Abstract
A physiological role in satiety is proposed for glucagon-like peptide-1 (GLP-1), secreted by the distal intestine in response to ingested nutrients. Here we report that in rats, ip injection of the GLP-1 receptor (GLP-1-R) antagonist exendin 9-39 (Ex9) elicited hyperphagia, but only at times of day when intake is otherwise low. Furthermore, ip administration of Ex9 attenuated satiety induced by either a voluntarily consumed sucrose meal (by 100%) or an intragastric glucose load (by 40%). To determine whether these effects involve blockade of GLP-1-R in brain or at a peripheral site, we injected Ex9 either centrally (into the third ventricle) or peripherally (ip) prior to GLP-1 injected either centrally or peripherally. Anorexia induced by peripheral GLP-1 was fully blocked by peripheral, but not central, pretreatment with Ex9, whereas the opposite was true for anorexic effect of central GLP-1. Thus, ip Ex9 appears to attenuate satiety via peripheral GLP-1-R blockade. Finally, anorexia induced by ip injection of exendin-4 (a GLP-1-R agonist) was due to both reduced meal size and increased duration between meals. We conclude that GLP-1 released from the intestine in response to ingested nutrients is a physiologically active satiety signal.
Peripheral blockade of glucagon-like peptide-1 (GLP-1) receptors increases spontaneous food intake and attenuates nutrient preload-induced feeding suppression, suggesting that endogenously released GLP-1 is a physiological satiety signal.
The delivery of nutrients to the gastrointestinal tract during a meal activates neural and hormonal responses that promote metabolic homeostasis by both limiting the amount of food consumed and enhancing the clearance of glucose and other nutrients from the bloodstream. Gut-derived mediators that inhibit feeding and lead to meal termination are collectively referred to as satiety signals, whereas a separate set of signals known as incretins augment nutrient-induced insulin secretion, thereby enhancing nutrient uptake (1,2). Prominent among incretin peptides is glucagon-like peptide (GLP)-1, which is secreted by intestinal L cells in response to ingested nutrients. In addition to its incretin effect, systemic administration of GLP-1 or long-acting GLP-1 receptor (GLP-1-R) agonists, such as exendin-4 (Ex4), reduce food intake and body weight, effects that confer therapeutic benefit to humans that receive Ex4 for the treatment of diabetes (1).
These findings raise the possibility that endogenously released GLP-1 serves as both a satiety signal and an incretin, a combination of functions not previously ascribed to any other molecule. In the current studies, therefore, we wished to determine whether GLP-1 plays a physiological role in satiety sensation, a function suggested but not actually confirmed by previous studies. To accomplish this goal, we sought to determine whether blockade of GLP-1 receptors using the GLP-1-R antagonist, exendin (9-39) (Ex9) either increases spontaneous food intake or attenuates the satiety effect of ingested nutrients. The results of these studies provide clear evidence that endogenous GLP-1 signaling is required for intact satiety sensation.
Because GLP-1 and its receptors are present in both the central nervous system (CNS) and peripheral tissues, the feeding effects of GLP-1 could be mediated at either or both sites. Indeed, GLP-1 reduces food intake after either central or systemic administration (3,4,5). In the brain, GLP-1 is synthesized exclusively in neurons in the nucleus of the solitary tract (6) that project to the hypothalamus, hindbrain, and other brain areas where GLP-1-R are expressed (7). GLP-1-R expressed by autonomic fibers innervating the gastrointestinal tract, portal vein, pancreatic islets, and other tissues are also targets for mediating the action of gut-derived GLP-1 (8,9). We therefore sought to determine whether gut-derived GLP-1 exerts its anorexic effects through a peripheral mechanism or by crossing the blood-brain barrier and binding to central GLP-1-R. Our findings that third-intracerebroventricular (3rd-icv) injection of Ex9 did not impair the response to peripheral GLP-1, and conversely, that peripheral Ex9 administration had no effect on the response to 3rd-icv GLP-1, support a model in which meal-related GLP-1 release by the intestine reduces food intake via a peripheral site of action. Combined with our finding that ip Ex4 injection reduces meal size and increases the interval between meals, we conclude that in addition to its function as an incretin peptide, GLP-1 released from the gastrointestinal tract plays a critical physiological role in the detection of satiety by the CNS.
Materials and Methods
Subjects
Naive male Wistar rats (Charles River Laboratories, Inc., Wilmington, MA) were individually housed in ventilated Plexiglass cages in a temperature-controlled room under a 12-h light; 12-h dark cycle. Water and standard rat chow (Purina, St. Louis, MO) were available ad libitum except where otherwise noted. All subjects were handled daily and habituated to ip injection of 1.5 ml saline and measurement of food intake on at least three occasions before the experiments began. Body weight and food intake were measured daily. Study procedures were approved by the Animal Care Committee at the University of Washington and conformed to standards described in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Drugs
GLP-1 was obtained from Bachem (Torrance, CA) and Ex4 and Ex9 were obtained from California Peptide Research (Napa, CA). Each was dissolved in sterile 0.9% saline.
Effect of GLP-1-R blockade on food intake
To determine whether ip Ex9 increases food intake during the light cycle, rats were split into three weight-matched groups (n = 6–7/group) and were treated with either vehicle or Ex9 at a dose of 30 μg/kg ip or 100 μg/kg ip. On the day of the experiment, food was removed from rats' cages 4 h before injections. ip injections were administered 8 h into the light phase. Food was immediately returned and then intake was measured 1 and 2 h later. To examine the effects of Ex9 at a time when rats consume a relatively large amount of food, we subjected three new groups of rats (n = 6/group) to the same procedure, except that injections were delivered immediately before the start of the dark phase. To assess whether Ex9 affects food intake when delivered during the dark but at a time when spontaneous intake is relatively low, rats were deprived of food for 4 h before dark onset, as in the previous studies, and were given their food back immediately before dark with no injections at that time. One hour into the dark phase, food intake was measured and animals received ip injections of vehicle, 30 μg/kg Ex9, or 100 μg/kg Ex9. Food was returned immediately and intake was measured 1 and 2 h later. This protocol used a within-subject design, in which each rat (n = 10) received all three drug conditions on consecutive days in counterbalanced order.
Effect of GLP-1-R blockade on satiety induced by an oral preload
Food was removed from rats' (n = 11) cages 4 h before the dark cycle began. The rats were trained to consume 12.5% sucrose solution over 15 min before the onset of the dark phase and were habituated to receiving ip injections immediately after this sucrose intake session. Within 5 days, the amount of sucrose consumed over this 15-min interval was highly reproducible (mean 10.4 ± 0.4 kcal) and thus constituted a reliable oral preload.
On experiment days, rats received either no access to the sucrose (no load condition) or sucrose access as they had during training (load condition). These conditions were presented in counterbalanced order on consecutive days. Immediately before dark phase onset, rats were given ip injections of saline or Ex9 (100 μg/kg), and preweighed chow was returned to their cages. Food intake was measured 30, 60, and 120 min later. This study used a mixed between/within-subject design, in which rats received either ip saline (n = 6) or ip Ex9 (n = 5) after both the no-preload and preload conditions.
Effect of GLP-1-R blockade on satiety induced by an intragastric (IG) preload
One limitation of a study design in which a nutrient preload is provided through voluntary consumption of a sucrose meal is the inability to include a control preload condition for comparison. To address this limitation, we investigated whether the satiating effect of a nutrient load delivered directly into the stomach is dependent on GLP-1-R signaling. Rats (n = 5) were fitted with a SILASTIC brand (Dow Corning, Midland, MI) IG catheter according to a technique adapted from Davis and Campbell (10).
After at least 1 wk of recovery from surgery, rats were habituated to IG infusion of warm saline followed by access to vanilla Ensure liquid diet (Abbott, Abbott Park, IL) in their home cages for 2 h during the midlight cycle. Chow was removed from their cages 6 h before the start of infusions. These habituation infusions were administered on three occasions before the experiment.
The infusion and intake test procedure was as follows. Tygon tubing from syringes filled with infusate (see below) was connected to the rats' IG catheters, and a motorized syringe pump (World Precision Instruments, Sarasota, FL) was used to infuse 10 ml at a rate of 1 ml/min (chosen to approximate rats' average rate of ingestion). Five minutes after the end of the infusion, the IG catheters were flushed with 0.5 ml saline to ensure that the infusate was delivered to the stomach in its entirety. The infusion tubing was then disconnected, and each rat received an ip injection. Preweighed bottles of vanilla Ensure liquid diet were made available 5 min after injections. Intake of Ensure 30, 60, and 120 min later provided a measure of satiety induced by the IG infusion.
On experimental days, rats received IG infusions of either saline or 10 kcal glucose (10 ml 25% glucose delivered at 1 ml/min), followed by an ip injection of either saline or the GLP-1-R antagonist Ex9 (100 μg/kg). Each rat received all four possible conditions: IG saline/ip saline; IG glucose/ip saline; IG saline/ip Ex9; IG glucose/ip Ex9, presented on consecutive days in counterbalanced order.
Effect of central Ex9 on central vs. peripheral GLP-1-induced anorexia
To verify that the dose of Ex9 selected for 3rd-icv injection attenuates the anorexic effect of central GLP-1, rats were stereotaxically implanted with a 26-gauge cannula (Plastics One, Roanoke, VA) aimed at the third cerebral ventricle. Cannula placement was verified before the start of the experiment through the measurement of a sympathetically mediated increase in plasma glucose 60 min after 3rd-icv injection of 210 μg of 5-thio-d-glucose. After recovering from surgery for at least 1 wk, rats were fasted overnight before the experiment. Overnight fasting was chosen on the basis of previous studies showing reliable effects of 3rd-icv GLP-1 on fasting-induced food intake. Each rat was assigned to one of two groups: icv saline or icv Ex9 (n = 6–7/group). One hour before the onset of the dark phase, rats were given an icv injection of saline or Ex9 (20 μg in 2 μl) followed by a second icv injection of saline or GLP-1 (3 μg in 2 μl) within 30 min before dark onset. Food was returned immediately before dark and intake was measured 30 and 60 min later. The GLP-1 injection conditions were presented in counterbalanced order and separated by 1 wk.
To determine whether blockade of central GLP-1-R can block the anorexic effects of ip GLP-1, rats with 3rd-icv cannulas were assigned to one of two weight-matched groups: icv vehicle or icv Ex9 (n = 5–7/group). On experimental days, food was removed 4 h before the dark cycle. Thirty minutes before the start of the dark phase, rats received a 3rd-icv injection of vehicle or Ex9 (20 μg in 2 μl). Immediately before dark onset, saline or GLP-1 (100 μg/kg ip) was administered and food was returned to the cages. Food intake was measured 30 min later. Each rat received both the saline and GLP-1 ip injection conditions, separated by at least 2 d.
Effect of peripheral Ex9 on central vs. peripheral GLP-1-induced anorexia
To test whether the dose of ip Ex9 (100 μg/kg) used in studies investigating the role of GLP-1 in satiety, described above, can block the effect of the same dose of ip GLP-1 (100 μg/kg) given in the last experiment, food was removed from rats' cages 4 h before the dark cycle began. On the day of the experiment, an ip injection of vehicle or Ex9 was followed by a second ip injection of saline or GLP-1. After the second ip injection, food was returned to the rats' cages. Intake was measured 30 and 60 min later. This study used a between-subject design, such that each rat was placed into one of four groups (n = 12–13/group): saline/saline, Ex9/saline, saline/GLP-1, or Ex9/GLP-1.
To ask whether the dose of ip Ex9 that we used in our preload experiments blocks the effects of central GLP-1 on intake, we split 3rd-icv cannulated rats into two groups, one receiving icv saline and the second receiving icv GLP-1. Rats were fasted overnight before the experiment. On the day of the study, ip saline or Ex9 (100 μg/kg) injections were delivered 15 min before icv injections of saline or GLP-1 (3 μg). The icv injections took place within 30 min before the onset of the dark phase. Immediately before dark, food was returned to the cages, and food was weighed again 30 min later. An additional group of rats received ip Ex9 45 min (rather than 15 min) before 3rd-icv GLP-1 injections to determine whether more prolonged exposure to a systemically administered GLP-1-R antagonist influences the feeding effect of centrally administered GLP-1. The ip conditions were presented in counterbalanced order and separated by one week.
Meal pattern analysis
To determine the size and frequency of meals, rats (n = 6) were housed for 7 d in calorimetry chambers equipped with the Feed-Scale system (Columbus Instruments, Columbus, OH) for continuous monitoring of food intake. The rats were given several days to habituate to these chambers before the experiment began. On experimental days, food was not available during the 4 h before the onset of the dark phase. With order of injection condition counterbalanced across animals, rats were injected with saline or Ex4 (1 μg/kg ip) 5 min before the dark phase, and food was returned after the injections. Conditions were separated by 2 d.
Analysis of meal patterns was performed with a customized computer program (Visual Basic, Microsoft, Redmond, WA) using the following criteria. An individual meal was defined as the consumption of at least 0.2 g of chow separated from the end of the previous meal by at least 10 min. Average meal size was calculated by dividing the number of individual meals by the total amount of food consumed over the test period. We defined meal duration as the time from the beginning to the end of a single meal, and the intermeal interval was defined as the time from the end of one meal to the beginning of the next.
Statistical analysis
The effects of Ex4 on food intake, meal frequency, and meal size were assessed by one-tailed paired-samples Student's t tests. The effect of Ex4 on the size of individual meals during the test period was analyzed by within-subject two-way ANOVA (drug × meal), and the least significant differences test was used for post hoc pairwise comparisons. P < 0.05 was taken to be significant.
In all other experiments, the data were analyzed via two-way ANOVA (between subjects, within subjects, or mixed design, depending on the study), and least significant differences tests were used to perform post hoc pairwise comparisons. P < 0.05 was taken to be significant.
Results
GLP-1-R blockade increased food intake
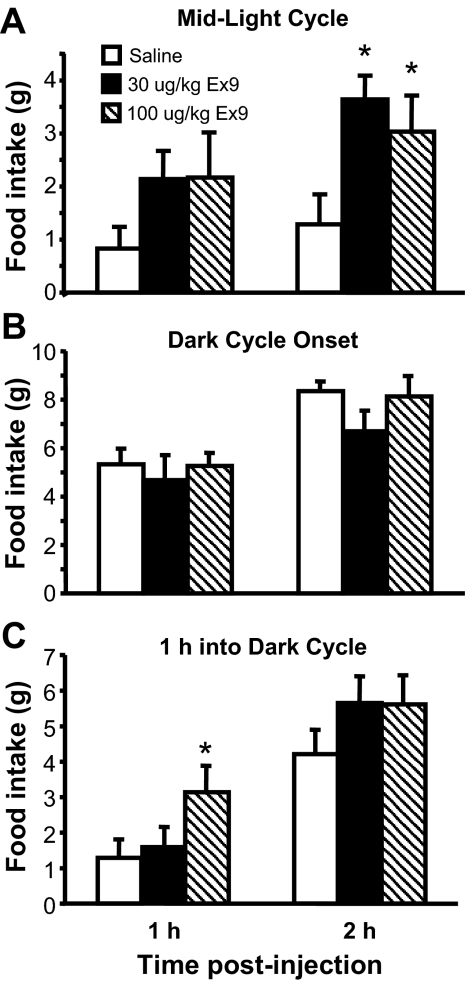
Administration of either of two different doses of the GLP-1-R antagonist Ex9 (30 μg/kg ip and 100 μg/kg ip) during the light phase, when spontaneous food intake is relatively low, increased food intake relative to vehicle [F(2,17) = 6.08, P < 0.05] during the 2 h after treatment (Fig. 1A). In contrast, injection of either dose of Ex9 immediately before the onset of the dark phase, at which point rats normally begin to eat a large meal, had no effect on food intake (Fig. 1B). By comparison, when Ex9 was given 1 h after dark onset, food consumption was increased during the 1 h after ip injection of both doses of Ex9 [F(2,18) = 3.82, P < 0.05] (Fig. 1C), similar to the effect observed during mid-light cycle (Fig. 1A).
Figure 1.
Ex9 effect on food intake. Food intake was measured in rats at 1 and 2 h after ip injection of either saline vehicle or Ex9 (30 or 100 μg/kg) during the middle of the light phase (A); immediately before the dark phase (B), or 1 h into the dark phase (C). Data are mean ± sem. *, P < 0.05 vs. vehicle.
GLP-1-R blockade attenuated nutrient preload-induced satiety
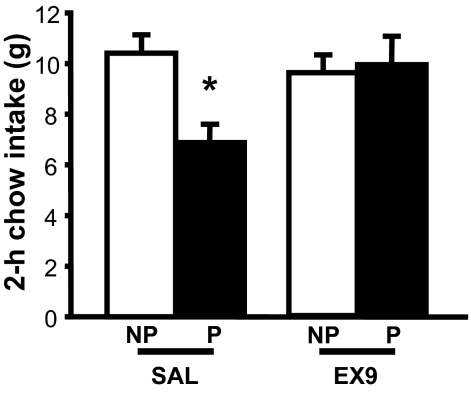
To determine whether spontaneously consumed nutrients reduce subsequent food intake via a GLP-1-dependent mechanism, we asked whether the GLP-1-R antagonist, given immediately before dark cycle onset, blunts the satiating effect of a voluntarily consumed sucrose preload. When rats were injected with saline, the sucrose preload reduced chow intake over the next 2 h (P < 0.05) (Fig. 2). Whereas Ex9 (100 μg/kg ip) had no effect when delivered in the absence of a preload, the effect of the sucrose preload to reduce intake was completely prevented by antagonist treatment [preload × Ex9 interaction, F(1,8) = 22.35, P < 0.01] (Fig. 2).
Figure 2.
Effect of GLP-1-R blockade on oral preload-induced satiety. After training rats to receive access to a sucrose solution for 15 min at the same time each day, chow intake was measured after either no preload (NP) or an orally consumed sucrose preload (P). Preloads were followed immediately by ip injection of either saline (SAL) or Ex9 (100 μg/kg), after which standard chow was returned to the cage and intake measured over the subsequent 2 h period. Data are mean ± sem. *, P < 0.05.
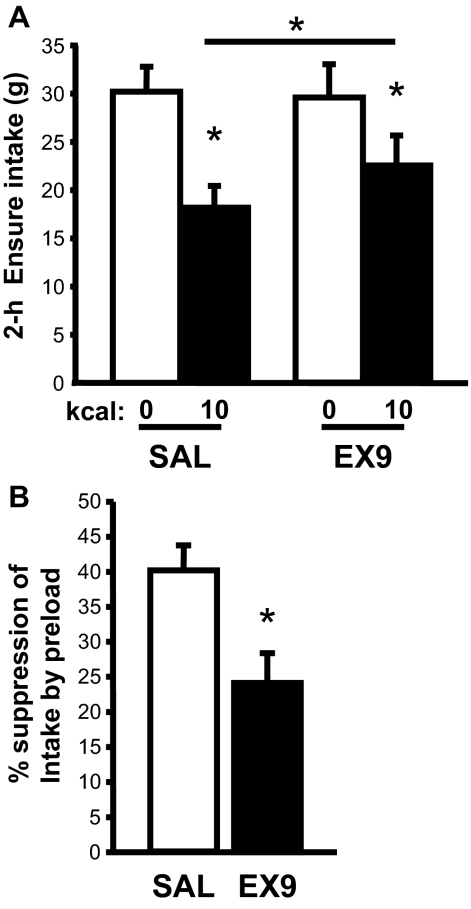
IG infusion of a 10-kcal glucose preload significantly suppressed subsequent liquid diet intake relative to IG saline during a 2-h test session (P < 0.05). When Ex9 (100 μg/kg ip) was administered immediately after the IG infusion ended, the satiating effect of the glucose preload (relative to IG saline) was attenuated (by 40%, Fig. 3) compared with the ip saline condition [preload × Ex9 interaction, F(1,4) = 9.03, P < 0.05].
Figure 3.
Effect of GLP-1-R blockade on IG preload-induced satiety. A, Intake of a palatable liquid diet was measured after IG infusion of either saline (SAL) or glucose, followed immediately by ip injection of either saline or Ex9 (100 μg/kg). B, The percentage of intake suppression induced by the glucose preload shown in A differed after ip saline vs. ip Ex9. Data are mean ± sem. *, P < 0.05.
Central Ex9 did not block peripheral GLP-1-induced anorexia
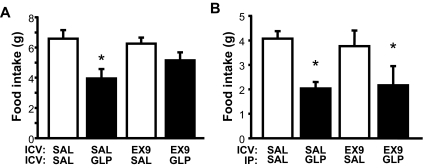
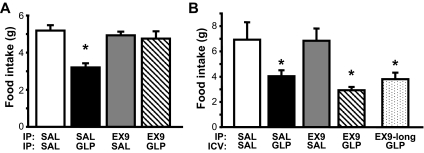
We determined that 3rd-icv pretreatment with Ex9 at a dose of 20 μg strongly attenuated the ability of 3rd-icv GLP-1 to reduce food intake in previously fasted rats, such that the anorexic effect of 3rd-icv GLP-1 was no longer significant at 30 min [Ex9 × GLP-1 interaction, F(1, 11) = 5.95, P < 0.05] (Fig. 4A) and was completely blocked by 3rd-icv Ex9 pretreatment after 1 h (data not shown). By contrast, the effect of systemic GLP-1 (100 μg/kg ip) to reduce food intake (by ∼50%) was not altered by 3rd-icv Ex9 pretreatment at the same dose [main effect of GLP-1, F(1, 10) = 55.15, P < 0.001; interaction with Ex9, P = NS] (Fig. 4B).
Figure 4.
Effect of central GLP-1-R blockade on central vs. peripheral GLP-1-induced anorexia. Food intake was measured in rats that were pretreated with a 3rd-icv injection of either saline (SAL) or Ex9 (20 μg) and then given either a 3rd-icv injection of saline or GLP-1 (3 μg) (A) or an ip injection of saline or GLP-1 (100 μg/kg) (B). Data are mean ± sem. *, P < 0.05.
Peripheral Ex9 did not block CNS GLP-1-induced anorexia
The anorexic response to peripheral GLP-1 (100 μg/kg ip) was fully blocked by the same dose of peripheral Ex9 (100 μg/kg ip) used in our preload experiments [Ex9 × GLP-1 interaction, F(1,46) = 9.13, P < 0.01] (Fig. 5A). By contrast, pretreatment with the same dose of ip Ex9 had no effect on the anorexic response to 3rd-icv GLP-1, regardless of whether the antagonist was delivered 15 or 45 min before the central GLP-1 injection (P < 0.05) [main effect of GLP-1, F(1,8) = 12.65, P < 0.01; interaction with Ex9, P = NS] (Fig. 5B).
Figure 5.
Effect of peripheral GLP-1-R blockade on central vs. peripheral GLP-1-induced anorexia. Food intake was measured in rats that received an ip injection of either saline (SAL) or Ex9 (100 μg/kg) before either an ip injection of saline or GLP-1 (100 μg/kg) (A) or 3rd-icv injection of saline or GLP-1 (3 μg) (B). The icv GLP-1 injection was given either 15 or 45 min (Ex9-long) after ip injection. Data are mean ± sem. *, P < 0.05.
Ex4 reduced meal size in the rat
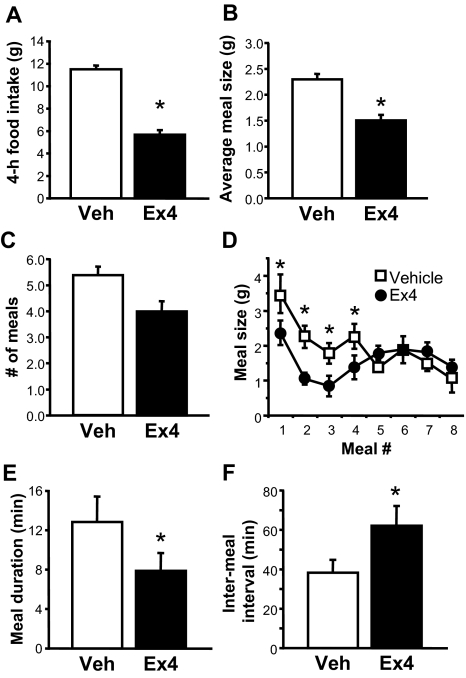
To determine whether anorexia mediated by GLP-1-R stimulation involves reductions of meal size (as expected for a satiety signal), meal frequency, or both, we monitored food intake continuously after ip injection of Ex4 (1 μg/kg) or saline vehicle immediately before the onset of the dark phase. Ex4 reduced food intake over the next 4 h [t (5) = 7.56, P < 0.001] (Fig. 6A), and during this time, average meal size was decreased by drug treatment [t (5) = 3.11, P < 0.05] (Fig. 6B), whereas the number of meals taken was not significantly different across conditions (Fig. 6C). A closer analysis of meal patterns shows that each of the first four meals taken after the onset of the dark phase was reduced in both size [meal × drug interaction, F(6, 30) = 3.02, P < 0.05, post hoc P < 0.05] (Fig. 6D) and duration [t (5) = 2.09, P < 0.05] (Fig. 6E) after Ex4 administration, whereas meals taken thereafter were similar in size across conditions. Moreover, the time required to complete the first four meals after Ex4 was increased by 50% compared with vehicle treatment [vehicle mean 2.36 ± 0.61 h vs. Ex4 mean 3.58 ± 0.56 h, t (5) = −2.71, P < 0.05], due largely to an Ex4-induced increase in the average time between those meals [62%, t (5) = −2.47, P < 0.05] (Fig. 6F).
Figure 6.
Effect of Ex4 on meal pattern. Effect of ip injection of vehicle (Veh) or Ex4 at dark cycle onset on feeding parameters measured during the first 4 h of the dark phase, including food intake (A), average meal size (B), and number of meals (C). D, Effect of Ex4 on meal size varied over time, affecting both the duration (E) and intermeal interval (F) of the first four meals taken. Data are mean ± sem. *, P < 0.05.
Discussion
The pharmacological effect of GLP-1-R agonists to reduce food intake is well established, but the question of whether GLP-1 plays a physiological role as a satiety signal has not been addressed by previous studies. In the current work, we provide strong, direct support for the conclusion that endogenous meal-related GLP-1 plays a physiological role in the control of food intake. First, we confirmed that food intake suppression induced by the GLP-1-R agonist Ex4 arises from a combination of reduced meal size and increased intermeal interval. Conversely, systemic blockade of GLP-1-R with Ex9 increases food intake in rats, an indication that endogenous GLP-1 signaling is a factor that limits intake under normal feeding conditions. Furthermore, systemic administration of Ex9 completely blocked satiety induced by an orally consumed sucrose preload. A second preload study used a strategy in which satiety is induced by an involuntary IG glucose infusion, which allowed us to include the control condition of a nonnutritive preload for comparison. This experiment confirmed the effect of systemic GLP-1-R blockade to attenuate nutrient preload-induced satiety, although the effect was less pronounced than when a sucrose preload was consumed voluntarily. Taken together, our results constitute direct evidence that endogenous GLP-1 released from the GI tract in response to meals plays a physiological role in satiety.
Although many peptide hormones can reduce food intake, most do not meet the criteria, first described by Gibbs (11) and colleagues (2,12), to be considered a physiologically relevant satiety signal. These criteria stipulate that the circulating level of a putative satiety signal must be increased by food intake, have a rapid onset and brief duration of action, and reduce meal size. Its anorexic effects must not be caused by nausea or malaise, and if the signal is thought to act humorally, it must reduce food intake when delivered at physiological doses. Finally, blockade of the signal must increase food intake. Although many gut peptides reduce food intake when delivered pharmacologically, cholecystokinin (CCK) remains the only molecule known to fulfill each of these criteria and to therefore be considered a true satiety signal (2).
It is instructive to compare GLP-1 with CCK with respect to these criteria. It has long been clear that GLP-1 is secreted from intestinal L cells in response to food intake and that its effects, although rapid in onset, are short-lived in part because the active form of GLP-1 is rapidly degraded by the circulating protease dipeptidyl peptidase IV (1). CCK is also released rapidly upon food ingestion and has a short duration of action (13). Second, although high pharmacological doses of GLP-1-R agonists cause viscerosensory stress, evidenced by their ability to support conditioned taste aversion, like CCK, lower GLP-1 doses can effectively reduce food intake without nonspecific or aversive effects (14,15). These observations suggest that at physiological levels, GLP-1's effects on feeding are not due to the sensation of gastrointestinal malaise. A third similarity between the actions of GLP-1 and CCK is evidence that GLP-1's effects on food intake are not humorally mediated but are instead mediated by the vagus nerve. As for CCK, either vagotomy or vagal sensory ablation by capsaicin blocks GLP-1-R stimulation-induced anorexia (16,17,18). The final criterion, that blockade of the signal must increase food intake, has been met for GLP-1 with the results of the studies described here. These findings parallel previous work in which the CCK-A receptor antagonist devazepide was shown to increase food intake on its own and also to blunt satiety induced by intraduodenal nutrient infusion, observations that were critical in the determination that CCK is indeed a physiologically relevant satiety signal (19,20,21). These considerations buttress the proposal that GLP-1 can be added to CCK as the only gut peptides that are clearly documented to participate in satiety. Combined with previous evidence identifying GLP-1 as an endogenous regulator of meal-induced insulin secretion, our findings suggest a unique, physiological role for GLP-1 as both an incretin and a satiety signal.
The very short plasma half-life of GLP-1 (<2 min) is among several findings that support a local enteric action of endogenous, gut-derived GLP-1, rather than an action in the CNS. In addition, the GLP-1-albumnin fusion protein Albugon, which does not cross the blood-brain barrier, reduces feeding when administered systemically (22), and vagal sensory ablation prevents peripherally administered GLP-1- or Ex4-induced anorexia in rodents (17,18), findings that imply a role for vagal afferent fibers in its feeding effects. To investigate whether our findings with the GLP-1-R antagonist are the result of peripheral or central GLP-1-R blockade, we asked whether peripherally administered Ex9 blocks the anorexic effects of central as well as peripheral GLP-1-R stimulation. We found that ip injection of Ex9 increased spontaneous food intake and blocked the satiating effects of IG-infused or orally consumed nutrients and ip GLP-1, yet had no effect on anorexia induced by 3rd-icv GLP-1 administration. These observations are compatible with a peripheral site of Ex9 action in these studies. The converse is true as well; central GLP-1-R blockade was not sufficient to impair the response to peripheral GLP-1 treatment. Similarly, Barrera et al. (23) found that 3rd-icv delivery of the GLP-1-R antagonist des His1, Glu8 exendin-4 did not block the anorexic effects of peripheral GLP-1 treatment, and Hayes and Grill (24) have shown that caudal brain stem application of Ex9 does not reverse the inhibitory effects of IP Ex4 on either food intake or gastric emptying. Taken together, these findings implicate a physiological role for peripheral endogenous GLP-1 and its receptors in the satiety response to ingested nutrients. This conclusion does not rule out the possibility that the CNS GLP-1 system also plays a role. Indeed, caudal brain stem GLP-1-R blockade impairs the satiety response to an orally consumed preload (25). Thus, activation of peripheral GLP-1-R may in turn increase GLP-1 signaling in the CNS (presumably involving activation of GLP-1 neurons in the nucleus of the solitary tract), and this sequence of events may contribute to satiety. This model resembles a mechanism proposed for the incretin effect of peripheral GLP-1-R stimulation and, therefore, warrants further investigation (26).
In the present study, we found that in freely feeding animals, peripherally administered Ex9 increased intake when delivered in the mid-light phase when baseline food intake is low but not when delivered immediately before the dark phase when spontaneous intake is high. Rather than being driven by factors related to circadian rhythm or ambient light, these differences in the feeding response to GLP-1-R blockade appear to be related to differences in recent food consumption and/or baseline spontaneous food intake at those times. This interpretation is based on our finding that Ex9 delivered 1 h after the onset of the dark phase, when animals have already taken their first large meal of the night and baseline intake is low, resulted in increased food intake. These findings provide additional support for the hypothesis that endogenous GLP-1 plays a more dominant role in satiety at times when food intake is low and is less important when animals are highly motivated to eat.
We also observed a seemingly greater effect of GLP-1-R blockade to attenuate preload-induced satiety when a preferred food was consumed voluntarily than when nutrients were infused into the stomach. Whereas it is tempting to speculate about the light that these findings cast on the physiological role of GLP-1 in satiety, differences in experimental design (e.g. glucose vs. sucrose preload, involuntary vs. voluntary consumption of the preload, test meal of Ensure vs. chow, test meal administered during light vs. dark phase) may also have contributed to the outcome. Thus, additional studies are warranted to clarify whether the role played by GLP-1 in satiety is influenced by the nutrient composition or caloric content of foods, the route or timing of ingestion, or other factors.
One limitation to the interpretation of our experiments with Ex9 is that GLP-1 is not the sole ligand for GLP-1-R. Oxyntomodulin (OXM), a peptide closely related to GLP-1 that is also released by the L cells of the distal intestine, also binds to GLP-1-R, albeit with reduced affinity (27). Like GLP-1, OXM is secreted in response to food intake and reduces feeding when delivered pharmacologically (28). Whether peripherally delivered OXM reduces food intake via peripheral GLP-1-R activation has not been determined, and available data implicate an as-yet-unidentified receptor in at least some OXM effects (29). Moreover, OXM binds and activates GLP-1-R with more than 50-fold weaker affinity (27) and more than 300-fold lower efficacy (30) compared with GLP-1. Although these estimates derive from studies in heterologous cell systems, these data suggest that plasma OXM levels, although typically greater than levels of GLP-1, are insufficiently high to explain the satiety action of GLP-1-R signaling. Lastly, available evidence points to a central mechanism for OXM effects on feeding. For example, Dakin et al. (28) reported that peripheral OXM-induced anorexia was blunted by intrahypothalamic (arcuate nucleus) Ex9 pretreatment. Therefore, although we cannot exclude the possibility that some portion of the Ex9 effects that we observed is attributable to endogenous OXM action, we view this possibility as unlikely.
Our finding that Ex4 reduces meal size in rats is consistent with the findings that iv administration of GLP-1 reduces meal size in rats and that Ex4 reduces meal size in nonhuman primates (31,32). In addition, we found the intermeal interval was also prolonged by Ex4 administration. Thus, pharmacologic GLP-1-R stimulation appears to induce satiation (the decision to terminate a meal) as well as promote satiety (the interval after a meal that precedes the emergence of hunger and the onset of the next meal). In this context, we note that our preload experiments using Ex9 also support a role for endogenous GLP-1 in satiety because the design of those studies examines preload-induced satiety more so than satiation. The preloads in those experiments took place before the test meal and so were not designed to enhance satiation during the test meal. Thus, the effect of GLP-1-R blockade in these experiments may be attributed to an effect on satiety. Although we acknowledge the limitations inherent in studies that measure pharmacological effects of GLP-1R ligands, we conclude that the relevant literature combined with our studies using both agonists and antagonists supports the hypothesis that GLP-1 plays a role in both satiety and satiation.
A priority for future research will be to clarify the mechanisms by which peripheral GLP-1-R mediates its effects on feeding behavior. GLP-1's incretin effects have been hypothesized to occur via a local action on afferent autonomic fibers in the gut and/or hepatic portal vein, as opposed to a direct action on β-cells (33). Although the present studies did not investigate the specific sites of action for GLP-1's satiety effects, our findings combined with available literature support a similar model. This analysis raises the intriguing possibility that both satiety and incretin effects of GLP-1 are mediated by a single common pathway involving autonomic communication between the gut and brain.
Acknowledgments
We are grateful for technological expertise provided by Dr. Kayoko Ogimoto, Miles Matsen, and Iaela David.
Footnotes
This work was supported by National Institutes of Health Grants DK52989, DK083042, and DK68384 (to M.W.S.) and 7K99DK078779 (to D.L.W.) and a New Investigator Award from the Obesity Society (to D.L.W.). This material is also based on work supported in part by the Office of Research and Development Medical Research Service, Department of Veterans Affairs. D.G.B. is senior research career scientist, Research and Development Service, Department of Veterans Affairs Puget Sound Health Care System, Seattle, Washington. Meal pattern analysis was performed with support from the Clinical Nutrition Research Unit at the University of Washington supported by National Institutes of Health Grant P30 DK035816. This research was also supported by the facilities of the Cellular and Molecular Imaging Core of the University of Washington National Institutes of Health Diabetes Endocrinology Research Center, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-17047.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 12, 2008
Abbreviations: CCK, Cholecystokinin; CNS, central nervous system; Ex4, exendin-4; Ex9, exendin 9-39; GLP, glucagon-like peptide; GLP-1-R, GLP-1 receptor; IG, intragastric; OXM, oxyntomodulin; 3rd-icv, third-intracerebroventricular.
References
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Woods SC 2005 Signals that influence food intake and body weight. Physiol Behav 86:709–716 [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW 2006 Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55:3387–3393 [DOI] [PubMed] [Google Scholar]
- Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gomez R, Munoz RM, Eng J, Blazquez E 2000 Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49:709–717 [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath, MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR 1996 A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C 1997 Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77:257–270 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P 1999 Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K 2004 Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110:36–43 [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Taylor RG, Fuller PJ 1998 Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 141:179–186 [DOI] [PubMed] [Google Scholar]
- Davis JD, Campbell CS 1975 Chronic intrajugular, intraportal, gastric, and duodenal cannulae for the rat. In: Singh D, Avery DD, eds. Physiological techniques in behavioral research. Monterey, CA: Brooks Cole; 163–177 [Google Scholar]
- Gibbs J, Young RC, Smith GP 1973 Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84:488–495 [DOI] [PubMed] [Google Scholar]
- Smith GP 1999 Introduction to the reviews on peptides and the control of food intake and body weight. Neuropeptides 33:323–328 [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP 2004 Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 286:G183–G188 [DOI] [PubMed] [Google Scholar]
- Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA 2006 Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes 30:1332–1340 [DOI] [PubMed] [Google Scholar]
- Perez C, Sclafani A 1991 Cholecystokinin conditions flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol 260:R179–R185 [DOI] [PubMed] [Google Scholar]
- Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ 1997 Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272:R1245–R1251 [DOI] [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR 2005 The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, and Brubaker PL 2005 Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146:3748–3756 [DOI] [PubMed] [Google Scholar]
- Yox DP, Brenner L, Ritter RC 1992 CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol 262:R554–R561 [DOI] [PubMed] [Google Scholar]
- Brenner LA, Ritter RC 1996 Type A CCK receptors mediate satiety effects of intestinal nutrients. Pharmacol Biochem Behav 54:625–631 [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Castellanos DA, Hulce M 2003 Effects of peripheral CCK receptor blockade on food intake in rats. Am J Physiol Regul Integr Comp Physiol 285:R429–R437 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ 2004 A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53:2492–2500 [DOI] [PubMed] [Google Scholar]
- Barrera JG, Woods SC, Seeley RJ, Central GLP-1 receptor antagonism does not block peripheral exendin-4-induced anorexia. Keystone Symposia (J2), Obesity: Peripheral and Central Pathways Regulating Energy Homeostasis, Keystone, CO, 2007, p 144 (Abstract P104) [Google Scholar]
- Hayes MR, Grill HJ 2007 The caudal brainstem is sufficient to mediate inhibition of gastric emptying by peripheral glucagon-like-peptide-1 receptor activation. Appetite 49:296 (Abstract) [Google Scholar]
- Hayes MR, Grill HJ 2007 Caudal brainstem integration is sufficient for anorectic responses to central and to peripheral GLP-1R agonist treatment with endogenous hindbrain GLP-1R activity contributing to satiation control. Obesity 15:A15 (Abstract) [Google Scholar]
- Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R 2005 Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehmann HC, Jiang J, Schweinfurth J, Wheeler MB, Boyd III AE, Goke B 1994 Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I (7–36)-amide, oxyntomodulin, exendin-4, and exendin (9–39). Peptides 15:453–456 [DOI] [PubMed] [Google Scholar]
- Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR 2004 Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 145:2687–2695 [DOI] [PubMed] [Google Scholar]
- Sowden GL, Drucker DJ, Weinshenker D, Swoap SJ 2007 Oxyntomodulin increases intrinsic heart rate in mice independent of the glucagon-like peptide-1 receptor. Am J Physiol Regul Integr Comp Physiol 292:R962–R970 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE 2007 Oxyntomodulin differentially affects glucagon-like peptide-1 receptor β-arrestin recruitment and signaling through G-α-s. J Pharmacol Exp Ther 322:148–154 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD 2005 Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- Scott KA, Moran TH 2007 The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293:R983–R987 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman, JP, D'Alessio DA 2007 Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]