Abstract
Thyroid hormone is necessary for cochlear development and auditory function, but the factors that control these processes are poorly understood. Previous evidence indicated that in mice, the serum supply of thyroid hormone is augmented within the cochlea itself by type 2 deiodinase, which amplifies the level of T3, the active form of thyroid hormone, before the onset of hearing. We now report that type 3 deiodinase, a thyroid hormone-inactivating enzyme encoded by Dio3, is expressed in the immature cochlea before type 2 deiodinase. Dio3−/− mice display auditory deficits and accelerated cochlear differentiation, contrasting with the retardation caused by deletion of type 2 deiodinase. The Dio3 mRNA expression pattern in the greater epithelial ridge, stria vascularis, and spiral ganglion partly overlaps with that of thyroid hormone receptor β (TRβ), the T3 receptor that is primarily responsible for auditory development. The proposal that type 3 deiodinase prevents premature stimulation of TRβ was supported by deleting TRβ, which converted the Dio3−/− cochlear phenotype from one of accelerated to one of delayed differentiation. The results indicate a protective role for type 3 deiodinase in hearing. The auditory system illustrates the considerable extent to which tissues can autoregulate their developmental response to thyroid hormone through both type 2 and 3 deiodinases.
Development of the auditory system requires thyroid hormone, but the system is protected from premature stimulation by a thyroid hormone-inactivating enzyme, type 3 deiodinase.
The development of hearing is critically dependent upon thyroid hormone. Congenital hypothyroidism and iodine deficiency are known causes of human deafness, with the greatest risk occurring from hormone insufficiency at fetal and neonatal stages (1,2). Thyroid hormone must be available during this period because provision at later stages cannot reverse the auditory deficits. In rodents the corresponding developmental window spans from late embryonic to early postnatal stages before the initiation of auditory function at approximately 2 wk of age (3,4,5). A major target within the auditory system is the cochlea, in which thyroid hormone promotes terminal differentiation of a variety of cell types, including the sensory epithelium (5,6,7,8). However, the factors that mediate these functions of thyroid hormone in auditory development remain poorly understood.
Thyroid hormone receptor β (TRβ), encoded by the Thrb gene, is required for hearing in humans (9,10,11) and mice (12,13). TRβ is expressed in the rodent cochlea in the greater epithelial ridge (GER) and sensory epithelium, and at lower levels in the spiral ganglion and other areas (14). Thrb−/− mice have delayed differentiation of the GER and malformation of the tectorial membrane (6). Many features of cochlear maturation in Thrb−/− mice eventually “catch-up” with normal development, but auditory function remains permanently impaired, indicating a critical role for TRβ in determining the timing of development. TRβ is expressed in the cochlea from midgestation, but the phenotype in Thrb−/− mice occurs postnatally, suggesting that other factors influence TRβ activity.
Type 2 and 3 deiodinases, respectively, amplify and deplete levels of thyroid hormone, and are thought to determine tissue-specific events that are relatively independent of systemic thyroid hormone levels (15,16). Thyroid hormone is present most abundantly in the circulation as T4 and at lower levels as T3, the major ligand for the receptor. Studies in mice indicate that one of the most prominent functions for type 2 deiodinase, which generates T3 from T4 by outer ring 5′-deiodination, is in auditory development. Type 2 deiodinase, encoded by Dio2, is induced in the cochlea before auditory function begins. Dio2−/− mice, like Thrb−/− mice, exhibit deafness with delayed cochlear differentiation, suggesting that this enzyme amplifies local T3 levels to stimulate the onset of hearing (17,18). Here, we report that type 3 deiodinase, encoded by Dio3, is also critical for auditory development. Type 3 deiodinase depletes active sources of thyroid hormone by inner ring 5-deiodination of T4 and T3, producing reverse T3 and 3,3′-diiodothyronine, respectively (19). In contrast to the retarded phenotype in Dio2−/− mice, Dio3−/− mice display deafness with premature cochlear differentiation, indicating a protective role for type 3 deiodinase in auditory development. The results suggest the underlying importance of type 3 deiodinase in restricting hormonal responses, even in a system such as the auditory system that is highly dependent upon thyroid hormone.
Materials and Methods
Mouse strains
The Dio3 mutation inactivates the catalytic site of type 3 deiodinase (19). The mutation was present on a 129/SvJ × C57BL/6 mixed background, and was backcrossed for one to two generations onto the C57BL/6J strain that has minimal hearing loss before 6 months of age. The Thrb mutation was on a C57BL/6J background (6). Genotyping was performed by PCR as described (12,19). Dio3+/− parents were crossed to generate Dio3−/−, +/−, and +/+ littermates for study. After backcrossing onto the C57BL/6J strain, Dio3−/− pups represented approximately 25% of postnatal d 0 (P0) neonates born to Dio3+/− parents, although less than one in six of these survived beyond P1. This was a poorer survival rate than described previously (19), suggesting that genetic background or environmental conditions in different housing facilities influence survival. Pups that did survive thrived well. Daily T3 injections were given to wild-type C57BL/6J pups from P0–3 by sc injection at the back of the neck. For determination of resultant serum T3 concentration, daily injections were extended to P5 to allow collection of larger volumes of serum for analysis; groups contained two or three pools, with each pool representing four to six pups. Animal studies were conducted under approved protocols at Dartmouth Medical School, Oregon Health & Sciences University, and National Institutes of Health.
Auditory function analyses
The auditory evoked brainstem response (ABR) was measured with a SmartEP system (Intelligent Hearing Systems, Miami, FL) in 2- to 3-month-old mice under Avertin anesthesia (0.25 mg/g body weight, ip), as described (6). Statistical comparison was performed using the Student's t test. The Distortion Product Otoacoustic Emission (DPOAE) was measured with a Smart DPOAE system (Intelligent Hearing Systems) in 2-month-old mice under Avertin (Aldrich Chemical, Milwaukee, WI) anesthesia, as described previously. Two Etymotic Research (ER-2) speakers with a 10B+ microphone were placed in the ear canal with a tight plastic seal. The DPOAE was evoked with two pure tones at a lower test frequency (f1) and a higher test frequency (f2) (f2 > f1 and f2/f1 = 1.22), and recorded using f1 at 65 decibel (dB) sound pressure level (SPL) and f2 at 55 dB SPL. The endocochlear potential (EP) was measured in 4- to 7-month-old mice under ketamine anesthesia (120 mg/kg, im). A glass microelectrode with a tip diameter of approximately 0.5 μm and filled with 300 mm KCl was inserted into the scala media through the round window membrane and basilar membrane. The stable DC voltage between the glass electrode and an Ag/AgCl reference electrode in neck tissue was considered to be the EP. The recording was confirmed by demonstrating that the potential returned to approximately 0 mV when the electrode tip was retracted to the scala tympani.
Compound action potential (CAP)
Anesthesia and surgical procedures were the same as those for the EP measurement. A recording electrode, made of Teflon-coated silver wire (DuPont Co., Wilmington, DE) with a diameter of 75 μm, was placed in the round window niche against the media-posterior bony wall. The reference electrode was in the soft tissues next to the bulla. Tone bursts of 10 msec duration and 1 msec increase/decrease time at different frequencies were used to evoke the CAP. The signal at the round window was amplified, filtered, and displayed on an oscilloscope. The CAP threshold, i.e. the SPL needed to generate an N1/P1 voltage of 10 μV (20,21), was visually identified at each stimulus frequency.
Histology and in situ hybridization
For histology, cochleae were fixed in 3% glutaraldehyde/2% paraformaldehyde, decalcified, embedded in methacrylate plastic, sectioned at 3 μm as described (6), and stained with aqueous hematoxylin (Biomeda Corp., Foster City, CA). For each age, four to six cochleae from three or more mice were analyzed. Cryosections of paraformaldehyde-fixed tissues were used for in situ hybridization with digoxigenin-labeled riboprobes generated from mouse cDNA sequences for type 2 (bases 590-1383, accession no. AF177196) and type 3 (bases 78-526, accession no. BC106847) deiodinases and TRβ (1.6 kb) (17).
Northern blot analysis
RNA samples prepared from pools of cochleae from C57BL/6J mouse embryos or pups were analyzed with mouse cDNA probes for Dio3 (bases 56-446, accession no. BC106847), Dio2, and control G3pdh, labeled with 32P by random priming, as described (17). For cochlea, total RNA was analyzed due to the small amounts of tissue available. For control brain and liver samples, poly A-selected RNA was used.
Deiodination assays and RIAs
Activities of type 3 and 2 deiodinases in wild-type mice were determined as described (18,22) using three to four pools of cochleae with each pool representing an entire litter. For Dio3 mutants at embryonic d 18.5 (E18.5), individual pairs of cochleae from seven +/+, 22 +/−, and 11−/− embryos were analyzed. A repeat experiment at E17.5 gave similar results. Briefly, type 2 deiodinase assays included [125I]T4 substrate, 1.2 mm EDTA, and 20 mm dithiothreitol as cofactor, incubated for 1 h at 37 C with or without 1 mm 6n-propyl-2-thiouracil, an inhibitor of type 1 deiodinase; [125I]I− product was detected after separation on Bio-Rad AG 50WXG (H+) resin (Bio-Rad Laboratories, Inc., Hercules, CA). Type 3 deiodinase activity was determined by measuring, after separation by paper chromatography, the amount of [125I]3,3′-diiodothyronine produced after incubation of tissue homogenate (25–50 μg protein) for 1 h with 2 nm [125I]T3 in the presence of 50 mm dithiothreitol as cofactor, in a 50-μl volume. Radiolabeled substrates were obtained from PerkinElmer Inc. (Norwalk, CT). Serum total T4 and total T3 levels were determined by RIA with Coat-A-Count reagents (Diagnostic Systems Laboratories, Inc., Webster, TX). T4 and T3 values are shown as means ± sem.
Results
Differential expression of type 2 and 3 deiodinases in the cochlea
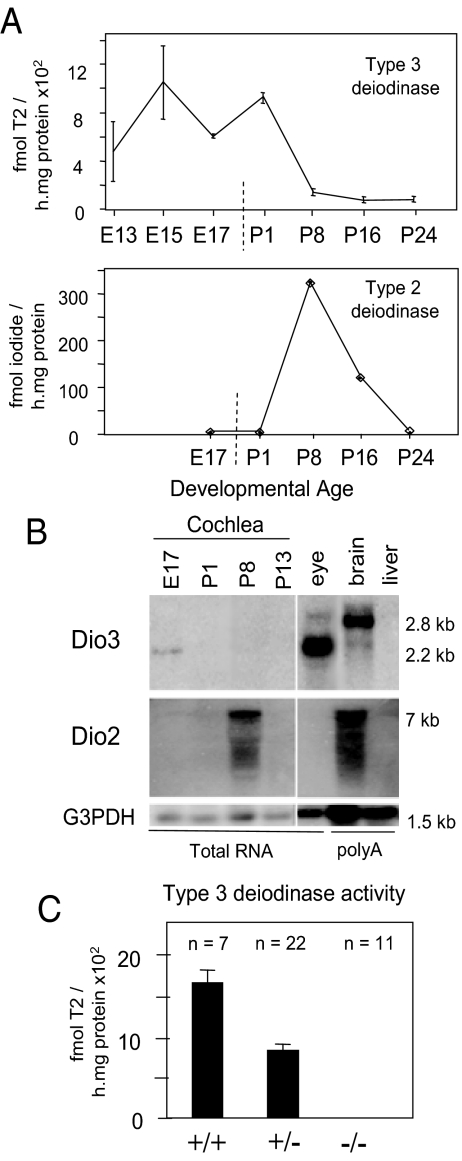
Cochlear homogenates from mouse embryos showed significant levels of type 3 deiodinase activity, which decreased postnatally and persisted into adulthood at low levels (Fig. 1A). The peak of type 3 deiodinase activity preceded that of type 2 deiodinase, which increased postnatally to peak at P8, as shown previously (17). Northern blot analysis detected a Dio3 mRNA band of approximately 2.2 kb in cochlear RNA at E17, corresponding to the major band in the eye but differing from the major 2.8-kb band in adult brain (Fig. 1B). The 2.2- and 2.8-kb Dio3 mRNAs are each thought to encode active enzyme and contain alternative upstream sequences. Both mRNAs correlate with type 3 deiodinase activity in different tissues (23,24). Dio2 mRNA levels peaked at P8, correlating with the type 2 deiodinase activity peak. Dio3−/− mice at E18 lacked cochlear type 3 deiodinase activity, confirming that the enzyme activity was encoded by the Dio3 gene (Fig. 1C).
Figure 1.
Developmental expression of type 3 and 2 deiodinases in cochlea. A, Type 3 activity was present in mouse cochlear homogenates at embryonic and neonatal stages. Type 2 activity peaked postnatally when type 3 activity was in decline. Note: scale on x-axis differs before and after birth (vertical dashed line). B, Northern blot analysis of Dio3 and Dio2 mRNA in cochlea. Dio3 mRNA was detectable in embryonic cochlea, whereas Dio2 mRNA peaked postnatally. Adult eye and brain, positive controls for Dio3 and Dio2, respectively; liver, negative control for Dio2 and Dio3. Poly-A-enriched RNA samples, 2 μg/lane; total RNA, 15 μg/lane. G3PDH, control probe. C, Cochlear homogenates from Dio3−/− mice lacked D3 activity, shown for samples at E18.5.
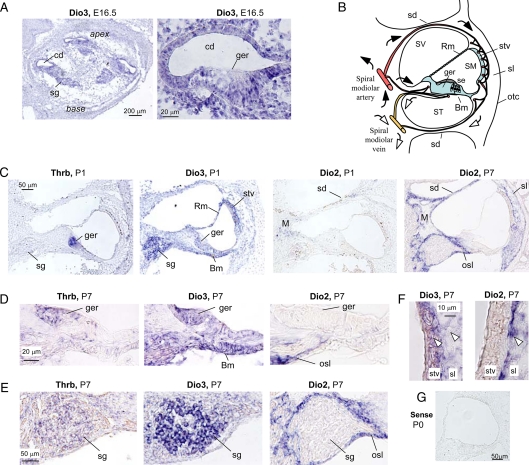
In situ hybridization localized Dio3 mRNA at E16.5 to the epithelium around the cochlear duct, including the GER, the adjacent sensory epithelium, supporting cells, and spiral ganglion (Fig. 2A). There was also a low-level expression of Dio3 mRNA in surrounding tissues in the cochlear capsule. At P1, Dio3 mRNA was detected in the GER, sensory epithelium, tympanic border cells below the basilar membrane, stria vascularis, and Reissner's membrane (Fig. 2C). Dio3 expression in these structures surrounding the scala media suggested that type 3 deiodinase contributes to a functional boundary that limits exposure of internal tissues to T3 and T4 at immature stages. The Dio3 expression pattern partly overlapped with that of Thrb, which was prominent in the GER at P1 (14,17). In contrast to the pattern of Dio3 expression in the neonatal cochlea, Dio2 was minimally expressed at P1 but increased later in a distinct pattern from that of Dio3. At P7, Dio2 mRNA was detected in the modiolus, osseous spiral lamina, and septal divisions between the scala vestibuli and scala tympani, structures that support the internal sensory tissues. Dio2 mRNA was also detected in the spiral ligament located laterally to the stria vascularis, as reported previously (17). At P7, Dio3 expression was reduced, but Dio3 and Thrb mRNAs were still detectable in the GER (Fig. 2D) and spiral ganglion (Fig. 2E). In contrast, Dio2 mRNA was absent from the GER and spiral ganglion. Dio2 mRNA was expressed in the spiral ligament but was largely absent from the stria vascularis, whereas Dio3 mRNA was detected in both of these structures (Fig. 2F). The Dio2-expressing tissues are exposed to the inflow of blood-borne T4 substrate in the arterial vasculature of the cochlea (25) (Fig. 2B) and presumably convert T4 into T3 for release into the interior tissues at appropriate postnatal stages. In contrast, the Dio3-expressing structures may represent an internal protective zone that limits exposure of target tissues to T3 at earlier immature stages (see diagram in Fig. 2B).
Figure 2.
In situ hybridization analysis of Dio3 mRNA in the cochlea. A, Antisense riboprobes detected Dio3 signal (dark blue/purple) in the embryonic cochlear duct (cd) and spiral ganglion (sg) in all turns of the cochlea from base to apex (left). Higher-power magnification (right) shows Dio3 signal in the cochlear duct and GER (ger). Lower level signal was present in tissues surrounding the cochlear duct. B, A diagram of a postnatal cochlear section shows major arterial and venous blood flow (filled and open arrows, respectively) and summarizes the expression pattern of Dio3 mRNA, shaded in blue (data from panels C and D). The sensory epithelium (se) containing the hair cells does not receive direct blood flow. A capillary network exists between the spiral ligament (sl) and the stria vascularis (stv) (25). C, Comparison of Thrb, Dio3, and Dio2 mRNA expression. At P1, Thrb signal is mainly in the GER and adjacent epithelia, and more weakly in the spiral ganglion. Dio3 signal [in the stria vascularis, Reissner's membrane (Rm), tympanic border cells below basilar membrane (Bm), GER, and sensory epithelium] localizes to tissues that enclose the scala media (SM) and partly overlaps with Thrb signal. At P1, minimal Dio2 signal is detected in the modiolus (M) and septal divisions of the cochlea. By P7 (right panel), Dio2 signal is stronger in the modiolus, spiral ligament, osseous spiral lamina (osl), and septal divisions. Dio3 and Dio2 patterns were largely noncoincidental. D, Coexpression of Thrb and Dio3 but not Dio2 mRNA in the GER at P7. E, Coexpression of Thrb and Dio3 but not Dio2 mRNA in the spiral ganglion at P7. Thrb expression is relatively weaker in the spiral ganglion than GER. Dio2 signal is in the osseous spiral lamina. F, Expression of Dio3 but not Dio2 mRNA in the stria vascularis at P7. Dio2 mRNA is detected only in the spiral ligament (single arrowhead), whereas Dio3 mRNA is in the stria vascularis and spiral ligament (dual arrowheads). Brown coloration is from blood and vascular tissues in the stria vascularis. G, Control hybridization of cochlear section with Dio3 sense probe at P0. Scale bars, as marked (A and G), 50 μm (C), 20 μm (D), 50 μm (E), and 10 μm (F), otc, Otic capsule; sd, septal divisions between turns of the cochlear spiral; ST, scala tympani; SV, scala vestibuli.
Auditory defects in Dio3−/− mice
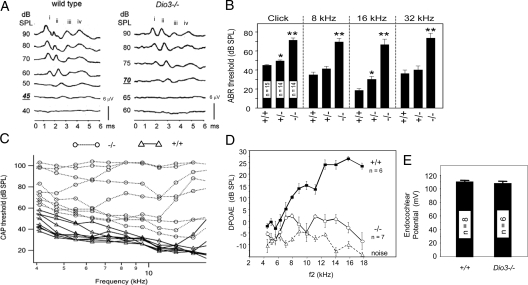
The functional requirement for type 3 deiodinase was investigated in Dio3−/− mice. The ABR, a measure of overall auditory function, was severely impaired in adult Dio3−/− mice (Fig. 3A). For a click stimulus, the mean SPL required to evoke a response in wild-type and Dio3−/− mice was 44.9 ± 0.9 and 71.3 ± 2.2 dB SPL, respectively. The residual waveforms in Dio3−/− mice had reduced magnitude and prolonged duration, suggestive of defects in the relay of the response from the cochlea through the brainstem auditory nuclei. For a click stimulus at 90 dB SPL, the mean latencies of the waveform for wild-type and Dio3−/− mice, measured from time of stimulus to peak iv (the latest major peak in the response), were 4.16 ± 0.06 and 5.14 ± 0.17 msec, respectively (n = 9 Dio3+/+ and 12 Dio3−/−; P < 0.001). Dio3−/− mice also had impaired responses to pure tone stimuli of 8, 16, and 32 kHz that span the sensitive hearing range of mice (Fig. 3B), indicating a consistent deficit across frequencies. The ABR thresholds of Dio3+/− mice showed a trend toward slightly elevated thresholds that were significant for click and 16 kHz stimuli. The Dio3 gene is subject to genomic imprinting (26), but further analyses that distinguished between the contribution of maternal and paternal alleles indicated that the slight threshold shift in Dio3+/− mice was not due to imprinting (data not shown). Instead, the marginal impairment in Dio3+/− mice is more likely to reflect a mild impact of haploinsufficiency.
Figure 3.
Auditory defects in Dio3−/− mice. A, ABR thresholds were approximately 30 dB higher in Dio3−/− mice than +/+ mice (adults, 2-3 months old). Representative waveforms for +/+ and Dio3−/− mice with thresholds of 45 and 70 dB SPL, respectively, for a click stimulus. Normalized voltage scale shown. Main waveform peaks are marked i–iv. B, Mean ABR thresholds ± sem for click and 8, 16, and 32 kHz pure tone stimuli. Numbers of mice noted within columns. Significance of difference from wild type: *, P < 0.01; **, P < 0.001. C, Tone-evoked CAPs. Lines represent response thresholds for individual wild-type (triangles/solid lines) and Dio3−/− (circles/dotted lines) mice over a range of frequencies. D, DPOAEs were defective in Dio3−/− mice for f2 frequencies over a frequency range of 4-18 kHz. Dashed line indicates background noise. E, Mean EPs did not differ between wild-type and Dio3−/− adult mice (P = 0.61).
To investigate the contribution of cochlear dysfunction to the phenotype, tone-evoked CAPs were recorded from the auditory nerve in the proximity of the cochlear round window (Fig. 3C). In wild-type mice, CAP thresholds were broadly comparable to ABR thresholds for a given frequency with lowest thresholds in the 16-kHz region. In contrast, Dio3−/− mice showed elevated thresholds ranging from slightly to severely elevated. During auditory transduction, sound stimulates the basilar membrane on which the hair cells reside. Basilar membrane motion deflects the stereociliary bundles of the hair cells against the tectorial membrane to activate hair cell transducer currents. Outer hair cells possess an intrinsic electromotility that can amplify basilar membrane motion. DPOAEs are a measure of this activity and can be detected as the sound products evoked by two distinct tone stimuli, f1 and f2 (27). DPOAEs were impaired in Dio3−/− mice over an f2 frequency range of 4-18 kHz (Fig. 3D), indicating that nonlinear basilar membrane motion was impaired.
The stria vascularis is involved in generating the EP in the endolymph of the scala media that is necessary for auditory transduction. In mice lacking all thyroid hormone receptors (TRβ and TRα1) (6), or hypothyroid mice (28), the EP is reduced. The EP was measured in Dio3−/− mice to investigate if Dio3 expression in the stria vascularis was related to the generation of the EP. The EP was normal in Dio3−/− mice (Fig. 3E), suggesting that the main role of the Dio3 gene in the stria vascularis is to regulate exposure of internal tissues to T3 rather than regulating an intrinsic function of the stria vascularis in generating the EP. In summary, Dio3−/− mice exhibit marked auditory deficits with a component of the defect residing in cochlear function, although not in the EP.
Accelerated cochlear differentiation in Dio3−/− mice
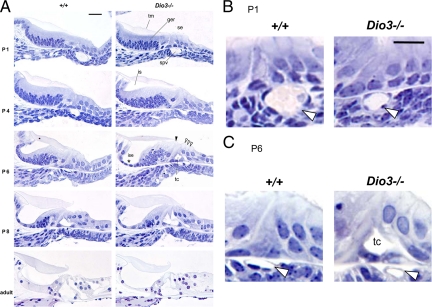
The activity of type 3 deiodinase in inactivating thyroid hormone (both T3 and T4) suggests that in Dio3−/− mice, the immature auditory system would be subject to premature hormonal stimulation. Dio3−/− mice also have dysfunction of the hypothalamic-pituitary-thyroid axis, such that there is a premature postnatal increase of serum T3 levels that peak at P10 rather than P15 (19). T3 levels decrease to near normal by P12, but the elevation (2- to 5-fold) in the first postnatal week could also add to inappropriate stimulation of the auditory system in the absence of type 3 deiodinase. An alternative hypothesis is suggested by the low serum T4 level in Dio3−/− pups, such that the lack of T4 substrate would limit the local generation of T3 by type 2 deiodinase, leading to a hypothyroid-like state within the cochlea (18). Morphological examination revealed premature rather than retarded cochlear differentiation of Dio3−/− mice, supporting the former hypothesis (Fig. 4).
Figure 4.
Cochlear morphology in Dio3−/− mice, shown for midturns of the cochlea. A, In Dio3−/− mice at P1, the spiral vessel (spv) was diminished in diameter. At P4, premature cochlear differentiation was evident. The GER (ger) had begun to regress to form the inner sulcus (is) below the tectorial membrane (tm) at P4 rather than P6. A thin inner sulcus epithelium (ise) (asterisk) was forming by P6. The tunnel of Corti (tc) opened at P6 rather than P8. Open arrowheads, three rows of outer hair cells; filled arrowhead, single row of inner hair cells. In Dio3−/− adults, there was little overt abnormality except that the ise was of lower height than in +/+ mice. Groups, n ≥ 3 mice per age. B and C, Higher magnification of the spiral vessel (open arrowhead) in Dio3−/− pups at P1 (B) and P6 (C) and prematurely opened tunnel of Corti at P6 (C). Scale bars, 20 μm.
In neonatal wild-type mice, the cochlear cell types have been generated but are immature. At P1, the tectorial membrane adheres to the underlying GER, which is subsequently remodeled to form the cavity of the inner sulcus. By P8, the tunnel of Corti has opened between the pillar cells that flank the inner and outer hair cells. These events proceed along the cochlear spiral from base to apex with a 1- to 2-d time lag (3). In Dio3−/− pups at P1, cochlear morphology showed little abnormality, except that the spiral vessel below the basilar membrane was small (Fig. 4, A and B). Normally, in mice this blood vessel enlarges transiently at P1, after which it shrinks over the next few days (29). In Dio3−/− mice, the diameter of the vessel was diminished over this period. At P1, mean widths in Dio3+/+ and Dio3−/− mice were 26.2 ± 1.7 and 17.1 ± 1.7 μm, respectively (P < 0.01; n = 6 sections per cochlea for six cochleae from three mice).
By P4, Dio3−/− mice exhibited premature cochlear differentiation that was advanced by about 2 d compared with wild-type mice. The GER began to regress, and the inner sulcus began to open at P4 rather than at P6, as in wild-type mice. The tunnel of Corti opened at P6 instead of P7-8, and a thin inner sulcus epithelium formed as early as P6 in Dio3−/− mice (Fig. 4, A and C). After P8, cochlear differentiation in Dio3−/− mice decreased to a more normal rate of progression. In adult Dio3−/− mice, there was no overt morphological malformation, although the epithelial cells of the inner sulcus appeared to be of lower height than in wild-type mice.
Premature cochlear differentiation in Dio3−/− mice is mediated by TRβ
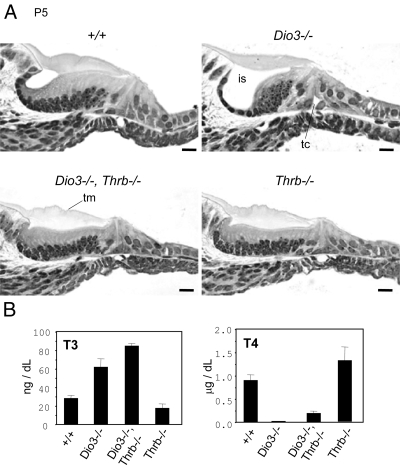
The premature differentiation of the cochlea in Dio3−/− mice suggested that the phenotype resulted from stimulation by T3 in the absence of protection by type 3 deiodinase. This proposal was in accord with the fact that premature development in Dio3−/− mice in the GER and sensory epithelium occurred in the same structures that show delayed development in Dio2−/− or hypothyroid mice (18). To test the hypothesis that the Dio3−/− phenotype was indeed mediated by T3, the major receptor for T3 in the cochlea, TRβ, was removed from Dio3−/− mice. According to the hypothesis, the cochlea in Dio3−/−;Thrb−/− combined mutants would be resistant to such actions of T3. At P5, the cochlea in Dio3−/−;Thrb−/− mice showed a delayed rather than premature phenotype that closely resembled the resistant phenotype in Thrb−/− mice (Fig. 5A). The tectorial membrane was swollen and adhered to the underlying epithelium, the inner sulcus had not formed, and the tunnel of Corti was unopened, demonstrating reversal of the cochlear phenotype from a prematurely sensitive state to a resistant state. The results support the conclusion that the Dio3−/− phenotype is mediated by T3.
Figure 5.
Premature cochlear differentiation in Dio3−/− pups is mediated by TRβ. A, Deletion of the Thrb gene reversed the cochlear phenotype of Dio3−/− mice from a premature state to a state of delayed differentiation, shown for equivalent midturns of the cochlea. At P5, Dio3−/− mice showed premature formation of the inner sulcus (is) and opening of the tunnel of Corti (tc). In contrast, Dio3−/−;Thrb−/− double mutants showed retarded differentiation, with an unopened inner sulcus and malformed tectorial membrane (tm), as in Thrb−/− mice; n = 6 cochleae from three pups per genotype. B, Serum T3 and T4 levels at P5 in Dio3−/−;Thrb−/− double and Dio3−/− or Thrb−/− single mutants. Mean ± sem; groups of three to four pools of serum, each from three to four pups. The introduction of the Thrb−/− mutation only slightly modified T3 and T4 levels in Dio3−/− mice. Scale bars, 20 μm.
To exclude possible indirect influences of systemic hormonal changes on the phenotype of Dio3−/−;Thrb−/− double mutants, serum T3 and T4 levels were determined in the postnatal period (Fig. 5B). Although Dio3−/− single and Dio3−/−;Thrb−/− double mutant mice showed opposite outcomes of accelerated and delayed cochlear differentiation, respectively, serum T3 levels were similarly elevated (2- to 3-fold) in both genotypes at P5. Similarly, T4 levels in Dio3−/− and Dio3−/−;Thrb−/− strains were only moderately different, being very low and low, respectively. In contrast, in Thrb−/− single mutants, the T4 level was increased above normal consistent with the known hyperactivity of the hypothalamic-pituitary-thyroid axis in this strain (12). The opposite cochlear abnormalities in Dio3−/− and Dio3−/−;Thrb−/− pups, despite their broadly similar hormonal profiles, suggest that the systemic hormonal status is not the primary determinant of the cochlear phenotype.
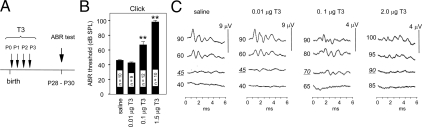
To test further the hypothesis that premature stimulation by T3 at neonatal stages causes auditory defects, wild-type mice of the C57BL/6J strain were treated transiently with T3 between P0 and 3. This window encompasses the period during which type 3 deiodinase would limit T3 stimulation but precedes the later period near P8, during which type 2 deiodinase would amplify T3 levels. After treatment, auditory function was tested later at 4 wk of age by determining ABR thresholds (Fig. 6A). It was predicted that low or moderate T3 doses would produce little or no defect, whereas excessive T3 would overcome the protective role of type 3 deiodinase to impair auditory function.
Figure 6.
Excessive T3 exposure in neonatal mice results in auditory deficits. A, C57BL/6J pups were injected (sc) with a given T3 dose each day from P0-3. The ABR was measured on treated mice at approximately P28. B, ABR thresholds (mean ± sem) for treated groups. Numbers of mice indicated within columns. **, P < 0.001, compared with saline-treated group. C, Representative ABR waveforms for a click stimulus. SPL (dB) is indicated to the left of each trace. Thresholds (underlined) are normal for treatment with saline and 0.01 μg T3 but are increased for 0.1 and 2.0 μg T3. Waveform amplitudes are diminished for mice treated with high T3 doses (note different fixed scale responses of 4 μV for high T3 and 9 μV for saline and low T3). ms, msec.
Figure 6B shows that injections of saline vehicle or a moderate T3 dose (0.01 μg/d, sc) did not alter ABR thresholds, which were similar to those for untreated mice (∼40 dB SPL). Very high T3 doses that gave 80-fold or more increases above normal serum T3 levels impaired the ABR, with the highest doses giving the worst impairment. A dose of 0.1 μg T3/d resulted in thresholds of approximately 70 dB SPL, whereas 1.5 or 2.0 μg T3/d resulted in thresholds of more than or equal to 90 dB SPL. The highest T3 doses resulted in poorly defined ABR waveforms with diminished amplitude (Fig. 6C). T3 injections resulted in expected increases in serum T3 concentrations. Daily injections of 0.01, 0.1, and 2.0 μg T3 until P5 resulted in serum T3 levels that were approximately 12-, 80-, and 280-fold, respectively, above levels in saline-treated pups when measured 1–2 h after injection on the last day of treatment. (Serum concentrations of T3 were 59 ± 1, 4,200 ± 1,014, and 16,000 ± 426 ng/dl after treatment with saline or 0.1 or 2.0 μg T3.) The auditory impairment caused by excessive T3 supported the conclusion that the deafness in Dio3−/− mice resulted from inappropriate stimulation by T3 in development.
Discussion
Adequate amounts of thyroid hormone must be present in the circulation to allow the development of hearing. The mouse cochlea itself augments this process through the activity of type 2 deiodinase, which can amplify the T3 level locally to meet increased demands shortly before the onset of hearing (18). The present study shows that type 3 deiodinase, a thyroid hormone-inactivating enzyme, also has a role in the auditory system in preventing premature response to T3. The comparably severe hearing loss that results from inactivation of either types 3 or 2 deiodinase in mice suggests that these enzymes prevent too much or too little hormonal stimulation at inappropriate stages in development. At immature stages, type 3 deiodinase limits stimulation by T3. Postnatally, a double switch occurs with a decline in type 3 and an increase in type 2 deiodinase, such that the cochlea progresses rather abruptly from a relatively protected to an actively responsive state. This switch would allow a surge in T3 levels to trigger the onset of auditory function. The immature cochlea expresses TRβ and is primed to respond to T3 but is constrained from doing so until the deiodinase switch occurs.
The physiological role of the cochlea includes supporting the nutritional needs of the hair cells as well as the regulation of the ionic composition of the endolymph that is necessary for auditory transduction (30). Our study suggests that another critical role of the cochlea is in the endocrine regulation of its thyroid hormone supply by type 3 and 2 deiodinases. The internal target tissues of T3 action in the cochlea receive little or no direct blood supply (25) and rely upon type 2 deiodinase in the cochlear support tissues for provision of adequate T3 (Fig. 2B). The separation of the tissues that express type 2 deiodinase and the internal tissues that respond to T3 points to the existence of transport mechanisms that would take up T4 for conversion to T3 and that would release T3 internally. Thyroid hormone transporters such as monocarboxylate transporter 8 are implicated in the brain and other systems (31,32), but none is yet known in the cochlea.
The phenotypes of premature and retarded cochlear differentiation in Dio3−/− and Dio2−/− strains, respectively, although opposites in some respects, have in common a lack of coordinated development with the result that function is impaired in both cases. Thus, this developmental program follows an intrinsic timetable under the control of type 3 and 2 deiodinases that can neither be hurried nor delayed without a deleterious outcome. Developmental timing is critical in sensory systems, and the initiation of sensory input at appropriate stages is necessary for maturation of central pathways in the visual (33) and auditory systems (34). An analogous role for thyroid hormone in coordinating developmental timing is evident in amphibian or fish species that undergo metamorphosis. Treatment with thyroid hormone accelerates metamorphosis, but the individual does not become a full-sized, normally functioning adult because tissues and organs are not allowed sufficient time to mature correctly (35).
Perhaps the most obvious function of T3 in the cochlea concerns the remodeling of the GER, a transient and enigmatic structure that loses most of its cell mass postnatally in mice. Regression of the GER involves caspase 3-mediated cell death (36) and leads to formation of the inner sulcus epithelium that borders the cavity below the tectorial membrane. The resulting dynamic suspension of the tectorial membrane, which contacts the hair cells, is critical for hearing because this permits the conversion of sound-induced movement into hair cell stimulation. Little is known of the function of the GER beyond it being the passive target of this remodeling. However, a recent study suggests that the GER in rats actively induces maturation of the nearby inner hair cells by ATP signaling (37). Conceivably, thyroid hormone also triggers signals between the GER and the sensory epithelium at a key postnatal stage to promote auditory function. Thyroid hormone also stimulates neuronal maturation in the brainstem and higher auditory centers (38), and deiodinase expression in central pathways may contribute to the overall development of hearing (39). Nonetheless, the current results showing premature cochlear differentiation, decreased otoacoustic emissions, and impaired CAPs indicate that cochlear dysfunction plays a major part in the hearing loss in Dio3−/− mice.
This study suggests that premature exposure to T3 causes hearing loss. However, another conclusion is that type 3 deiodinase protects the auditory system from such impairment during normal development and probably also in congenital disorders with mild elevation of thyroid hormone levels. Thus, treatment with very high but not low doses of T3 impaired auditory function in wild-type mice (Fig. 6). Treatment of immature rodents with thyroid gland extracts or T4 is reported to have only minor or transient consequences on auditory function (28,40). In rats, T4 injections modestly advanced the onset of auditory function, but adult thresholds for the ABR and DPOAE were ultimately normal (41,42). It is unknown how much of this T4 is converted into T3 by type 2 deiodinase in the auditory system, but the lack of a major impact on hearing may be attributed to the protection given by type 3 deiodinase, which can inactivate both T4 and T3. In contrast, the severe, permanent deafness in Dio3−/− mice can be explained by the auditory system being oversensitive to T3 in the absence of type 3 deiodinase. A contribution by the moderately increased T3 levels in Dio3−/− mice is not excluded. However, this contribution may be small because wild-type mice only acquired ABR defects after treatment as neonates with very high but not moderate T3 doses (Fig. 6). Genetic background in mice has been reported to modify the sensitivity of the auditory system to thyroid hormone, implying the involvement of other currently unknown factors in determining tissue-specific responses to this hormone (28).
In addition to hearing in mammals, type 3 deiodinase controls a range of functions in other species. These include changes in eye structure during Xenopus metamorphosis (43). In Japanese quail, reciprocal changes in Dio2 and Dio3 expression in the hypothalamus accompany changing daylight length and are thought to control seasonal reproductive function (44). Type 3 deiodinase is also expressed in the rodent and human uterus, where it is thought to limit general exposure of the fetus to thyroid hormone (45,46). Type 3 deiodinase mutations are currently unknown in humans but may be predicted to impair hearing, as well as growth and thyroid gland function, as suggested by phenotypes in mice (19,47).
The phenotype of premature cochlear differentiation in Dio3−/− mice is unusual but is remarkably similar to defects described in rats exposed to cocaine in utero (48). In both cases, regression of the GER and opening of the tunnel of Corti occur prematurely. Prenatal cocaine exposure can impair the auditory response in rats (49), and is associated with neurological impairment and auditory defects in human infants (49,50). The possibility that related mechanisms underlie the developmental abnormalities caused by cocaine exposure or by the absence of type 3 deiodinase may deserve further study.
Supplementary Material
Acknowledgments
We thank F. Celi and J. Nunez for comments on this study. We dedicate this article to the memory of our colleague Jack Robbins.
Footnotes
This work was supported in part by March of Dimes Birth Defects Foundation and National Institutes of Health Grants DC-03441 (to D.F.), HD-09020 (to V.A.G.), DK-42271 (to D.L.S.), and DC-004554 (to T.R.), and by the Intramural Program at National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (to D.F.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 18, 2008
Abbreviations: ABR, Auditory evoked brainstem response; CAP, compound action potential; dB, decibel; DPOAE, Distortion Product Otoacoustic Emission; E18.5, embryonic d 18.5; EP, endocochlear potential; f1, lower test frequency; f2, higher test frequency; GER, greater epithelial ridge; P0, postnatal d 0; SPL, sound pressure level; TRβ, thyroid hormone receptor β.
References
- DeLong GR, Stanbury JB, Fierro-Benitez R 1985 Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev Med Child Neurol 27:317–324 [DOI] [PubMed] [Google Scholar]
- Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R 1996 Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr 128:776–783 [DOI] [PubMed] [Google Scholar]
- Deol MS 1976 The role of thyroxine in the differentiation of the organ of Corti. Acta Otolaryngol 81:429–435 [DOI] [PubMed] [Google Scholar]
- Hébert R, Langlois JM, Dussault JH 1985 Permanent defects in rat peripheral auditory function following perinatal hypothyroidism: determination of a critical period. Brain Res 23:161–170 [DOI] [PubMed] [Google Scholar]
- Uziel A 1986 Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol Suppl 429:23–27 [DOI] [PubMed] [Google Scholar]
- Rüsch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D 2001 Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci 21:9792–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zimmermann U, Winter H, Mack A, Kopschall I, Rohbock K, Zenner HP, Knipper M 2002 Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci USA 99:2901–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bulankina AV, Riedel D, Moser T 2007 Maturation of ribbon synapses in hair cells is driven by thyroid hormone. J Neurosci 27:3163–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S, DeWind LT, DeGroot LJ 1967 Familial syndrome combining deaf-mutism, stippled epiphyses, goiter, and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab 27:279–294 [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni M-A, Koby M, Weintraub BD 1996 Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab 81:2768–2772 [DOI] [PubMed] [Google Scholar]
- Phillips SA, Rotman-Pikielny P, Lazar J, Ando S, Hauser P, Skarulis MC, Brucker-Davis F, Yen PM 2001 Extreme thyroid hormone resistance in a patient with a novel truncated TR mutant. J Clin Endocrinol Metab 86:5142–5147 [DOI] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T 1996 Thyroid hormone receptor β is essential for development of auditory function. Nat Genet 13:354–357 [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Szymko YM, Kaneshige M, Quinonez RE, Kaneshige K, Heintz KA, Mastroianni MA, Kelley MW, Cheng SY 2002 Knock-in mouse model for resistance to thyroid hormone (RTH): an RTH mutation in the thyroid hormone receptor β gene disrupts cochlear morphogenesis. J Assoc Res Otolaryngol 3:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young 3rd WS 1994 α and β thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci USA 91:439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- St Germain DL, Hernandez A, Schneider MJ, Galton VA 2005 Insights into the role of deiodinases from studies of genetically modified animals. Thyroid 15:905–916 [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D 2000 Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci USA 97:1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D 2004 Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA 101:3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D 2006 Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T 2002 Longitudinal pattern of basilar membrane vibration in the sensitive cochlea. Proc Natl Acad Sci USA 99:17101–17106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham MA, Huynh KH, Gao J, Zuo J, Dallos P 2004 Cochlear function in Prestin knockout mice. J Physiol 560(Pt 3):821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA 2001 Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- Hernandez A 2005 Structure and function of the type 3 deiodinase gene. Thyroid 15:865–874 [DOI] [PubMed] [Google Scholar]
- Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D 2007 Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104:14466–14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL 2003 Disorders of cochlear blood flow. Brain Res Brain Res Rev 43:17–28 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D 2002 The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology 143:4483–4486 [DOI] [PubMed] [Google Scholar]
- Parham K, Sun X-M, Kim D 2001 Noninvasive assessment of auditory function in mice: auditory brainstem response and distortion product otoacoustic emissions. In: Willott J, ed. Handbook of mouse auditory research. Boca Raton, FL: CRC Press; 37–58 [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Parlow AF, Raphael Y, Dolan DF, Camper SA 2007 Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm Genome 18:596–608 [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding DA 1967 The spiral vessel and stria vascularis in Shaker-1 mice. Electron microscopic and histochemical observations. Acta Otolaryngol 63:395–410 [DOI] [PubMed] [Google Scholar]
- Dallos P, Popper A, Fay R 1996 The cochlea. New York: Springer [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ 2004 Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ 1996 Synaptic activity and the construction of cortical circuits. Science 274:1133–1138 [DOI] [PubMed] [Google Scholar]
- Ruben RJ, Rapin I 1980 Plasticity of the developing auditory system. Ann Otol Rhinol Laryngol 89:303–311 [DOI] [PubMed] [Google Scholar]
- Inui Y, Miwa S 1985 Thyroid hormone induces metamorphosis of flounder larvae. Gen Comp Endocrinol 60:450–454 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kamiya K, Urase K, Suga M, Takizawa T, Mori H, Yoshikawa Y, Ichimura K, Kuida K, Momoi T 2001 Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res 894:359–367 [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE 2007 The origin of spontaneous activity in the developing auditory system. Nature 450:50–55 [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Crane AM, Rosloff B, Kennedy C, Sokoloff L 1986 Local cerebral glucose utilization in the adult cretinous rat. Brain Res 373:139–145 [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Escámez M, Rausell E, Bernal J 1999 Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory neurons. J Neurosci 19:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle PM, McGee J, Bertoni JM, Walsh EJ 2001 Prevention of auditory dysfunction in hypothyroid Tshr mutant mice by thyroxin treatment during development. J Assoc Res Otolaryngol 2:348–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard PA, Hebert R, Coulombe P 1982 Influence of tetraiodothyronine on hearing maturation in rats. Arch Otorhinolaryngol 234:181–186 [DOI] [PubMed] [Google Scholar]
- Freeman S, Cherny L, Sohmer H 1996 Thyroxine affects physiological and morphological development of the ear. Hear Res 97:19–29 [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD 1999 Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 24:871–878 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S 2003 Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426:178–181 [DOI] [PubMed] [Google Scholar]
- Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL 1999 Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest 103:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR 2003 Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 88:1384–1388 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL 2007 Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148:5680–5687 [DOI] [PubMed] [Google Scholar]
- Trigueiros-Cunha N, Leao P, Renard N, Tavares MA, Eybalin M 2006 Prenatal cocaine exposure accelerates morphological changes and transient expression of tyrosine hydroxylase in the cochlea of developing rats. Brain Res 1086:55–64 [DOI] [PubMed] [Google Scholar]
- Church MW, Crossland WJ, Holmes PA, Overbeck GW, Tilak JP 1998 Effects of prenatal cocaine on hearing, vision, growth, and behavior. Ann NY Acad Sci 846:12–28 [PubMed] [Google Scholar]
- Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL 2003 The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr 142:279–285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.