Abstract
Cortisol is an important mediator of physiological stress responses. Hypothalamic CRH and arginine vasopressin (AVP) and pituitary ACTH, in addition to hypothalamic and pituitary cortisol feedback, regulate cortisol secretion. Importantly, joint interactions among the four, rather than the signal of any one hormone, govern this life-preserving axis. Quantifying in vivo strength of such joint interactions has been difficult, especially without direct injection of cortisol, CRH, AVP, or ACTH. The goal of the present research was to estimate these joint feedback and feedforward interactions in vivo in the conscious horse during low-cortisol and hypoglycemic stress. Pituitary venous sampling of ACTH, CRH, and AVP was performed every 0.5–1 min and jugular venous sampling of cortisol every 15–20 min. Estimation of hypothalamic dynamics revealed that: 1) hypocortisolemia amplifies CRH and AVP secretion, when mean (slow) and rate-adjusted (rapid) cortisol feedback concentrations decrease by 0–25%; and 2) reduced peptide feedback augments CRH and AVP secretion, when CRH and AVP secretion each decreases by 0–25 and 50% of its respective maximum. Thus, low-cortisol feedback enhances CRH outflow in part by relieving CRH's autoinhibition. Estimation of pituitary dynamics disclosed that: 1) endogenous CRH and AVP synergize in evoking ACTH secretion, and 2) hypocortisolemia potentiates individual and conjoint stimulation of ACTH secretion by CRH and AVP. Formulations such as the present one should have application to evaluating other complex endocrine dynamics.
This report discusses a general model for estimating feed-back and feed-forward in vivo.
Stress adaptations comprise multifold endocrine, metabolic, and autonomic nervous system adjustments to abrupt changes in the external environment and internal chemical milieu (1,2). Adaptations include mobilization of amino acids, glucose, and fatty acids as metabolic fuels, immune regulation, suppression of inflammation, behavioral fight or flight responses, memory consolidation, and cardiovascular reactions. Glucocorticoids, such as cortisol, are crucial mediators of the physiological stress response and primary regulators of feedback (3,4).
Cortisol is secreted by the adrenal zona fasciculata under intermittent stimulation by pituitary ACTH (5,6). ACTH secretion is driven individually and synergistically by CRH and arginine vasopressin (AVP), which originate in hypothalamic paraventricular nuclei (7,8,9). Cortisol feeds back to repress CRH, AVP, and ACTH secretion via direct and indirect pathways, which include the limbic system, hypothalamus, and pituitary gland (10,11,12,13). Thus, reciprocal interactions among CRH, AVP, ACTH, and cortisol, rather than signaling by any one hormone alone, determine the dynamics of this life-preserving axis (11,14).
Analytical constructs have been designed recently to estimate in vivo feedforward dose-response functions linking ACTH → cortisol, GnRH → LH, and LH → testosterone noninvasively (3,6,15,16,17,18,19). Other important simulation models and analytical methods exist (5,20,21,22,23,24). However, none permits quantitative estimation of multiple in vivo regulatory pathways simultaneously. In particular, no model exists in the literature in any species to: 1) assess fast/slow feedback dose-response functions in vivo without infusing cortisol; 2) estimate autofeedback by endogenous peptides on their own release; and 3) quantify endogenous CRH/AVP synergy (6,37). The present work frames an analytically tractable model of the CRH-AVP-ACTH-cortisol ensemble as a prototype of complex reciprocal interactions. The objective was to estimate endogenous feedforward and feedback dynamics in a tetrapartite (four hormone) nonlinearly coupled system. The hypothesis was that ensemble dynamics exhibit quantifiable: 1) time evolution after an endogenous stressor, such as acute cortisol depletion or hypoglycemia; 2) individual and joint feedback of cortisol on ACTH, CRH, and AVP outflow; 3) synergy between CRH and AVP in driving ACTH secretion; and 4) possible in vivo autofeedback by hypothalamic CRH and AVP. Direct high-frequency pituitary blood sampling in the procedurally adapted, conscious, unmedicated, and unrestrained horse is ideal for evaluating a new model of hypothalamo-pituitary regulation.
Materials and Methods
Animals
Six standard-bred horses (Lincoln University Equine Research Unit) weighing 500–550 kg were used. Horses were maintained outdoors on pasture and did not require supplementary feeding. At least 24 h before the start of each experiment, a cannula (40 cm, 7 French Berestein; Bard International, Murray Hill, NJ) was placed in the intercavernous sinus using the method of Irvine and Alexander (25) to enable collection of pituitary venous (PV) blood. This nonsurgical technique involves cannulation of a venous pathway unique to equids, and is performed under local anesthesia and mild xylazine tranquilization (0.2 mg/kg, iv). The procedure was approved by the Lincoln University Animal Ethics Committee, which adheres to guidelines similar to those of the National Institutes of Health. A cannula (Angiocath, 16 gauge, 5.2 in.; Deseret Co., Sandy, UT) was also placed in a jugular (JUG) vein. Animals were returned to pasture overnight until study the next morning (0938–1222 h).
Experimental method
Experiments commenced between 0938 and 1222 h. Each animal was placed in a small outside yard to which it was accustomed. Heparin (100,000–125,000 U) was administered iv before and after approximately 2 h sampling. PV blood was collected continuously by peristaltic pump and divided into 30-sec 2.5- to 3.0-ml segments for 0.5 h, after which insulin (0.4 U/kg iv bolus; Novo Nordisk, Bagsværd, Denmark) or metyrapone (Metopiron; Ciba-Geigy, Basel, Switzerland) was given (bolus iv injection of 1.25 g, 0.1% wt/vol solution in a 0.44 m tartaric acid, followed by a constant iv infusion of 8.75 g metyrapone given over 2.5–3.3 h, rate 0.5 g metyrapone in 0.5 ml/min). JUG blood was collected by syringe every 15 or 20 min for glucose and cortisol measurements.
Sample handling
Blood was collected into polystyrene tubes packed in a water and ice bath, and was centrifuged at 4 C within 20 min collection. Plasma was aliquoted, snap frozen on dry ice, and stored at −20 C until assay.
Hormone assays
ACTH was assayed in PV samples by RIA without prior extraction as described previously (25). The detection limit of the assay was 13 pmol/liter plasma. The within-assay coefficient of variation (CV) was 7.6%, and the between-assay CV was 8.5%.
CRH was assayed by RIA in PV samples after methanol extraction (26). The detection limit of the assay was 0.2 pmol/liter plasma. Within- and between-assay CVs were 4.5 and 8.4%, respectively.
AVP was assayed by RIA in PV samples without prior extraction (26). The detection limit was 2.4 pmol/liter plasma. Within- and between-assay CVs were 7.0 and 9.2%, respectively.
Cortisol was assayed in JUG samples by RIA using antibody Z (gift from C. Munro, University of California, Davis, CA) at a final dilution of 1:180,000. The detection limit of the cortisol assay was 2.5 nmol/liter plasma. Within- and between-assay CVs were 3.4 and 4.7%, respectively. Glucose was measured in JUG plasma by the glucose-oxidase technique.
All assays except glucose and CRH were performed in duplicate.
Data in six horses
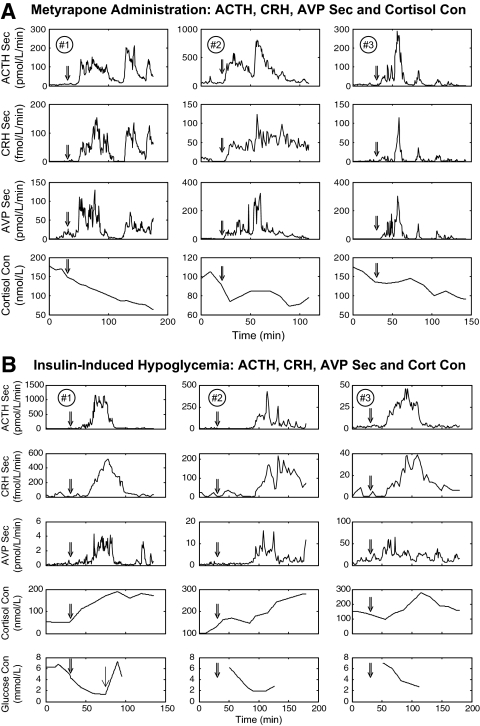
Data comprised ACTH, CRH, and AVP secretion rates and cortisol concentrations previously measured by intensive sampling of PV blood in six normal horses: two estrous mares, two luteal mares, and two castrate stallions (27,28). Three animals were observed before and during iv administration of metyrapone to lower cortisol concentrations, and three other animals were sampled before and during insulin-induced hypoglycemia (Fig. 1). In each horse, ACTH, CRH, and AVP secretion (flow × concentration) and cortisol concentrations were monitored for 0.5 h before and approximately 2.5 h after the onset of the stimulus.
Figure 1.
Pituitary blood ACTH, CRH, and AVP secretion (Sec) rates and cortisol (Cort) concentrations (Con) monitored for approximately 2 ½ h in three horses administered metyrapone (A) (30 sec sampling) and three others subjected to hypoglycemia (B) (30 sec and 1 min sampling). Double arrow denotes start of metyrapone infusion or bolus insulin injection. Single arrow (bottom left) denotes infusion of 24% glucose at 66 min in horse no. 1. Cortisol was measured every 10–15 min, and glucose as shown.
Analyses
Hypoglycemia resulted in initial concomitant secretion of CRH, AVP, ACTH, and cortisol, therein making it difficult to determine hypothalamic feedback. Metyrapone administration stimulated ACTH, CRH, and AVP secretion due to cortisol disinhibition, thus permitting estimates of both hypothalamic and pituitary feedback.
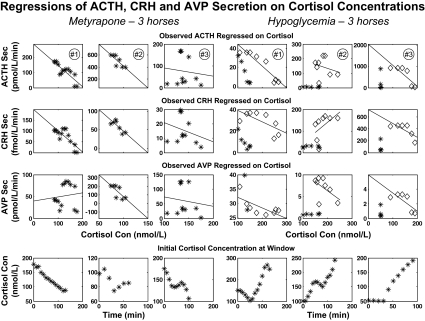
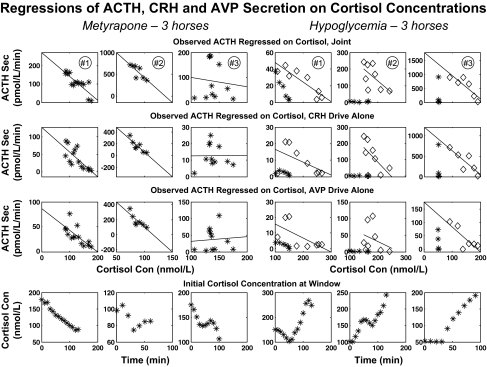
To make a preliminary assessment of cortisol feedback on ACTH, CRH, and AVP secretion, we constructed successive windows of data length 40 min that were shifted by 10 min throughout the full time series. Within each window, the 84th percentile values (mean + 1 sd for a one-tailed distribution) for ACTH, CRH, and AVP secretion rates were calculated. The cortisol concentration at the start of each window was taken as the putative feedback signal. The resulting set of window values in each horse was used to regress ACTH, CRH, and AVP secretion rates on cortisol concentrations (Fig. 2). This approach estimates inhibition of ACTH, CRH, and AVP individually by cortisol but does not quantify more complex interactions controlling ACTH secretion. Thus, the next step was to partition regulation of the time-evolving ACTH secretion rate into the component due solely to CRH stimulation, solely to AVP stimulation, joint CRH and AVP synergism, and cortisol's inhibition of pituitary synergism (Fig. 3). The supplemental appendix, which is published as supplemental data on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, gives a mathematical model of these CRH-AVP-ACTH-cortisol feedback and feedforward interactions.
Figure 2.
Linear regression of ACTH, CRH, and AVP secretion (Sec) rates on cortisol concentrations (Con) in 40-min windows successively advanced by 10-min increments. Each data point corresponds to the 84th percentile value of the ACTH, CRH, or AVP secretion rate in a given window and the matching cortisol concentration at the start of that window (from Fig. 2). The six columns correspond to the six horses: the left three for metyrapone and the right three for hypoglycemia. Top to bottom, The upper three rows show individual window values for ACTH secretion rate vs. cortisol, CRH secretion rate vs. cortisol, and AVP secretion rate vs. cortisol. The bottom row is a plot of cortisol concentrations vs. time. For hypoglycemia, the first hour of euglycemia was excluded from least-squares linear regression (excluded time points are plotted as asterisks, the remaining points as diamonds). Estimated slopes, se values, and P values (testing that slope is negative against nonnegative) are given in Table 1.
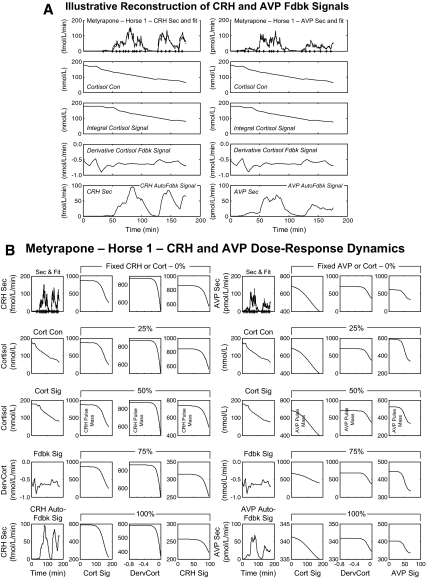
Figure 3.
A, Model-based estimates (fits) of hypothalamic CRH (left) and AVP (right) secretion (Sec), and associated feedback (Fdbk) signals (Sig) in a horse (no. 1) given metyrapone. Top row, Experimentally observed CRH and AVP secretion rates (solid lines) and estimated fits (dashed lines). The next rows depict (top to bottom): measured cortisol (Cort) concentrations (Con); estimated slow (time convolved) and rapid [time derivative (Derv)] cortisol feedback signals; and (estimated) CRH and AVP autofeedback signals. B, Secretory burst mass (y-axes) modeled as a logistical dose-response function of time-delayed (slow) and rate-dependent (fast) cortisol feedback and homologous peptide autofeedback (x-axes) in the same metyrapone-treated horse. The left four columns present CRH dose-response dynamics and the right four those for AVP. The first column of each set contains the same plots as panel A to facilitate interpretation. The second and third columns in each set represent, respectively, the effects of slow and rapid feedback by cortisol at fixed CRH or AVP levels. The fourth column in each set displays the effects of CRH or AVP autofeedback at fixed cortisol strata. The five rows in each column correspond to fixed feedback levels: 0 (lowest observed), 25, 50, 75, and 100% (maximal observed). Note different y-axis scales.
Two conceptual steps were involved in representing two-peptide synergy and feedback. First, pulsatile secretion was estimated for the two hypothalamic neuropeptides, CRH and AVP (29,30). Secretory burst mass was then modeled as a logistical function of the cortisol concentration (slow negative feedback), the rate of change in cortisol concentrations (rapid negative feedback), and negative autofeedback by each peptide. To accommodate physiological variations in burst mass, random effects were allowed in pulse-by-pulse dose-response efficacy (18). Second, for each 40-min window, ACTH secretion was constructed as a logistical dose-response function of individual and combined feedforward by CRH and AVP. This analytical construction permits quantification of the dose-responsive effects of: 1) CRH, and 2) AVP on ACTH secretion individually, and 3) together.
The input into Figs. 2 and 4–6 is calculations over moving windows of length 40 min using the ACTH, CRH, and AVP secretion models (formulated in the supplemental appendix). The models allow one to partition observed ACTH secretion rates into portions due to CRH alone, AVP alone, and CRH and AVP jointly. Associations between measured and modeled parameters were assessed by linear regression. The P values given in Tables 1–3 are the result of testing whether slopes of the linear regressions are nonzero.
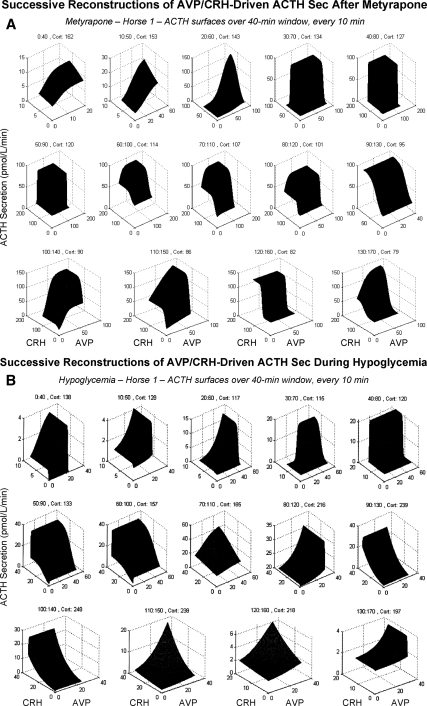
Figure 4.
Time-evolving ACTH secretory dynamics modeled via individual and combined CRH and AVP feedforward under noncompetitive cortisol (Cort) feedback. A, Data from one horse administered metyrapone. B, Data from another horse subjected to hypoglycemia. Because cortisol concentrations change much more slowly than CRH or AVP secretion (Sec) rates, overall time was divided into successive 40-min windows each advanced by a 10-min increment (left to right and top to bottom). Data contained in each window were used to estimate CRH and AVP drive of ACTH secretion through logistical dose-response functions. Therefore, consecutive dose-response surfaces reflect the effects of time and changing concentrations of cortisol, as stated above each window.
Figure 5.
Estimation of feedback by cortisol onto overall and individual drive of ACTH secretion (Sec) by CRH and AVP. Analyses are based upon logistical dose-responses surfaces constructed from 40-min time windows (illustrated in Fig. 4). For overall (joint) peptide stimulation (top row), points were calculated from successive surfaces at the 84th percentile of CRH and the 84th percentile of AVP secretion to illustrate the dynamics. Matching cortisol is from the start of each window. For CRH drive alone (second row), the 84th percentile of CRH and the zero percentile of AVP secretion were used. The converse applies for AVP action alone (third row). The six columns correspond to the six horses: left metyrapone (n = 3), right hypoglycemia (n = 3). Lines are least-squares regressions. For hypoglycemia, the first hour of englycemia was excluded from least-squares linear regression (excluded time points are plotted as asterisks, the remaining points as diamonds). Estimated slopes, se values, and P values are given in Table 2. Con, Concentration.
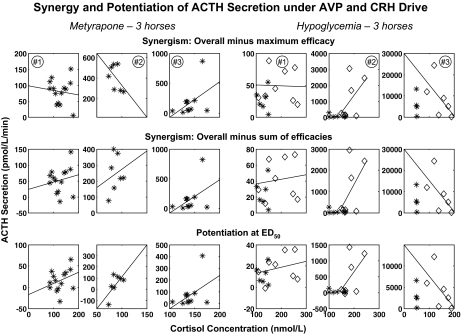
Figure 6.
Synergism was defined as the difference between the overall (combined peptide) estimate and: the maximum of that obtained individually for CRH and AVP (top), or the sum of CRH and AVP efficacies (middle); and potentiation as (bottom) the difference between the joint potency effect and the sum of the ACTH effects of CRH and AVP at their potencies. Data are presented otherwise as described in the legend of Fig. 5. Statistical analyses and synergy/potentiation estimates are given in Table 3. For hypoglycemia, the first hour of euglycemia was excluded from least-squares regression (excluded time points are plotted as asterisks, the remaining points as diamonds).
Table 1.
Slope estimates for linear regressions in Fig. 2
| Metyrapone
|
Hypoglycemia
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Horse 1 | Horse 2 | Horse 3 | Overall | Horse 1 | Horse 2 | Horse 3 | Overall | |
| Slope of ACTH on cortisol | −1.41 | −7.2 | −0.37 | −3.0 | −0.23 | −0.54 | −9.9 | −3.6 |
| (0.26) | (2.4) | (1.2) | (0.90) | (0.04) | (0.85) | (3.3) | (1.1) | |
| df = 12 | df = 5 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (a) | (b) | (NS) | (a) | (a) | (NS) | (b) | (b) | |
| Slope of CRH on cortisol | −0.98 | −1.2 | −0.13 | −0.76 | −0.11 | +0.94 | −2.4 | −0.51 |
| (0.22) | (0.37) | (0.16) | (0.15) | (0.048) | (0.63) | (1.1) | (0.41) | |
| df = 12 | df = 52 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (a) | (b) | (NS) | (a) | (b) | (NS) | (b) | (NS) | |
| Slope of AVP on cortisol | +0.09 | −5.1 | −0.30 | −1.8 | −0.044 | −0.040 | −0.025 | −0.034 |
| (0.25) | (2.2) | (0.90) | (0.81) | (0.015) | (0.027) | (0.011) | (0.011) | |
| df = 12 | df = 5 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (NS) | (b) | (NS) | (b) | (b) | (NS) | (b) | (b) | |
Least-squares linear regression-estimated slopes, in parentheses their se values errors, degrees of freedom (df), and P values (testing that slope is negative against its being nonnegative) for data shown in Fig. 2 (left metyrapone, right hypoglycemia). The overall estimate (fourth and eighth columns) is the average estimate for the three animals. NS, Not significant.
P < 0.01.
P < 0.05.
Table 2.
Slope estimates for regressions in Fig. 5
| Metyrapone
|
Hypoglycemia
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Horse 1 | Horse 2 | Horse 3 | Overall | Horse 1 | Horse 2 | Horse 3 | Overall | |
| Slope of ACTH on CRH and AVP jointly | −1.29 | −11.5 | −0.37 | −4.4 | −0.23 | −1.5 | −8.3 | −3.3 |
| (0.25) | (4.1) | (1.3) | (1.4) | (0.04) | (0.79) | (2.5) | (0.89) | |
| df = 12 | df = 5 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (a) | (b) | (NS) | (b) | (a) | (NS) | (b) | (a) | |
| Slope of ACTH on CRH alone | −0.71 | −8.4 | −0.002 | −3.0 | −0.08 | −1.8 | −5.8 | −2.6 |
| (0.18) | (2.1) | (0.13) | (0.70) | (0.048) | (0.97) | (2.1) | (0.75) | |
| df = 12 | df = 5 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (a) | (a) | (NS) | (a) | (NS) | (NS) | (b) | (b) | |
| Slope of ACTH on AVP alone | −0.44 | −6.6 | +0.15 | −2.3 | −0.089 | −0.40 | −0.93 | −0.47 |
| (0.12) | (2.0) | (0.62) | (0.71) | (0.04) | (0.53) | (0.34) | (0.21) | |
| df = 12 | df = 5 | df = 9 | df = 5 | df = 6 | df = 6 | df = 4 | df = 4 | |
| (a) | (b) | (NS) | (b) | (b) | (NS) | (b) | (b) | |
Statistical summary of regression analysis presented in Fig. 5. Data are sample means, se values, degrees of freedom (df), and P values as described in Table 1. NS, Not significant.
P < 0.01.
P < 0.05.
Table 3.
Mean synergy estimates from regressions in Fig. 6
| Metyrapone
|
Hypoglycemia
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Horse 1 | Horse 2 | Horse 3 | Overall | Horse 1 | Horse 2 | Horse 3 | Overall | |
| Joint effect minus maximum of CRH and AVP effect | 80.1 | 405 | 172 | 219 | 48.9 | 987 | 8570 | 3200 |
| (10.3) | (47.2) | (73.0) | (29.2) | (9.28) | (431) | (3670) | (1230) | |
| df = 13 | df = 6 | df = 10 | df = 6 | df = 7 | df = 7 | df = 5 | df = 5 | |
| P < 0.001 | P < 0.001 | P = 0.02 | P < 0.001 | P < 0.001 | P = 0.028 | P = 0.033 | P = 0.020 | |
| Joint effect minus sum of maximal effects of CRH and AVP | 52.6 | 2451 | 155 | 151 | 41.8 | 945 | 8530 | 3170 |
| (11) | (44) | (69) | (28) | (8.9) | (426) | (3660) | (1230) | |
| df = 13 | df = 6 | df = 10 | df = 6 | df = 7 | df = 7 | df = 5 | df = 5 | |
| P < 0.001 | P < 0.001 | P = 0.025 | P = 0.0008 | P = 0.0011 | P = 0.031 | P = 0.034 | P = 0.02 | |
| Potentiation at ED50 | 15.8 | 46.5 | 73.7 | 45.3 | 18.5 | 466 | 4250 | 1570 |
| (6.7) | (34) | (35) | (16) | (4.6) | (210) | (1820) | (611) | |
| df = 13 | df = 6 | df = 10 | df = 6 | df = 7 | df = 7 | df = 5 | df = 5 | |
| P = 0.018 | P = 0.11 | P = 0.03 | P = 0.02 | P = 0.0025 | P = 0.033 | P = 0.034 | P = 0.02 | |
Data are individual and overall (group) synergy parameters defined in Materials and Methods. Below each parameter is its se in parentheses, the number of statistical degrees of freedom (df), and the corresponding P value against the null hypothesis of zero interaction.
A fourth term, synergy, was estimated as the difference between conjointly driven ACTH secretion and the larger of that induced by either peptide alone. In this definition, nonzero synergy exceeds the effects of each individual stimulus. Feedback effects of cortisol on ACTH secretion, which are mediated at both the hypothalamic and pituitary levels, were estimated by regressing each of the four types of estimates of CRH- and AVP-driven ACTH secretion rates on cortisol concentrations over successive 40-min time windows. The steepness of the resulting slopes defines the degree to which cortisol concentrations inhibit each pathway to ACTH secretion. Dilution from pituitary to peripheral venous blood exceeds 1:4000, rendering the effect of recirculation negligible. The estimated time delay in pituitary blood is less than 12 sec, making hormone transit time relatively insignificant.
Results
Figure 1 shows all 18 frequently sampled ACTH, CRH, and AVP secretion-rate individually and associated glucose and cortisol-concentration time series in the six animals. Visual inspection indicated that cortisol depletion induced by administration of metyrapone, a steroidogenic inhibitor, evokes prompt and marked secretion of pituitary ACTH and hypothalamic CRH and AVP (Fig. 1A). Responses to insulin-induced hypoglycemia were analogous, except that cortisol concentrations and ACTH production increased together initially, thus eclipsing estimation of negative feedback during the first hour (Fig. 1B).
The question whether cortisol concentrations determine feedback on ACTH, CRH, and AVP secretion independently of time was addressed first. The analysis related the peptide secretion rate in each 40-min window (dependent variable) to the cortisol concentration at the start of the same window (independent variable) (Fig. 2). To encompass a dynamic range, 84th percentile values of ACTH, CRH, and AVP secretion rates were determined in each window for each peptide in each of the six horses separately. Peptide secretion rates were inversely related to cortisol concentrations in a linear fashion for both metyrapone (Fig. 2, left three columns) and hypoglycemia (Fig. 2, right three columns). Table 1 reports the slopes of the 18 individual regressions (ACTH/cortisol, CRH/cortisol, and AVP/cortisol), and the mean slopes of the regressions in the metyrapone and hypoglycemia settings. Analyses of combined data demonstrated that cortisol exerts concentration-dependent inhibition of: 1) ACTH secretion (P < 0.01 for metyrapone, P < 0.05 for hypoglycemia); 2) AVP secretion (P < 0.05 for metyrapone and P < 0.05 for hypoglycemia); and 3) CRH secretion (P < 0.01 for metyrapone, and P > 0.10 for hypoglycemia). In the last case, CRH secretion was negatively related to cortisol concentrations in two of the three hypoglycemic animals. The exception was due to a positive slope of CRH on cortisol in horse no. 2, reflecting concomitant increases in CRH and cortisol output during much of the observation interval.
Data analyses in Fig. 2 and Table 1 did not explicitly require modeling CRH, AVP, and ACTH pulses. Accordingly, the next step was to estimate pulsatile peptide secretion during metyrapone-induced CRH and AVP secretion (see supplemental appendix). The motivation was that a low-cortisol feedback milieu might permit detection of hypothalamic CRH and AVP autofeedback, if present. Figure 3A illustrates analytical reconstruction of integral (mean) and derivative (rate-of-change) cortisol-concentration feedback signals, as well as time-varying CRH (left) and AVP (right) autoinhibitory signals (horse no. 1). Knowledge of the feedback signals was used to estimate dose-response negative feedback on CRH (and analogously on AVP) secretion by integral and derivative cortisol concentrations, and by CRH (or AVP) itself as continuous logistical functions. To illustrate the analyses graphically, Fig. 3B depicts feedback dose-response functions segmented into cortisol-feedback strata. The strata represent the lowest cortisol feedback state in a given animal (Fig. 3B, top row, 0%), and stepwise increments of 25% to include the highest (Fig. 3B, bottom row, 100%) cortisol feedback signal. The first column reproduces (from Fig. 1A) observed CRH (or AVP) secretion profiles and feedback signals for orientation. The second through fourth columns give inhibitory dose-response estimates, wherein y-axis values denote the mass of CRH (left) and AVP (right) secreted per burst (note different absolute scales). The three x-axes (left to right) define the integral cortisol signal (nmol/liter), derivative cortisol signal (nmol/liter·min), and CRH signal (nmol/liter·min). There is marked augmentation of estimated CRH secretory burst mass when the strength of each of the three feedback signals (integral cortisol, derivative cortisol, CRH itself) decreases by 0–25%, viz. from 100% (maximal inhibition) to 75% of the maximum. Similar high sensitivity to cortisol feedback withdrawal (0–25% change) pertains to both time modes of inhibition of AVP secretion. However, unlike CRH, disinhibition of AVP secretion becomes prominent only when AVP concentrations decrease to 50% of maximally observed feedback values. Thus, negative feedback at the hypothalamus is quantifiable for integral and derivative cortisol concentrations, and for homologous peptides with greater feedback sensitivity to CRH than AVP.
Given analytical estimates of pulsatile secretion of the hypothalamic regulatory peptides, CRH and AVP, and their feedback control by two time modes of cortisol inhibition and homotypical peptides, the next goal was to quantify how CRH, AVP, and cortisol individually and jointly regulate ACTH secretion. Cortisol feedback was modeled as noncompetitive inhibition of the efficacy of CRH and AVP in inducing ACTH secretion (31,32). The interaction between CRH and AVP was constructed as a potentiating effect of AVP on CRH efficacy (33,34). Each signal was assumed to act by way of a logistical dose-response function representing stimulation (CRH and AVP) and inhibition (cortisol) of ACTH secretion, which was estimated analytically in each 40-min time window during metyrapone and hypoglycemic stimulation. Regressions related ACTH secretion to: 1) cortisol feedback concentrations at the start of each window, 2) combined CRH and AVP stimulation, 3) individual CRH feedforward (input of AVP set to zero), and 4) individual AVP drive (input of CRH set to zero). To visualize the time evolution of the resulting dose-response functions, three-dimensional surfaces were constructed linking ACTH secretion to CRH and AVP secretion at given cortisol concentrations in a successive 40-min time window. Figure 4A illustrates such sequential ACTH dose-response contours in a horse administered metyrapone, and Fig. 4B in an animal subjected to insulin-induced hypoglycemia. The analysis unveils remarkably dynamic dose-response variations as cortisol feedback changes over time. The dynamics are distinguishable among individual CRH- and AVP-stimulated and synergistically evoked ACTH secretion.
Statistical analysis showed that from time zero (metyrapone) and time 1 h (hypoglycemia), higher cortisol concentrations predict each of the following: 1) reduced conjoint (overall endogenous) stimulation of ACTH secretion via the combined hypothalamic release and pituitary actions of CRH and AVP in four of six horses, 2) decreased CRH-driven ACTH secretion (AVP signal set to zero) in three of six animals, 3) attenuated AVP-stimulated (CRH signal set to zero) ACTH secretion in four of six horses, and 4) significant calculated CRH-AVP synergy in five of six horses. Figure 5 depicts and Table 2 summarizes corresponding linear-regression estimates in each animal. P values for the group corroborated that higher cortisol concentrations significantly inhibit conjoint (all pathways combined) ACTH stimulation in both the metyrapone (P < 0.05) and hypoglycemia (P < 0.01) interventions, and repress individual CRH and AVP drive (P < 0.01 and P < 0.05, respectively, for metyrapone and P < 0.05 and P < 0.05, respectively, for hypoglycemia).
Using the data from Fig. 5, synergism was defined in three alternative forms: first, as the difference between the overall (combined peptide) efficacy and the maximum of that obtained individually for CRH and AVP; second, as the difference between the overall ACTH efficacy and the sum of CRH and AVP efficacies; and third (a potentiation synergy), as the difference between the joint potency effect and the sum of the ACTH effects of CRH and AVP at their potencies. By each of these three definitions, estimated synergy (average of data points in each of the three corresponding rows of Fig. 6) was significantly positive and nonzero (Table 3) after both the administration of metyrapone (P < 0.01, P < 0.01, and P < 0.05, respectively) and induction of hypoglycemia (P < 0.05 for each of the three). Synergy was not significantly inversely related to cortisol concentrations in any of the six horses. Thus, higher cortisol concentrations inhibit individual CRH and AVP and conjoint (total endogenous) CRH/AVP stimulation of ACTH secretion in both the metyrapone and hypoglycemia models. In contrast, calculated synergy is relatively resistant to suppression by the cortisol concentrations realized here.
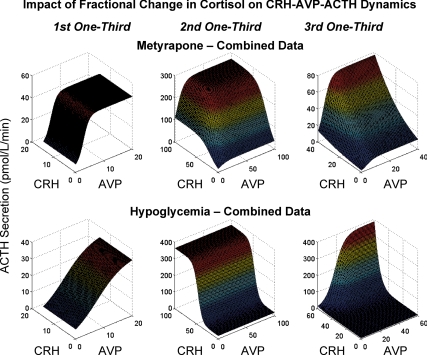
To make inferences for each cohort of horses, ACTH, CRH, and AVP data were combined in each cohort by tertiles (0–33, 34–67, and 68–100%) of cortisol concentrations, and analyzed as just described. Figure 7 depicts the six surfaces (three for metyrapone, top; three for hypoglycemia, bottom). In the case of metyrapone, the highest (starting) stratum of cortisol concentrations was associated with predominantly AVP-dependent feedforward, which maintained a 10-fold range of ACTH secretion rates (Fig. 7, top left). The middle (decreasing) stratum of cortisol concentrations evoked combined CRH and AVP outflow, and synergistic (70-fold) augmentation of ACTH secretion. The lowest tertile of cortisol concentrations resulted in a steep CRH dose-response function, especially at high AVP outflow, indicating strong two-peptide synergy. In the case of hypoglycemia, the lowest stratum (baseline) of cortisol levels was marked by predominantly AVP drive of ACTH secretion (Fig. 7, bottom left), analogous to the baseline metyrapone condition. Increasing cortisol concentrations (middle stratum) were associated with CRH-predominant stimulation of ACTH secretion (Fig. 7, bottom middle), whereas maximal cortisol concentrations (highest stratum) were accompanied by prominent synergy due to simultaneously high CRH and AVP secretion (Fig. 7, bottom right).
Figure 7.
Cohort-based analyses of ACTH secretion rates as logistical dose-response functions of CRH and AVP secretion. To combine data within a cohort, the total change in cortisol concentrations (maximum minimum) was calculated and divided into thirds in each horse for each intervention. Data within an intervention were then combined for analysis at each cortisol stratum. The top row displays the three (one for each one third change in cortisol) surfaces for the metyrapone study, and the bottom row those for the hypoglycemia study.
Both ACTH (overall P < 0.01) and CRH (overall P < 0.005) pulse frequency were negatively correlated with cortisol concentrations in the metyrapone experiment. The 33 and 110% acceleration in ACTH and CRH pulse frequencies, respectively, somewhat exceed that inferred for ACTH in humans (15–30%) under low-cortisol feedback (35). In contrast, AVP pulse frequency decelerated by 50% during low-cortisol feedback in each animal (overall P < 0.001). The basis for the divergence in CRH and AVP pulse frequencies during hypocortisolemia is not yet clear.
Discussion
Salient facets of the new analytical formalism include: 1) formulation of a dynamic four-effector ensemble model of hormone interactions; 2) analytical estimation of time-evolving three-peptide response surfaces; 3) inclusion of two time modes of feedback, viz., rapid (derivative-dependent) and slow (integral-defined); 4) representation of aggregate dynamics using data from all animals; 5) confirmation of estimates of nonlinear dose-response dynamics by a simple regression model comprising repeated snapshots (time windows) of the system; 6) quantification of homologous peptide autofeedback (e.g. CRH on CRH secretion, AVP on AVP secretion); 7) estimation of in vivo two-peptide synergy; and 8) application to two distinct experimental paradigms, a low-cortisol milieu and hypoglycemia to assess generality.
In this modeling approach, one estimates interactions by calculating nonlinear (four-parameter sigmoidal) dose-response functions, which represent feedforward for individual CRH → ACTH, individual AVP → ACTH, combined CRH/AVP → ACTH, individual negative feedback by CRH and AVP on itself, and feedback by cortisol on each of CRH, AVP, and ACTH. Synergistic interactions were then defined in three ways: 1) whether joint CRH/AVP efficacy exceeds the individual efficacy of CRH and AVP, 2) whether joint CRH/AVP efficacy exceeds the sum of the efficacies of CRH and AVP, and 3) potentiation at ED50 (Table 3 gives summary). Estimation of nonlinear dose-response relationships distinguishes the new model from linear cross-correlation methods (36). All three definitions of CRH/AVP synergy revealed significant potentiating interactions between the two peptides in vivo.
Model-based analyses predicted significant negative feedback by both mean (slow) and derivative (rapid) cortisol concentrations at the hypothalamic level. In particular, time-averaged cortisol concentrations in the metyrapone intervention negatively determined the outflow of CRH and AVP as measured directly in pituitary blood. Rapidly increasing cortisol concentrations also negatively predicted the release of CRH and AVP. An unexpected analytical outcome was that the rapid derivative-dependent feedback effect was cortisol concentration dependent. Thus, a given rate of increase in cortisol concentrations was more inhibitory at higher absolute cortisol concentrations (Fig. 5). The physiological mechanism mediating this relationship is not known. Possible explanations are that type I (mineralocorticoid) and II (glucocorticoid) nuclear and/or putative membrane receptors differ in responsiveness to integral and rapid negative feedback (4,37). Corticosteroid-modulated neuronal CRH type 1 receptors and AVP type 1b receptors may further influence feedback control via limbic cortex-stria terminalis-hypothalamic connections (38).
Mean cortisol concentrations inhibited CRH- and AVP-driven and total endogenous pathway driven ACTH secretion dose dependently in both models. In contrast, calculated CRH-AVP synergy was not significantly repressed by the cortisol concentrations attained in the paradigms used here. Relative resistance to cortisol feedback is also evident after combined-peptide stimulation in vivo and in vitro (39). The precise basis for partial feedback independence of synergy is not clear but may involve the lesser sensitivity of AVP and other hypophysiotropic factors to glucocorticoid inhibition compared with CRH (40,41,42). Other mechanisms might contribute to pituitary escape from nonpharmacological glucocorticoid feedback, such as unequal brain-pituitary expression of cortisol-activating and cortisol-metabolizing enzymes and cortisol-binding globulin (43,44), selective corticotrope expression of nuclear corepressors and coactivators of the glucocorticoid receptor and ACTH gene (45), functionally distinguishable N-terminally modified glucocorticoid-receptor isoforms, and P-glycoprotein-mediated export of cortisol out of the central nervous system.
Glucocorticoid inhibition of pituitary ACTH secretion and hypothalamic CRH and AVP secretion was modeled as noncompetitive, which assumes that cortisol decreases the stimulatory efficacies (maximal effects) of CRH and AVP. In vitro and in vivo data reinforce this assumption in the case of mean and rapidly increasing cortisol concentrations (31,32,46,47). Because in vivo data demonstrating noncompetitive rapid cortisol feedback directly on the pituitary are sparse (48), this potential pathway was not modeled initially. Rapid neuronal repression may be mediated via inhibition of calcium and potassium currents, and/or via putative membrane-receptor signaling (46,47). A rapid positive (excitatory) neuronal effect of cortisol is also observed in some studies. The last observation might explain concurrent increases in all four of ACTH, AVP, CRH, and cortisol secretion during the initial response to hypoglycemia.
The accompanying model was able to quantify for the first time homotypical feedback by endogenous CRH and AVP (autoinhibition of hypothalamic peptide release). In vivo and in vitro experiments indicate that CRH and AVP can inhibit their own release from the paraventricular nucleus, putatively via neuronal CRH-R1 and AVP-1b receptors (49). Autoinhibitory CRH and AVP signals may also occur from brainstem projections to the paraventricular nucleus (50). Under some conditions, AVP may either inhibit or stimulate CRH secretion (heterotypical feedback) and vice versa (51,52,53). The framework developed here would allow later inclusion of such effects.
Hypoglycemia is thought to activate ascending brainstem signals, which converge in part on hypothalamic paraventricular nuclei that discharge CRH and AVP into pituitary portal blood (54,55). Microdialysis of brain nuclei would allow one to relate time-varying interstitial (extraneuronal) glucose concentrations to AVP and CRH release. When systemic cortisol is sampled frequently, one can also construct the dose-response function linking pulsatile ACTH concentrations to adrenal cortisol secretion (6), along with potential splanchnic neuronal potentiation of ACTH-adrenal stimulation of cortisol output (56). The basic mathematical model presented here, and novel interventions (such as consecutive administration of metyrapone and insulin), should permit further advances in these areas.
Aspects of CRH and AVP action not expressly modeled include homologous and heterologous receptor down-regulation, collateral inputs by putative inhibitors like atriopeptin and somatostatin (57), and putative costimulators like oxytocin and angiotensin II (32). As a caveat, the present platform does not yet link time-varying brain glucose concentrations to corticotropic-axis outflow or to other endocrine responses, or include specific neural circuits that supervise CRH/AVP release. Brain microdialysis of glucose and neurotransmitter concentrations over time would allow such estimates (58,59,60). The new model provides an analytical construct for estimating these relationships in future experiments, and thereby extending the understanding of more complex regulation.
In summary, the present work highlights the utility of a new analytical construct designed to assist in quantifying complex in vivo feedback interactions. Whereas most mathematical models emphasize simulation, the accompanying formalism permits analytical estimation of interactive regulatory pathways. The model presented here, albeit explored initially in the horse, should have utility in the sheep, rat, and human. For example, direct portal-venous sampling and microdialysis of selected brain regions have been accomplished in the sheep and rodent (54,60,61). Microdialysis data will allow one to use the model to reconstruct time-varying linkages among brain region-specific glucose, cortisol, neurotransmitter, and peptide concentrations (58,59). The mathematical formalism with minimal adaptation also will be applicable in humans to analyzing pulsatile ACTH secretion under exogenous AVP, CRH, and/or cortisol “clamps,” which constrain one or more of the main signals while estimating the other(s). Further applications could include analyses of other hormone ensembles, such as those comprising insulin-glucagon-somatostatin-glucose, calcium-phosphorus-parathormone-vitamin D, and estradiol-inhibin-FSH-GnRH.
Supplementary Material
Acknowledgments
We thank Donna Scott for support of manuscript preparation, Ashley Bryant for data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Footnotes
This work was supported in part via the Center for Translational Science Activities Grant 1 UL 1 RR024150 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD), and R01 NIDDK DK073148 and R21 NIA AG029215 from the National Institutes of Health (Bethesda, MD).
Disclosure Summary: The authors have nothing to declare.
First Published Online November 20, 2008
Abbreviations: AVP, Arginine vasopressin; CV, coefficient of variation; JUG, jugular; PV, pituitary venous.
References
- Selye H 1946 The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol 6:117–230 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F 2005 Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475 [DOI] [PubMed] [Google Scholar]
- Yates FE, Brennan RD, Urquhart J 1969 Application of control systems theory to physiology. Adrenal glucocorticoid control system. Fed Proc 28:71–83 [PubMed] [Google Scholar]
- Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL 2007 Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 148:5470–5477 [DOI] [PubMed] [Google Scholar]
- Dempsher DP, Gann DS, Phair RD 1984 A mechanistic model of ACTH-stimulated cortisol secretion. Am J Physiol 246(4 Pt 2):R587–R596 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Veldhuis JD 2004 Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab 287:E652–E661 [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Mezey E, Skirboll L 1984 Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci USA 81:1854–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale W 1983 Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305:325–327 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Orth DN 1987 Detailed kinetic analysis of adrenocorticotropin secretion by dispersed rat anterior pituitary cells in a microperifusion system: effects of ovine corticotropin-releasing factor and arginine vasopressin. Endocrinology 121:1133–1145 [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Shinsako J, Dallman MF 1983 Feedback inhibition of adrenocorticotropic hormone by physiological increases in plasma corticosteroids in conscious dogs. J Clin Invest 71:859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G, Robinson IC, Tannahill LA 1988 Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol [Erratum J Physiol (Lond) (1988) 405:785] 401:329–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia LG, Canny BJ, Leong DA 1992 Paracrine communication regulates adrenocorticotropin secretion. Endocrinology 130:534–539 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R 1991 The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134 [DOI] [PubMed] [Google Scholar]
- Tanimura SM, Watts AG 1998 Corticosterone can facilitate as well as inhibit corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Endocrinology 139:3830–3836 [DOI] [PubMed] [Google Scholar]
- Stokely EM, Howard LL 1972 Analog computer model for the ACTH-glucocorticoid system. IEEE Trans Biomed Eng 19:13–20 [DOI] [PubMed] [Google Scholar]
- Jusko WJ, Slaunwhite Jr WR, Aceto Jr T 1975 Partial pharmacodynamic model for the circadian-episodic secretion of cortisol in man. J Clin Endocrinol Metab 40:278–289 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD 2001 A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA 98:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD 2004 Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci USA 101:6740–6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD 2006 An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 147:2817–2828 [DOI] [PubMed] [Google Scholar]
- Gupta S, Aslakson E, Gurbaxani BM, Vernon SD 2007 Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor Biol Med Model 4:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Conrad M, Hubold C, Schweiger U, Fischer B, Fehm HL 2007 The principle of homeostasis in the hypothalamus-pituitary-adrenal system: new insight from positive feedback. Am J Physiol Regul Integr Comp Physiol 293:R83–R98 [DOI] [PubMed] [Google Scholar]
- Picard-Hagen N, Gayrard V, Alvinerie M, Smeyers H, Ricou R, Bousquet-Melou A, Toutain PL 2001 A nonlabeled method to evaluate cortisol production rate by modeling plasma CBG-free cortisol disposition. Am J Physiol Endocrinol Metab 281:E946–E956 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD 2001 Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol Regul Integr Comp Physiol 280:R1755–R1771 [DOI] [PubMed] [Google Scholar]
- Lenbury Y, Pornsawad P 2005 A delay-differential equation model of the feedback-controlled hypothalamus-pituitary-adrenal axis in humans. Math Med Biol 22:15–33 [DOI] [PubMed] [Google Scholar]
- Irvine CH, Alexander SL 1987 A novel technique for measuring hypothalamic and pituitary hormone secretion rates from collection of pituitary venous effluent in the normal horse. J Endocrinol 113:183–192 [DOI] [PubMed] [Google Scholar]
- Alexander SL, Irvine CH, Ellis MJ, Donald RA 1991 The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinology 128:65–72 [DOI] [PubMed] [Google Scholar]
- Alexander SL, Irvine CH, Livesey JH, Donald RA 1993 The acute effect of lowering plasma cortisol on the secretion of corticotropin-releasing hormone, arginine vasopressin, and adrenocorticotropin as revealed by intensive sampling of pituitary venous blood in the normal horse. Endocrinology 133:860–866 [DOI] [PubMed] [Google Scholar]
- Alexander SL, Roud HK, Irvine CHG 1997 Effect of insulin-induced hypoglycemia on secretion patterns and rates of corticotropin-releasing hormone, arginine vasopressin and adrenocorticotrophin in horses. J Endocrinol 153:401–409 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD 2003 Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol 285:R664–R673 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD 2005 Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236:242–255 [DOI] [PubMed] [Google Scholar]
- Giguere V, Labrie F, Cote J, Coy DH, Sueiras-Diaz J, Schally AV 1982 Stimulation of cyclic AMP accumulation and corticotropin release by synthetic ovine corticotropin-releasing factor in rat anterior pituitary cells: site of glucocorticoid action. Proc Natl Acad Sci USA 79:3466–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Burke JA 1987 Use of the reverse hemolytic plaque assay to study the regulation of anterior lobe adrenocorticotropin (ACTH) secretion by ACTH-releasing factor, arginine vasopressin, angiotensin II, and glucocorticoids. Endocrinology 120:439–444 [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Verleun T, Oosterom R, de Jong F, Hackeng WH 1984 Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J Clin Endocrinol Metab 58:298–303 [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W 1983 Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology 113:939–942 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD 2003 Cortisol feedback state governs adrenocorticotropin secretory-burst shape, frequency and mass in a dual-waveform construct: time-of-day dependent regulation. Am J Physiol Regul Integr Comp Physiol 285:R950–R961 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD 2003 Mathematical modeling of receptor-mediated interlinked systems. In: Encyclopedia of hormones. San Diego: Academic Press; 286–294 [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP 1996 Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 17:245–261 [DOI] [PubMed] [Google Scholar]
- Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G 2004 The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest 113:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnloser J, Von Werder K, Muller OA 1989 Acute dexamethasone suppression of ACTH secretion stimulated by human corticotrophin releasing hormone, AVP and hypoglycaemia. Clin Endocrinol (Oxf) 31:175–184 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Vale WW 1987 The cellular actions of vasopressin on corticotrophs of the anterior pituitary: resistance to glucocorticoid action. Mol Endocrinol 1:451–458 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, John CD, Solito E, Tierney T, Flower RJ, Christian H, Morris J 2006 Annexin 1, glucocorticoids, and the neuroendocrine-immune interface. Ann NY Acad Sci 1088:396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil CK, Keil LC, Chang D, Reid IA 1988 Effects of CRF and ANG II on ACTH and vasopressin release in conscious dogs. Am J Physiol 255(1 Pt 1):E46–E53 [DOI] [PubMed] [Google Scholar]
- Holmes Mc, Seckl JR 2006 The role of 11β-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol 248:9–14 [DOI] [PubMed] [Google Scholar]
- Petersen HH, Andreassen TK, Breiderhoff T, Brasen JH, Schulz H, Gross V, Grone HJ, Nykjaer A, Willnow TE 2006 Hyporesponsiveness to glucocorticoids in mice genetically deficient for the corticosteroid binding globulin. Mol Cell Biol 26:7236–7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der LS, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC 2008 Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology 149:725–732 [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R 2000 Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res 17:1273–1277 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang CA, Chen YZ 1995 Nongenomic effect of glucocorticoid on the release of arginine vasopressin from hypothalamic slices in rats. Neuroendocrinology 62:628–633 [DOI] [PubMed] [Google Scholar]
- Widmaier EP, Dallman MF 1984 The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology 115:2368–2374 [DOI] [PubMed] [Google Scholar]
- Arima H, Aguilera G 2000 Vasopressin and oxytocin neurones of hypothalamic supraoptic and paraventricular nuclei co-express mRNA for type-1 and type-2 corticotropin-releasing hormone receptors. J Neuroendocrinol 12:833–842 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, van Heerikhuize JJ, Arts M, van der Woude TP 1992 Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res 580:62–67 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Bruhn TO, Otto S 1985 Central modulation of immunoreactive arginine vasopressin and oxytocin secretion into the hypophysial-portal circulation by corticotropin-releasing factor. Endocrinology 116:1669–1671 [DOI] [PubMed] [Google Scholar]
- Maruyama H, Makino S, Noguchi T, Nishioka T, Hashimoto K 2007 Central type 2 corticotropin-releasing hormone receptor mediates hypothalamic-pituitary-adrenocortical axis activation in the rat. Neuroendocrinology 86:1–16 [DOI] [PubMed] [Google Scholar]
- Friedman TC, Yanovski JA, Nieman LK, Doppman JL, Cutler Jr GB, Oldfield EH, Gold PM, Chrousos GP, Kalogeras KT 1996 Inferior petrosal sinus arginine vasopressin concentrations in normal volunteers and patients with Cushing's disease. J Clin Endocrinol Metab 81:3068–3072 [DOI] [PubMed] [Google Scholar]
- Caraty A, Grino M, Locatelli A, Guillaume V, Boudouresque F, Conte-Devolx B, Oliver C 1990 Insulin-induced hypoglycemia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest 85:1716–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grino M, Oliver C 1992 Ontogeny of insulin-induced hypoglycemia stimulation of adrenocorticotropin secretion in the rat: role of catecholamines. Endocrinology 131:2763–2768 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC 2006 Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 290:R1128–R1135 [DOI] [PubMed] [Google Scholar]
- Antoni FA, Dayanithi G 1990 Secretion of ACTH by perifused isolated rat anterior pituitary cells: pulses of secretagogue enhance the secretory response and modify the effect of atriopeptin. J Endocrinol 125:365–373 [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Muller-Preuss P, Holsboer F, Reul JM 1995 Effect of bacterial endotoxin and interleukin-1 β on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci 15:2920–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groote L, Linthorst AC 2007 Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience 148:794–805 [DOI] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC 2008 Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149:3241–3243 [DOI] [PubMed] [Google Scholar]
- Eckland DJ, Todd K, Jessop DS, Biswas S, Lightman SL 1988 Differential effects of hypothalamic catecholamine depletion on the release of arginine vasopressin and CRF-41 into hypothalamo-hypophyseal portal blood. Neurosci Lett 90:292–296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.