < 0.01 vs. group not treated with E2 (ANOVA followed by Student-Newman-Keuls test).
P effects on rEap1 transcriptional activity
Because P contributes to enhancing the preovulatory surge of gonadotropins in rats (21), we tested the effects of P on rEap1 transcriptional activity using C6, C6α, and C6β cells. Because the previous experiment (shown in Fig. 4) demonstrated the inability of E2, administered at two different doses, to increase rEap1 gene transcription, we assessed the effect of P using as controls C6, C6α, and C6β cells treated with the highest dose of E2 (10 nm). All groups were examined in either the absence or presence of a transiently transfected plasmid expressing PR-A. Despite a spurious decline in rEap1 promoter activity seen in native C6 cells exposed to P alone or E2 plus P (Fig. 5A, left panel), neither treatment affected Eap1 transcription in C6 cells transfected with PR-A (Fig. 5A, right panel). In C6α cells transfected with PR-A, Eap1 promoter activity was slightly decreased by P alone or E2 plus P (P < 0.05) (Fig. 5B), whereas neither P alone nor E2 plus P changed Eap1-driven transcription in C6β cells, regardless of the presence of PR-A (Fig. 5C).
Figure 5.
Neither P alone nor P given to E2-treated cells increases in vitro rEap1 promoter activity in C6, C6α, or C6β cells. A, left, C6 cells transfected with rEap1-pGL3 and challenged with E2 (10 nm), P (200 nm), or a combination of E2 plus P; right, C6 cells transfected with rEap1-pGL3 and a vector expressing PR-A, and treated with E2, P, or a combination of E2 plus P. hPRA, Human progesterone receptor A. B, same conditions as in A, but applied to C6α cells; C, same conditions as in A, but applied to C6β cells. Numbers in parentheses above bars represent number of wells per group; vertical lines are sem. *, P < 0.05 vs. group treated only with E2 (ANOVA followed by Student-Newman-Keuls test).
The absence of discernible sex steroid effects on rEap1 transcriptional activity in vitro suggests that sex steroids may not be responsible for the increase in hypothalamic Eap1 gene expression seen in vivo at the time of puberty. To address this issue, we examined the expression of rEap1 mRNA in the POA and MBH under two different in vivo conditions: in rats OVX during early juvenile development and euthanized at the expected time of puberty and after inducing an E2-P-induced LH surge in OVX rats.
Hypothalamic Eap1 gene expression increases at the expected time of puberty in the absence of ovarian steroids
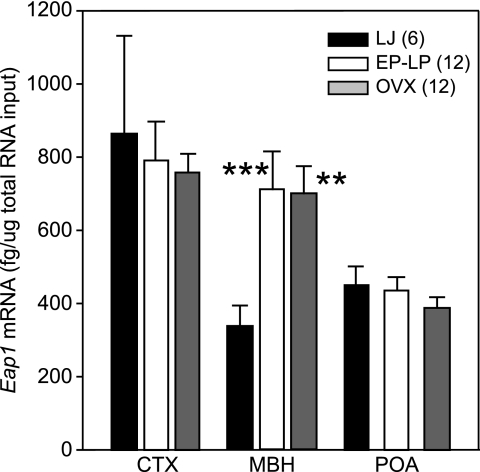
The MBH of intact rats that were in the EP and LP phases of puberty when euthanized between 32 and 34 d of age showed a significant (P < 0.01) increase in Eap1 mRNA levels compared with 29-d-old late juvenile controls (Fig. 6). This result is identical to that previously obtained using semiquantitative PCR (Fig. 1B). An identical increase was observed in age-matched OVX rats (Fig. 6), suggesting that the pubertal increase in Eap1 expression seen in the neuroendocrine brain at the time of puberty does not require ovarian steroids. No changes in Eap1 mRNA abundance were detected in the CTX or POA at the time of puberty in either intact or OVX rats (Fig. 6).
Figure 6.
Absence of ovarian steroids does not prevent the increase in Eap1 mRNA levels that occur in the MBH at the expected time of puberty. OVX indicates rats OVX on d 23 and killed at the same time as the animals undergoing natural puberty. Numbers in parentheses indicate number of animals per group; vertical lines represent sem. ***, P < 0.01; **, P < 0.02 vs. intact LJ controls (ANOVA followed by Student-Newman-Keuls test).
An E2-induced preovulatory surge of gonadotropins increases neither MBH nor POA rEap1 gene expression
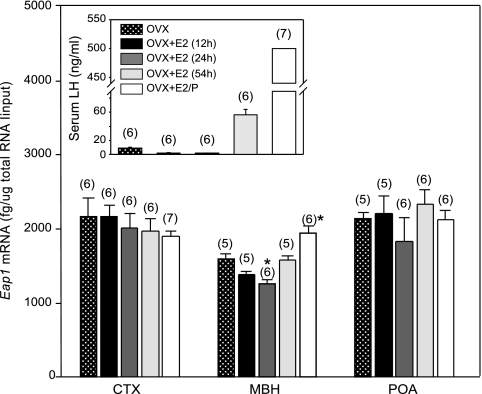
An injection of EB to 28-d-old OVX rats caused an initial decline in serum LH levels 12 and 24 h later (Fig. 7, inset). This decline was followed by a 30- to 40-fold increase in LH levels at 54 h, i.e. in the afternoon of the second day after the injection. As expected (22), administration of a single injection of P 6 h before killing greatly enhanced the EB-induced LH surge (Fig. 7, inset). EB injection resulted in a modest decrease in rEap1 mRNA levels in the MBH after 24 h but failed to affect MBH Eap1 mRNA abundance at the time of the E2-induced LH surge. Administration of P to EB-treated rats resulted in a small (P < 0.05) increase in Eap1 mRNA content in the MBH, but EB, either alone or in combination with P, was unable to alter Eap1 expression in the POA, the site of E2 positive feedback in the rat (23,24). Likewise, neither steroid treatment changed Eap1 mRNA levels in the CTX (Fig. 7).
Figure 7.
Effect of ovarian steroids (administered as a regime that induces a preovulatory surge of gonadotropins) on MBH and POA Eap1 mRNA levels measured by real-time PCR in 31-d-old OVX rats. Eap1 mRNA levels in the POA or CTX were unaffected by the steroid treatments. EB alone (100 μg/kg) caused a small (*, P < 0.05; ANOVA followed by Student-Newman-Keuls test) decrease in Eap1 mRNA levels in the MBH at 24 h but had no discernible effect at the time of the EB-induced preovulatory surge of LH 54 h after injection. P (1 mg/rat) administered to EB-treated rats 6 h before killing resulted in a small increase in Eap1 mRNA content in the MBH (*, P < 0.05) but not the POA. Numbers in parentheses on top of bars are number of animals per group. Vertical lines are sem. Inset, Changes in serum LH levels 12, 24, and 54 h after a single dose of EB or 6 h after injecting EB-treated rats with a single dose of P. Values beyond the upper limit of sensitivity of the assay were assigned an arbitrary value of 500 ng/ml.
Discussion
The present results indicate that Eap1 gene expression is not directly controlled by ovarian steroids and suggest that the increase in Eap1 mRNA abundance that occurs in the hypothalamus at the time of female puberty in both rodents and nonhuman primates is most likely determined by ovary-independent, centrally originated events. By showing this autonomy, Eap1 behaves like Oct2 and Ttf1, the two other presumptive upstream transcriptional regulators of the pubertal process, because hypothalamic expression of both of these genes changes during female prepubertal development in the absence of alterations in ovarian steroid output (3,4,25).
The inability of E2 to affect rEap1 gene transcription is somewhat surprising because of the presence of ERα in EAP1-containing neurons of the POA, VMH, and ARC and the in silico detection of ERE half-sites in the 5′-flanking region of the rEap1 gene. However, none of these incomplete EREs provides the canonical, complete palindromic sequence arrangement required for efficient ER binding (26). Although the ERE detected at −872 to −856 does contain both arms of the ERE palindromic sequence, the downstream arm is imperfect with the nucleotides CCTG in position +5 to +8 replaced by ACAA. An analysis of the impact that variations in the ERE sequence have on E2-induced transcriptional activity led to the conclusion that such substitutions would “decrease ERα binding even in the presence of a perfect ERE half-site in the imperfect palindrome” (27). The inability of E2 to increase Eap1 transcription at both physiological (1 nm) and supraphysiological (10 nm) concentrations provides experimental evidence supporting this prediction.
The 5′-flanking region of the rEap1 gene also contains AP1 and SP1 binding sites (Fig. 3). Although ERα has been shown to recognize such sites, and use them for regulation of gene transcription, such an interaction is not a required feature of estrogen action (18,19). A recent whole genome analysis of ERα binding sites (17) indicated that functional EREs may map to intronic regions or to DNA segments located more than 5 kb from the 5′ and/or 3′ end of the gene. Eap1 is an intronless gene, but we analyzed a large DNA fragment comprising 50 kb upstream and 50 kb downstream of TSS1. The absence of any complete palindromic ERE site in this large DNA segment further suggests that transcription of the rEap1 gene is not under direct estrogen control.
The in vivo studies faithfully corroborated the promoter assay results because EB, administered as a regime able to induce a preovulatory surge of gonadotropins, increased neither MBH nor POA Eap1 mRNA levels. If E2-dependent changes in Eap1 expression were required for the manifestation of the E2 positive feedback on GnRH/gonadotropin secretion, one would expect to see an increase in Eap1 expression in the POA, the primary site of E2 positive feedback in the rodent brain (23,24).
Our in vivo results did show, however, a modest decrease in MBH Eap1 mRNA levels 24 h after administration of EB, at the time when LH was reduced, and a similar modest increase 6 h after P administration, coinciding with the time of the massive increase in LH release induced by the steroid. The most likely interpretation of these data, when they are considered in conjunction with the promoter assay results, is that the small changes we observed in Eap1 mRNA levels after E2 or E2 plus P represent indirect effects exerted via cell-cell communication pathways set in motion by other E2- and P-responsive neuronal and/or glial populations. It is also possible that Eap1 expression in hypothalamic cells containing ERα is affected indirectly by transcriptional activators/repressors and/or posttranscriptional/posttranslational modifiers induced via E2 and/or P-dependent signaling pathways.
Such steroid-dependent interactions may, however, be important only to fine-tune Eap1 expression, because Eap1 mRNA levels increase in the MBH at the expected time of puberty in the absence of ovarian steroids, to levels similar to those seen in intact peripubertal animals. Moreover, E2 treatment of OVX immature rats elicits a robust surge of gonadotropins but does not increase Eap1 mRNA levels in either the MBH or POA. These findings strongly suggest that E2 is not a primary factor underlying the increase in hypothalamic Eap1 mRNA levels observed in the MBH of female rats and female rhesus monkeys at the time of puberty (5).
The age-related decrease in Eap1 mRNA levels detected in the MBH and CTX during neonatal-juvenile development, further argue for the independence of Eap1 expression from ovarian influences. These age-related changes are especially revealing, because hypothalamic Eap1 mRNA abundance begins to decline when estrogen negative feedback is not yet established (28) and continues to decrease unabated during the period when this feedback loop becomes fully functional (29), likely due to an increase in circulating levels of free E2 (30). The mechanisms underlying the age-related postnatal decrease in Eap1 abundance are not known, but they are likely to be driven by developmental events that are not region specific, because the decline is observed in both the CTX and MBH.
We previously reported that selective knockdown of hypothalamic Eap1 expression results in delayed puberty and disruption of the rat estrous cycle (5). The present results suggest that loss of hypothalamic Eap1 expression does not affect estrous cyclicity by interrupting estrogen positive feedback-dependent events. Instead, inhibition of Eap1 expression may affect some other basic regulatory mechanism, such as the circadian synchronization of pulsatile GnRH secretion that occurs during both puberty and the estrous cycle. Further studies are necessary to explore this issue. Also in need of experimental analysis is the potential role that Eap1 may play in the control of male puberty.
Altogether, these results are consistent with the notion that EAP1 may represent a potentially important component of the gonad-independent increase in central drive thought to initiate puberty in both rodents and primates (7,8). It would not be unreasonable to assume that this hierarchy of upstream transcriptional regulators to which Eap1 belongs may be a key molecular component of the central drive. Such a role would also be consistent with the concept that putative upper-echelon genes like Eap1 control the transcription of overlapping, but interacting, regulatory gene networks and that by doing so, they may establish the conditions required for the synchronized activation of neuronal and glial cellular networks involved in the control of puberty (1). It can be also envisioned that these transcriptional regulators may functionally converge with ovarian steroids to regulate targets genes important for the initiation of puberty and subsequent maintenance of reproductive cyclicity.
Supplementary Material
Acknowledgments
We thank Dr. Anda Cornea (Oregon National Primate Research Center Imaging and Morphology Core) for her expert assistance with confocal microscopy imaging. We also thank Ms. Maria Costa for performing the immunohistofluorescence experiments.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant HD25123, the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, RR00163 for the operation of the Oregon National Primate Research Center (to S.R.O.), and National Institute of Mental Health NS20311 (to D.M.D. and R.S.).
Present address for C.M.: Department of Psychiatry, University of Miami, Miami, Florida 33101.
Disclosure Summary: The authors have nothing to declare.
First Published Online November 20, 2008
Abbreviations: ARC, Arcuate nucleus; CTX, cerebral cortex; D1, first diestrus; E, first estrus; E2, estradiol; EAP1, enhanced at puberty 1; EB, E2 benzoate; EP, early proestrus; ERE, estrogen-responsive element; LJ, late juvenile; LP, late proestrus; MBH, medial basal hypothalamus; ME, median eminence; OVX, ovariectomized; P, progesterone; PN, postnatal; POA, preoptic area; PRE, progesterone response element; 5′-RACE, 5′-rapid amplification of cDNA ends; rEap1, rat Eap1; TSS, transcription start site; VMH, ventromedial nucleus.
References
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE 2006 Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 147:1166–1174 [DOI] [PubMed] [Google Scholar]
- Roth CL, Mastronardi C, Lomniczi A, Wright H, Cabrera R, Mungenast AE, Heger S, Jung H, Dubay C, Ojeda SR 2007 Expression of a tumor-related gene network increases in the mammalian hypothalamus at the time of female puberty. Endocrinology 11:5147–5161 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Hill J, Hill DF, Costa ME, Tapia V, Cornea A, Ma YJ 1999 The Oct-2 POU-domain gene in the neuroendocrine brain: a transcriptional regulator of mammalian puberty. Endocrinology 140:3774–3789 [DOI] [PubMed] [Google Scholar]
- Mastronardi C, Smiley GG, Raber J, Kusakabe T, Kawaguchi A, Matagne V, Dietzel A, Heger S, Mungenast AE, Cabrera R, Kimura S, Ojeda SR 2006 Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. J Neurosci 26:13167–13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger S, Mastronardi C, Dissen GA, Lomniczi A, Cabrera R, Roth CL, Jung H, Galimi F, Sippell W, Ojeda SR 2007 Enhanced at puberty 1 (EAP1) is a new transcriptional regulator of the female neuroendocrine reproductive axis. J Clin Invest 117:2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampazzo A, Pivotto F, Occhi G, Tiso N, Bortoluzzi S, Rowen L, Hood L, Nava A, Danieli GA 2000 Characterization of C14orf4, a novel intronless human gene containing a polyglutamine repeat, mapped to the ARVD1 critical region. Biochem Biophys Res Commun 278:766–774 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Terasawa E 2002 Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, eds. Hormones, brain and behavior. Vol 4. New York: Elsevier; 589–659 [Google Scholar]
- Plant TM, Witchel SF 2006 Puberty in nonhuman primates and humans. In: Neill JD, ed. The physiology of reproduction. 3rd ed. San Diego: Academic Press/Elsevier; 2177–2230 [Google Scholar]
- Terasawa E, Fernandez DL 2001 Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK 2006 Puberty in the rat. In: Neill JD, ed. The physiology of reproduction. 3rd ed. Academic Press/Elsevier: San Diego; 2061–2126 [Google Scholar]
- Nishikawa T, Ota T, Isogai T 2000 Prediction whether a human cDNA sequence contains initiation codon by combining statistical information and similarity with protein sequences. Bioinformatics 16:960–967 [DOI] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR 2002 Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology 143:1485–1494 [DOI] [PubMed] [Google Scholar]
- Mhyre AJ, Shapiro RA, Dorsa DM 2006 Estradiol reduces nonclassical transcription at cyclic adenosine 3′,5′-monophosphate response elements in glioma cells expressing estrogen receptor α. Endocrinology 147:1796–1804 [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL 1991 Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 2005 Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol 26:65–84 [DOI] [PubMed] [Google Scholar]
- Smale ST 1997 Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim Biophys Acta 1351:73–88 [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET 2007 Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P 2000 Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S 1997 Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569–1580 [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Cuba VL, Kim H, Wu K, Lee AV, Brown PH 2007 Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol Cell Endocrinol 277:13–25 [DOI] [PubMed] [Google Scholar]
- Freeman ME 2006 Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, ed. The physiology of reproduction. 3rd ed. San Diego: Academic Press/Elsevier; 2327–2388 [Google Scholar]
- Caligaris L, Astrada JJ, Taleisnik S 1972 Influence of age on the release of luteinizing hormone induced by oestrogen and progesterone in immature rats. J Endocrinol 55:97–103 [DOI] [PubMed] [Google Scholar]
- Simerly RB 2002 Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Lee BJ, Cho GJ, Norgren R, Junier MP, Hill DF, Tapia V, Costa ME, Ojeda SR 2001 TTF-1, a homeodomain gene required for diencephalic morphogenesis, is postnatally expressed in the neuroendocrine brain in a developmentally regulated and cell-specific fashion. Mol Cell Neurosci 17:107–126 [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC 2004 Anatomy of the estrogen response element. Trends Endocrinol Metab 15:73–78 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BD, Grazia YR, Kamberi IA, Porter JC 1971 Serum gonadotropin concentrations in intact and castrated neonatal rats. Endocrinology 88:771–776 [DOI] [PubMed] [Google Scholar]
- Andrews WW, Ojeda SR 1981 A quantitative analysis of the maturation of steroid negative feedbacks controlling gonadotropin release in the female rat: Transition from an androgenic to a predominantly estrogenic control. Endocrinology 108:1313–1320 [DOI] [PubMed] [Google Scholar]
- Andrews WW, Mizejewski GJ, Ojeda SR 1981 Development of estradiol-positive feedback on luteinizing hormone release in the female rat: a quantitative study. Endocrinology 109:1404–1413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.