Abstract
There have been several reports that TNF-related apoptosis-inducing ligand (TRAIL) has the ability to suppress the development of experimental autoimmune diseases, including a mouse model of experimental autoimmune encephalomyelitis, a rabbit model of rheumatoid arthritis, type 1 diabetes mellitus, in mice and experimental autoimmune thyroiditis (EAT) in mice. However, the mechanism underlying TRAIL effect is not well defined. In the present study, we specifically examined TRAIL effects on CD4+CD25+ regulatory T cells. CD4+CD25+ T cells prepared from mouse thyroglobulin (mTg)-immunized CBA/J mice proliferate in the presence of TRAIL and dendritic cells in vitro. These CD4+CD25+ T cells included both CD4+CD25+CD45RBLow (regulatory) and CD4+CD25+CD45RBHigh (effector) T cells. Our results demonstrated that mTg-immunized mice treated with TRAIL showed significant increases in the number of CD4+CD25+CD45RBLow T cells compared with mice immunized with mTg alone. CD4+CD25+CD45RBLow T cells expressed much higher levels of the forkhead family transcription factor, IL-10, and TGFβ1 than CD4+CD25+CD45RBHigh T cells, and these cells can completely suppress the proliferation of the mTg-primed splenocytes in lower concentrations than the unfractionated CD4+CD25+ T cells. Furthermore, transfer of these cells into CBA/J mice prior to mTg-primed splenocyte injection could markedly reduce the frequency and severity of EAT development. CD4+CD25+CD45RBLow T cells were more effective at suppressing histological thyroiditis than unfractionated cells. These results indicated that TRAIL can increase the number of mTg-specific CD4+CD25+CD45RBLow T cells, inhibiting autoimmune responses and preventing the progression of EAT. These findings reveal a novel mechanism by which TRAIL could inhibit autoimmune disease.
TRAIL can promote antigen-specific Treg cells by expanding their number of Foxp3-rich CD4+CD25+CD45RBLow T cells.
Hashimoto's thyroiditis (HT) is a well-known autoimmune disease characterized by immune cell infiltration into the thyroid, leading to the destruction of thyrocytes via apoptosis. Although the pathogenesis of HT is not entirely clear, our previous studies demonstrated that increasing the local production of inflammatory cytokines in the thyroid microenvironment plays a critical role in facilitating thyrocyte apoptosis (1,2,3); however, the factors controlling local thyroid inflammation are not well defined. Recent studies suggested that CD4+CD25+ regulatory T (Treg) cells can down-regulate Th1 cytokine-producing T cells (4,5) and thereby suppress the production of the inflammatory cytokines (6), such as TNF-α and interferon (IFN)-γ, which are involved in facilitating apoptosis in thyrocytes. Furthermore, the forkhead family transcription factor (Foxp3), which is crucial to the generation and survival of Treg cells (7), inhibits nuclear factor-κB-related inflammatory cytokine production, including IFN-γ and IL-1β (8). Treg cells also can prevent autoimmunity by suppressing autoreactive T cells and have been shown to be qualitatively and/or quantitatively deficient in human autoimmune diseases (9,10). Therefore, it is possible that the inflammatory cytokines underlying the development of HT result from a deficiency in Treg cells.
TNF-related apoptosis-inducing ligand (TRAIL) has been reported to suppress experimental autoimmune diseases, including a mouse model of experimental autoimmune encephalomyelitis (11), and a rabbit model of rheumatoid arthritis (12). It has been proposed that Treg cells may be involved in TRAIL-mediated prevention of experimental autoimmune encephalomyelitis (13) and suppression of the response to allogeneic skin grafts (14). In addition, blockage of TRAIL exacerbates the onset of type 1 diabetes in nonobese mouse model of autoimmune diabetes Severe Combined Immunodeficiency recipients of transferred diabetogenic T cells and in cyclophosphamide-treated NOD mice (15). Regulatory CD4+CD25+ T cells have been associated with expression of the memory T cell marker CD45RBLow (16,17). According to this marker, CD4+CD25+ T cells can be divided into CD4+CD25+CD45RBLow T cells and CD4+CD25+CD45RBHigh T cells. The former play a regulatory role whereas the latter function as effectors. The imbalance between these two populations of T cells is deemed to be critical in the development of autoimmune diseases, and the subsets may be differentially altered by TRAIL in different disease models.
Recent evidence suggests that reduced activity of Treg cells supports inflammation in experimental autoimmune thyroiditis (EAT), a mouse model for HT (18,19,20). Additional studies using a Graves' disease mouse model showed that these mice, depleted of Treg cells, developed severe thyroiditis (21). Removal of Treg cells from mice that do not develop autoimmunity can result in EAT (22), whereas the adoptive transfer of Treg cells prevents EAT (23,24,25). Therefore, a defect in Treg cells could enhance the production of inflammatory Th1 cytokines, such as IL-1β, IFN-γ, and TNF-α, in the thyroid, and this could facilitate thyroid cell apoptosis in HT (26,27). We previously showed that TRAIL-treated mice developed a milder form of the adoptive transfer EAT, with significant decreases in mononuclear cell thyroid infiltration with less follicular destruction and fewer apoptotic thyrocytes (28). Furthermore, mouse thyroglobulin (mTg)-specific Th1 responses in TRAIL-treated mice were much lower than those in the control animals. These findings suggested that TRAIL suppresses the development of EAT by altering the function of immune cells.
In the current studies, we examined the effect of TRAIL on the development of antigen-specific populations of T cells in EAT with specific focus on the ability of TRAIL to alter populations of CD4+CD25+ CD45RBLow T cells that suppress inflammation.
Materials and Methods
EAT induction and TRAIL treatment
CBA/J female mice were immunized twice with mTg (40 μg) and lipopolysaccharide (20 μg) at d 0 and 7. Some of the mice were also treated with recombinant human TRAIL as previously described (28) or a control protein (BSA) by ip injection starting at d 0 and then every other day at a dosage of 100 μg per mouse for 4 wk. The mice were killed 28 d after the first injection and their splenocytes were collected for the isolation of splenic T cells, CD4+, and CD4+CD25+ T cells.
Isolation of CD4+ and CD4+CD25+ T cells
Splenic T cells were isolated by T cell enrichment columns (R&D Systems, Minneapolis, MN), which can achieve a purity of greater than 95%. CD4+ and CD4+CD25+ T cells were isolated from the spleen-derived lymphocytes using a CD4+ or CD4+CD25+ T cell isolation kit (Miltenyi Biotec, Auburn, CA), respectively. The percentages of CD4+, CD4+CD25−, and CD4+CD25+ T cells in the total lymphocytes were determined by flow cytometry.
Cytokine production in vitro
Antibodies were immobilized on 96-well tissue-culture plates by incubating 5 μg/ml anti-CD3 (145-2C11) and 5 μg/ml anti-CD28 (37.51) in PBS overnight at 4 C (BD PharMingen, San Diego, CA). Excess antibody was removed by washing the wells with PBS. CD4+ T cells prepared from spleen were then added to anti-CD3- and anti-CD28-coated wells for 48 h in vitro, and their culture supernatants were collected and measured for cytokine production. The levels of IL-4, IL-10, and IFN-γ were detected by a mouse cytokine Lincoplex kit (Linco Research, St. Charles, MO) according to the manufacturer's instructions.
Sorting of CD4+CD25+, CD45RBLow, and CD4+CD25+CD45RBHigh T cells
CD4+ T cells isolated from the spleens were incubated with the CD16/32 antibody to block their Fc receptors, and then the cells were coincubated with phycoerythrin-anti-CD25 and fluorescein isothiocyanate (FITC)-anti-CD45RB antibodies (BD PharMingen). The stained cells were sorted by flow cytometry into two groups, based on the amount of CD45RB. The CD4+CD25+ T cells with a low level of the memory T cell marker CD45RB were identified as CD4+CD25+CD45RBLow T cells, and those with a high level of memory T cell maker CD45RB were identified as CD4+CD25+CD45RBHigh T cells.
Generation of dendritic cells (DCs)
Bone marrow (BM)-derived DCs were obtained from the femurs and tibiae of female CBA/J mice. After removal of the red blood cells, the BM cells were cultured in R-10 complete medium with the addition of 20 ng/ml of granulocyte macrophage colony-stimulating factor and 20 ng/ml of IL-4. Two days later, the nonadherent cells were removed, and a 1:1 volume of conditioned medium, fresh R-10 medium, granulocyte macrophage colony-stimulating factor, and IL-4 were added to the adherent cells. Three days later, the loosely adherent cells were gently agitated and harvested. The BM-DCs population was enriched by collecting the low-density interface after OptiPrep (Axis-shield, Norton, MA) density gradient centrifugation and was washed twice in Hanks' balanced salt solution. The BM-DCs were then further purified by positive selection, using CD11c microbeads (Miltenyi Biotec).
Determination of T cell proliferation
The CD4+CD25+ T cells or CD4+CD25+CD45RBLow T cells were cocultured with spleen cells or CD4+ CD25− T cells in the presence of TRAIL and DCs alone or in combination for 4 d in a 96-well plate. When DCs were used to stimulate T cells, the ratios of DCs to T cells were set at 1:20 and 1:30. Cell proliferation was assayed by the [3H]thymidine method (28). In the last 18 h, [3H]thymidine (ICN Biomedicals, Costa Mesa, CA) was added to each well. The cells were then harvested onto glass fiber filters, and radioactivities were determined by a flatbed β-counter.
mRNA expression of Foxp3, IL-10, and TGFβ1
The expression of Foxp3 mRNA was measured by RT-PCR. RNA isolated from the CD4+CD25+CD45RBLow or CD4+CD25+CD45RBHigh T cell was converted to cDNA by reverse transcription, using Moloney murine leukemia virus reverse transcriptase. The cDNA was then amplified by PCR, using the Cepheid smart cycler system (Cepheid, Sunnyvale, CA). The forward and backward primers for Foxp3 were 5′-ACT CGC ATG TTC GCC TAC TTC AGA-3′ and 5′-TGG CTC CTC TTC TTG CGA AAC TCA-3′, respectively. The primers for IL-10 and TGFβ1 were 5′-GGTTGCCAAGCCTTATCGGA-3′; 5′-ACCTGCTCCACTGCCTTGCT-3′ and 5′-AAGGGCTACCATGCCAACTT-3′; and 5′-TGTGTTGGTTGTAGAGGGCA-3′, respectively. The β-actin was also measured by RT-PCR from the same RNA samples and was used as an internal control. The mRNA expression was quantified using the comparative cross-threshold (CT; the PCR cycle number that crosses the signal threshold) method. The CT of the housekeeping gene β-actin was subtracted from the CT of the target gene (Foxp3) to obtain ΔCT. The normalized fold changes of the Foxp3, IL-10, and TGFβ1 mRNA expression were expressed as 2-ΔΔCT, where ΔΔCT is equal to the ΔCT sample minus the ΔCT control.
Detection of Foxp3 protein by flow cytometry
Spleen cells were prepared from mTg-immunized mice treated with or without TRAIL. Fc receptors on spleen cells were blocked by the CD16/32 antibody, and then the cells were incubated with APC-Cy7-anti-CD4, PerCp-Cy5.5-anti-CD25, and FITC-anti-CD45RB antibodies. After staining the surface molecules, the cells were incubated in the fixation/permeabilization solutions. Then the cells were further stained with anti-Foxp3-PE antibody (eBioscience, San Diego, CA) or an isotype control. Foxp3 protein expression was measured by flow cytometry.
Inhibition of EAT
A total of 3 × 105 of CD4+CD25+CD45RBLow T cells prepared from mTg-immunized mice were transferred iv to an irradiated (600 rads) CBA/J recipient before injection of mTg-primed spleen cells (1.5 × 107), which were activated with mTg (20 μg/ml) at 37 C for 72 h. After 21 d of CD4+CD25+CD45RBLow transfer, all mice were killed for assessment of thyroiditis. Thyroid glands were fixed, embedded, and sectioned by a standard method. The extent of mononuclear cell infiltration was based on a scale of 0–4 as described in Wang et al. (1). Scoring was performed blind to the animal treatment groups.
Statistical analysis
All values were expressed as mean ± se. The statistical significance of the differences between the control and the experimental group was analyzed with the Wilcoxon matched pair test or the Student's t test, using the software Stat View (Abacus Concept, Inc., Berkeley CA). P < 0.05 was taken as statistically significant.
Results
Increased production of IL-4 and IL-10 in EAT mice by TRAIL
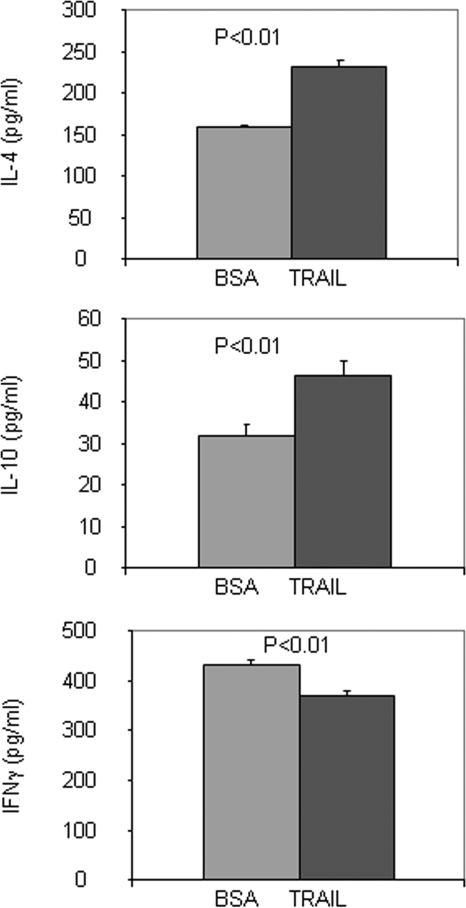
Given that our previous study showed that TRAIL could inhibit the adoptive transfer of EAT (28), we sought to determine whether TRAIL can also inhibit EAT directly induced by immunization with mTg and lipopolysaccharide. Mice were administered TRAIL or BSA (as a control) in mTg-immunized mice at d 0 and then every other day for 4 wk. Compared with the BSA control animals, the TRAIL-treated mice developed a milder form of EAT (n = 12, P < 0.01), which is identical with the effect of TRAIL on adoptive transfer EAT (28). Subsequently we determined the cytokine production of CD4+ T cells from these mice, after in vitro activation with anti-CD3 and anti-CD28 antibodies. Production of IL-4 and IL-10 by lymphocytes from the TRAIL-treated mice was significantly increased, whereas the level of IFN-γ was decreased (Fig. 1), suggesting that the TRAIL treatment favors CD4+ T cells to synthesize Th2 cytokines.
Figure 1.
Cytokine secretion profile of CD4+ T cells. CD4+ T cells isolated from mTg-immunized mice treated with TRAIL or BSA were activated with anti-CD3 and anti-CD28 monoclonal antibodies for 48 h in vitro. Their culture supernatants were collected and measured for IL-4, IL-10, and IFN-γ productions. The data are expressed as mean ± sd.
Inhibition of T cell proliferation by TRAIL
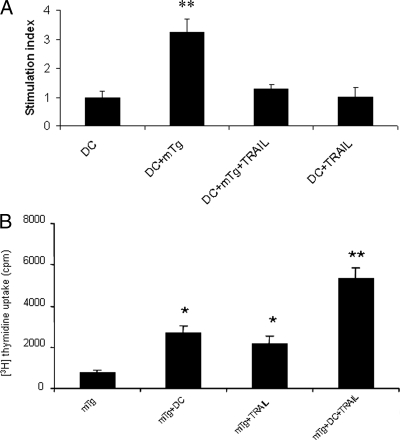
To determine whether TRAIL suppresses antigen-specific T cells, we then tested the effect of TRAIL on antigen-specific T cell proliferation in vitro. Splenic T cells isolated from mTg-primed splenocytes in the presence of DCs were incubated with TRAIL for 4 d in either the presence or absence of mTg. [3H]thymidine was added in the last 18 h. The results showed that mTg significantly enhanced the proliferation of splenic T cells (P < 0.01) (Fig. 2A). However, such an increase in the proliferation of splenic T cells was reduced to control levels by the addition of TRAIL. This indicates that TRAIL can effectively inhibit splenic T cell proliferation induced by mTg in vitro.
Figure 2.
TRAIL effect on splenic T cell or CD4+CD25+ T cell proliferation in vitro. Splenic T cells isolated from mTg-immunized mice were cultured with mTg, TRAIL, and mTg/TRAIL in the presence of DC. The proliferation of splenic T cells to mTg was determined by 3H-thymidine uptake. **, P < 0.01, compared with all three other groups (A). CD4+CD25+ T cells isolated from the splenocytes of mTg-immunized mice were cultured with mTg, DC, and TRAIL in the presence of IL-2 for 4 d. The proliferation of CD4+CD25+ T cells was determined by 3H-thymidine uptake. *, P < 0.05, compared with mTg group; **, P < 0.01, compared with mTg+DC or mTg+TRAIL groups (B).
Enhancement of CD4+CD25+ T cell proliferation by TRAIL
Because TRAIL was able to inhibit the proliferation of antigen-specific splenic T cell, we further tested whether the inhibitory effect was associated with the CD4+CD25+ T cell subpopulation that is thought to possess suppressive activity (9,10). We hypothesized that the increased mTg-specific CD4+CD25+ T cell population was responsible for the effect of TRAIL on splenic T cell proliferation. To this end, 2.5 × 105 of CD4+CD25+ T cells prepared from the spleen cells of mTg-immunized mice in the complete medium with the addition of 10 ng/ml of IL-2 were treated with TRAIL in the presence of mTg and DCs in vitro. We found that the proliferation was significantly higher in the CD4+CD25+ T cells treated with TRAIL and DCs in combination, compared with those treated with either agent alone (Fig. 2B; **, P < 0.01). Thus, the finding suggests that the inhibitory effect of TRAIL is associated with the increased proliferation of the CD4+CD25+ T cells in response to the antigen.
Inhibitory effect of CD4+CD25+ T cells
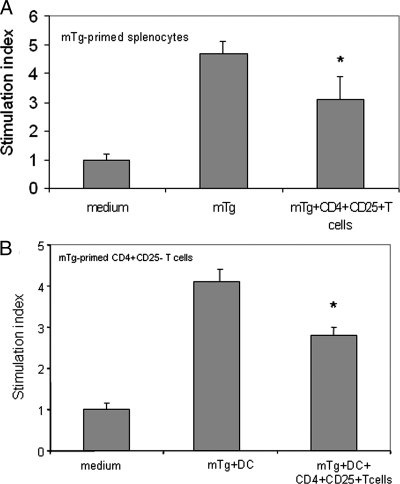
Further experiments were performed to evaluate the immune inhibitory effects of CD4+CD25+ T cells. We examined whether these cells could inhibit the proliferation of the spleen cells, in particular CD4+CD25− T cells. The purified CD4+CD25+ T cells were cocultured with spleen cells or CD4+CD25− T cells isolated from mTg-immunized CBA/J mice. In the coculture system, the CD4+CD25+ T cells were in 1:4 or 1:1 ratio to either the spleen cells or the CD4+CD25− T cells, respectively. The results showed that the CD4+CD25+ T cells partially blocked splenocyte proliferation (Fig. 3A). Using DCs as antigen-presenting cells, the proliferation of the CD4+CD25− T cells in the presence of mTg was also partially inhibited by the CD4+CD25+ T cells (Fig. 3B).
Figure 3.
Inhibitory effect of CD4+CD25+ T cells. mTg-primed splenocytes or CD4+CD25− T cells/DC were cocultured with CD4+CD25+ T cells isolated from mTg-immunized mice in the presence of mTg. The proliferation of splenocytes (A) or CD4+CD25− T cells (B) was determined by 3H-thymidine uptake. *, P < 0.05, compared with mTg or mTg+DC groups.
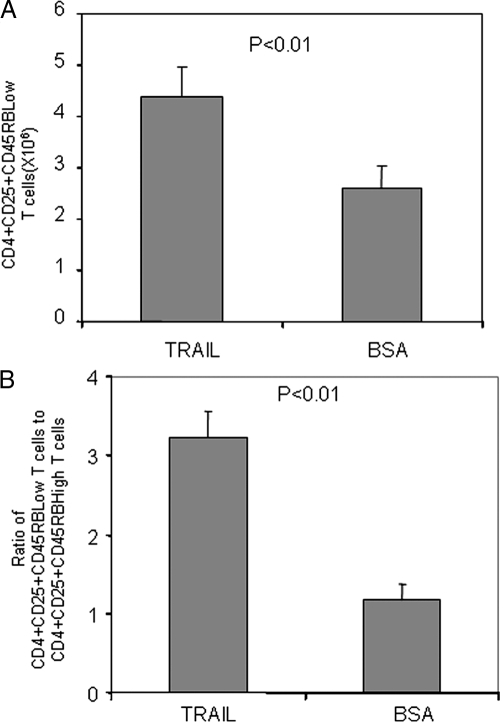
Expansion of CD4+CD25+CD45RBLow T cells by TRAIL
In mTg-immunized mice, the CD4+CD25+ T cells also include activated CD4+CD25+ effector T cells with high-level expression of CD45RB (16,17,20). Therefore, we further separated CD4+CD25+ T cells into CD4+CD25+ CD45RBLow and CD4+CD25+CD45RBHigh T cells, according to their expression of the T cell marker CD45RB. Of interest, the overall number of CD4+CD25+CD45RBLow T cells was significantly higher in the mTg-immunized mice treated with TRAIL than in those treated with BSA (Fig. 4A). In contrast to the CD4+CD25+CD45RBLow T cells, the number of CD4+CD25+CD45RBHigh T cells was slightly decreased (data not shown). The increase in CD4+CD25+CD45RBLow T cells and the decrease in CD4+CD25+CD45RBHigh T cells resulted in a significant increase in the ratio of CD4+CD25+CD45RBLow T cells to CD4+CD25+CD45RBHigh T cells in the mice treated with TRAIL, compared with the mice treated with BSA (Fig. 4B). The elevation of this ratio indicates an enhancement in the inhibitory effect of Treg cells because CD4+CD25+CD45RBHigh T cells are effector T cells, whereas CD4+CD25+CD45RBLow T cells are regulatory and inhibitory T cells (16,17).
Figure 4.
Increase of CD4+CD25+CD45RBLow T cell number by TRAIL treatment in vivo. CD4+ T cells were prepared from mTg-immunized mice with or without TRAIL treatment. The CD4+ T cells were stained with PE-anti-CD25 and FITC-anti-CD45RB antibodies. The number of CD4+CD25+CD45RBLow T cells was determined by flow cytometry (A). The ratio of CD4+CD25+CD45RBLow T cells to CD4+CD25+CD45RBHigh T cells was calculated (B).
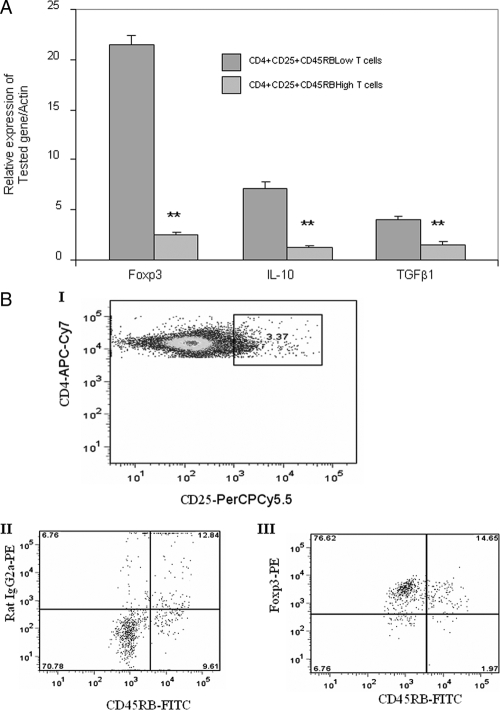
Foxp3 expression in CD4+CD25+CD45RBLow T cells
First, we examined the expression of Foxp3 in CD4+CD25+CD45RBLow T cells and CD4+CD25+CD45RBHigh T cells prepared from the spleen cells of mTg-immunized mice treated with TRAIL. The results showed that the CD4+CD25+CD45RBLow T cells expressed 8.6-fold more Foxp3 than the CD4+CD25+CD45RBHigh T cells in the mRNA level (Fig. 5A). The elevated expression of Foxp3 was also confirmed at the protein level as examined by flow cytometry (Fig. 5B). Under gating on the CD4 and CD25 expressions, 69.9% of CD45RBLow T cells expressed Foxp3, whereas 1.8% of CD45RBHigh T cells expressed Foxp3. Together, these data support that the inhibitory Treg cells predominantly consist of CD4+CD25+CD45RBLow T cells. Similar to CD4+CD25+CD45RBLow T cells prepared from the spleen cells of mTg-immunized mice treated with TRAIL, a higher percentage of CD4+CD25+CD45RBLow T cells prepared from the spleen cells of mTg-immunized mice without TRAIL treatment also expressed Foxp3 than that in the corresponding CD4+CD25+CD45RBHigh T cells. Therefore, the higher level of Foxp3 in CD4+CD25+CD45RBLow T cells appears to be an inborn feature of these cells. TRAIL may not directly affect the expression of Foxp3, but it dramatically increases the population of the Foxp3-rich CD4+CD25+CD45RBLow T cells (Fig. 5), as is evident by much higher numbers of CD4+CD25+CD45RBLow T cells in mTg-immunized mice treated with TRAIL than in those treated with BSA (Fig. 4). In addition to Foxp3, we also measured the levels of IL-10 and TGFβ1 in CD4+CD25+CD45RBLow T cells and CD4+CD25+CD45RBHigh T cells because both IL-10 and TGFβ1 are closely related to the function of Treg cells (24,29,30). The results showed that the mRNA levels of IL-10 and TGFβ1 were 5.7- and 2.5-fold higher, respectively, in the CD4+CD25+CD45RBLow T cells than in the CD4+CD25+CD45RBHigh T cells (Fig. 5A).
Figure 5.
Expression of Foxp3, IL-10, and TGFβ1 in CD4+CD25+CD45RBLow T cells and CD4+CD25+CD45RBHigh T cells. A, RNA was extracted from CD4+CD25+CD45RBLow T cells or CD4+CD25+ CD45RBHigh T cells isolated from mTg-immunized mice. The expression of Foxp3, IL-10, and TGFβ1 mRNA was measured by RT-PCR. **, P < 0.01, compared with CD4+CD25+D45RBLow T cell group. B, mTg-primed splenocytes were stained with the CD4, CD25, and CD45RB surface markers. Under gating on the CD4 and CD25 markers (I), the expression of Foxp3 protein in CD45RBLow and CD45RBHigh T cells (III) was analyzed by flow cytometry. The staining with rat IgG2a, an isotype control, was also shown (II). Data are representative of three independent experiments.
CD4+CD25+CD45RBLow T cell is a crucial population for inhibitory effect exerted by unfractionated CD4+CD25+ T cells
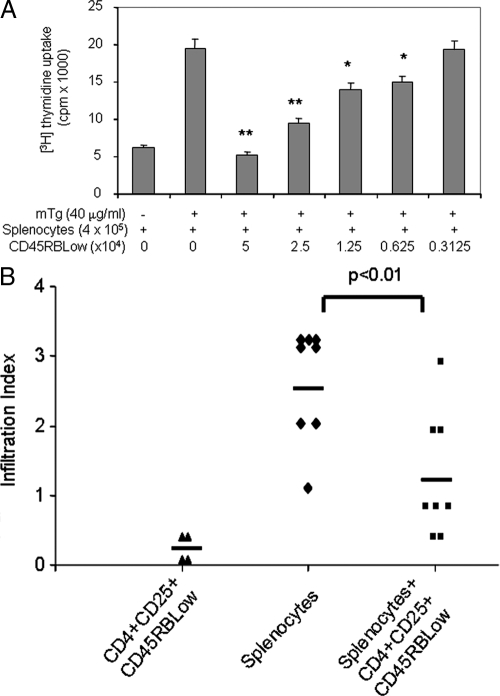
Second, we tested our hypothesis that the inhibitory effect of CD4+CD25+CD45RBLow T cells is much stronger than unfractionated CD4+CD25+T cells isolated from mTg-immunized mice. Different amounts of CD4+CD25+CD45RBLow T cells were cocultured with a total of 4 × 105 of spleen cells prepared from mTg-immunized mice in the presence of mTg for 4 d. The proliferation of splenocytes was determined by 3H-thymidine uptake. The results showed that the CD4+CD25+CD45RBLow T cells suppressed the proliferation of splenocytes in a dose-dependent manner (Fig. 6A). The proliferation of splenocytes was completely blocked under 1:8 or 1:16 ratios of CD4+CD25+CD45RBLow T cells to splenocytes and was partially blocked under 1:32 or 1:64 ratios. However, CD4+CD25+T cells can only partially block the proliferation of splenocytes under a 1:4 ratio (Fig. 3A). These data further confirm that the inhibitory function of CD4+CD25+CD45RBLow T cells is more potent than that of unfractionated CD4+CD25+ T cells.
Figure 6.
Suppressive effect of CD4+CD25+CD45RBLow T cells. Using flow cytometry, CD4+CD25+CD45RBLow T cells were sorted from CD4+ T cells isolated mTg-primed splenocytes. MTg-primed splenocytes were cocultured with CD4+CD25+ CD45RBLow T cells in the presence of mTg. The proliferation of splenocytes was determined by 3H-thymidine uptake. *, P < 0.05, **P < 0.01, compared with mTg + splenocyte group (A). CD4+CD25+CD45RBLow T cells (3 × 105) were transferred to an irradiated CBA/J recipient before injection of the mTg-primed splenocytes (1.5 × 107). After 21 d of CD4+CD25+CD45RBLow transfer, all mice were killed and the thyroid sections were analyzed for mononuclear cell infiltration (B). The index of infiltration was determined as previously described (1).
Therapeutic effect of CD4+CD25+CD45RBLow T cells
Finally, we examined whether the CD4+CD25+CD45RBLow T cells had any therapeutic effect on the EAT. We transferred the CD4+CD25+CD45RBLow T cells to syngeneic recipient mice before injection of spleen cells isolated from mTg-immunized CBA/J mice. The index of infiltration into the thyroid of EAT was compared between those with the input of the CD4+CD25+CD45RBLow T cells and those without. The CD4+CD25+CD45RBLow T cells were able to reduce the index significantly (P < 0.01, Fig. 6B), indicating the therapeutic potential of the CD4+CD25+CD45RBLow T cells in EAT. However, transfer of the same amount of unfractionated CD4+CD25+ T cells into CBA/J mice before mTg-primed splenocyte injection was unable to distinctly reduce the index of EAT. Thus, our data indicate that CD4+CD25+CD45RBLow T cells exert a crucial therapeutic effect in preventing the development of autoimmune thyroid disease.
Discussion
The pathogenesis of most human autoimmune diseases remains unclear. Although multiple genetic and environmental factors have been implicated, no single factor has been identified as the tipping point for this process. It is intriguing that some individuals develop autoimmune responses to organ-specific antigens but never develop disease characterized by end-organ destruction. In the case of autoimmune thyroid disease, many 65-yr-old women display thyroid autoantibodies but do not develop the disease and have normal thyroid function. In addition, women often develop postpartum thyroiditis that then resolves without the sequel of chronic thyroid dysfunction. Therefore, there must be immune regulatory processes that suppress the autoimmunity process after the immune recognition of autoantigens but before end-organ dysfunction occurs. Understanding these processes is crucial to developing therapeutics because almost all patients with autoimmunity are diagnosed after autoimmune responses have occurred.
This report provides a new paradigm to explain the immune-regulatory mechanisms that prevent organ damage in autoimmunity. It recapitulates our previous finding that the administration of TRAIL inhibits EAT produced by passive transfer of thyroid-reactive spleen cells into irradiated susceptible recipients (28), and it reveals a novel mechanism responsible for the inhibitory effect of TRAIL on thyroid autoimmune responses. TRAIL suppresses the proliferation of mTg-specific splenic T cells by the expansion of antigen-specific Treg cells. These cells can inhibit the proliferation of mTg-induced proliferation of splenocytes, particularly CD4+CD25− T cells, which are mainly naïve T cells in this model (31). We have shown that nondestructive thyroiditis can be converted to destructive thyroiditis based on the thyroid cytokine environment in the EAT model, and this process can be prevented by TRAIL (28). Therefore, these findings explain the variations in thyroid dysfunction in EAT.
Our present results are in agreement with the distinctive roles proposed for Treg cells and autoimmune-enhancing CD4+CD25− T cells reported for other autoimmune diseases (4,9,10). Treg cells play a critical role in controlling tolerance to self-antigens and alloantigens in humans and are also reported to be deficient in HT or EAT (19,20,21,22,23,24,25,32,33). Additionally, depletion of Treg cells from mice that do not develop autoimmunity after thyroglobulin immunization can result in EAT (22), and the adoptive transfer of Treg cells is able to prevent EAT (23,24,25). This demonstrates the central role of Treg cells in modulating the development of EAT. Given that Treg cells constitute only approximately 5–10% of the peripheral-blood CD4+ T cells (4,9,10), any alteration in the population of Treg cells would appear to have a significant effect on the development of destructive thyroiditis. This effect was documented in studies showing that the targeted engagement of cytotoxic T lymphocyte-associated antigen-4 resulted in an increase in the number of Treg cells, which in turn suppressed the development of EAT (34). Similar suppression of EAT was achieved by modifying the function of Treg cells through the selective induction of DCs (35). To have therapeutic value, the expansion of Treg cells must be achieved without the loss of their regulatory properties. The Treg cells induced by TRAIL are able to reduce not only the proliferation of mTg-primed splenic T cells in vitro but also the infiltration of mononuclear cells in the EAT thyroid, documenting that the TRAIL-expanded Treg cells are functional. The finding that TRAIL treatment can expand the number of Treg cells by promoting their proliferation provides evidence of how TRAIL modulated EAT and suggests this molecule might be a potent therapeutic agent for HT.
CD4+CD25+regulatory cells can inhibit the activity of T effector cells (36,37,38). In the current study, CD4+CD25+ T cells can be further divided into two subpopulations, based on the level of CD45RB: CD4+CD25+CD45RBLow and CD4+CD25+CD45RBHigh T cells. TRAIL treatment markedly increases the number of CD4+CD25+CD45RBLow T cells and slightly decreases the number of CD4+CD25+CD45RBHigh T cells, resulting in a significant increase in the ratio of CD4+CD25+CD45RBLow T cells to CD4+CD25+CD45RBHigh T cells. This should greatly enhance the suppressive effect of Treg cells, and our studies have provided evidence of three aspects of the inhibitory efficacy of the CD4+CD25+CD45RBLow T cells. First, CD4+CD25+CD45RBLow T cells express a high level of Foxp3, a molecule that confirms the inhibitory function of Treg cells (7,39,40). Second, the CD4+CD25+CD45RBLow T cells strongly inhibit the proliferation of activated effector T cells. Third, the CD4+CD25+CD45RBLow T cells significantly reduce mononuclear cell infiltration into the thyroids of EAT mice, the crucial pathologic marker of thyrocyte destruction in EAT. Our findings agree with reports that the CD4+CD25+CD45RBLow T cells are antiinflammatory and CD4+CD25+CD45RBHigh T cells are proinflammatory (17) and that only CD4+CD25+CD45RBLow T cells are capable of preventing the development of colitis in a mouse model of inflammatory bowel disease (41,42) and can inhibit graft rejection initiated by naïve CD45RBHigh CD4+ T cells (29,43). Therefore, the increase in the subpopulation of the CD4+CD25+CD45RBLow T cells by TRAIL is an important finding that supports the autoimmune-inhibitory function of Treg cells by TRAIL. Although here we shown that TRAIL-induced Treg cells can suppress Tg-specific T cells, further testing is required to determine whether these cells can also function against antigen nonspecific T cells.
Given that TRAIL suppresses and prevents EAT via enhancing the Treg cells, it is important to consider what role Treg cells play in the pathogenesis of EAT and HT. There is increasing evidence that Th1 or inflammatory cytokines in the thyroid microenvironment are a critical and necessary step in the initiation of apoptosis thyrocytes, which leads to thyroiditis and thyroid hypofunction (1,2,3). In relation to this finding, the production of proinflammatory Th1 cytokines is controlled by Treg cells at both the mRNA and protein levels (4,5,6,44). A recent study showed that Treg cells can inhibit the induction of Th1 cytokine mRNA as early as 1 h after stimulation (17). In addition, adding Treg cells markedly reduces the production of the inflammatory cytokines in synovial tissue cells from rheumatoid arthritis patients (45). Treg cells also actively suppress Th1 immunity, including the reduced production of IFN-γ, which can be reversed when Treg cells are depleted (46). Foxp3, the functional marker of Treg cells, has also been demonstrated to inhibit the inflammatory cytokine production by interfering with nuclear factor-κB activity (8). Because TRAIL treatment favors the production of Th2 cytokines and suppresses IFN-γ in CD4+ T cells, the major immune activity of TRAIL in EAT can be attributed to the augmentation of CD4+CD25+CD45RBLow T cells. Therefore, the enhancement of Treg cells by TRAIL may completely explain our prior results showing that splenocytes from TRAIL-treated EAT mice produce significantly lower levels of IFN-γ (28).
Although TRAIL can significantly expand Treg cells, the mechanism through which TRAIL can induce Treg cells is not clear at this time. However, we speculate that it may be occurring via enhanced conversion. The inducible Treg cells are derived from Foxp3-naïve, or even activated T cells in the periphery (47). CD4+CD25− T cells can be converted to have Treg cell functionality under particular conditions (48,49). There are no direct data showing how TRAIL increases the population of Treg. However, TRAIL-expressing embryonic stem (ES)-DCs inhibit autoimmune responses by enhancing Tregs. This increase in Treg cell population suggests that TRAIL may be involved in converting or stimulating conventional CD4+ T cells into Treg cells (13).
Taken together, our present study demonstrates that TRAIL can promote antigen-specific Treg cells by expanding their number of Foxp3-rich CD4+CD25+CD45RBLow T cells. These studies demonstrate a novel mechanism by which TRAIL is capable of suppressing or preventing the autoimmune process in EAT.
Acknowledgments
We thank Drs. Massimo Pietropaolo and Pascale R. Leroueil for useful discussions and Yih-Chieh Chen for preparation of mTg for this study.
Footnotes
This work was supported by National Institutes of Health Grant 2 R01 AI 37141.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 13, 2008
Abbreviations: BM, Bone-marrow; CT, cross-threshold; DC, dendritic cell; EAT, experimental autoimmune thyroiditis; FITC, fluorescein isothiocyanate; Foxp3, forkhead family transcription factor; HT, Hashimoto's thyroiditis; IFN, interferon; mTg, murine thyroglobulin; T1DM, type 1 diabetes mellitus; TRAIL, TNF-related apoptosis-inducing ligand; Treg, regulatory T cells.
References
- Wang SH, Bretz JD, Phelps E, Mezosi E, Arscott PL, Utsugi S, Baker Jr JR 2002 A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol 168:2470–2474 [DOI] [PubMed] [Google Scholar]
- Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, Thompson NW, Baker Jr JR 2005 Induction and regulation of Fas-mediated apoptosis in human thyroid epithelial cells. Mol Endocrinol 19:804–811 [DOI] [PubMed] [Google Scholar]
- Wang SH, Van Antwerp M, Kuick R, Gauger PG, Doherty GM, Fan YY, Baker Jr JR 2007 Microarray analysis of cytokine activation of apoptosis pathways in the thyroid. Endocrinology 148:4844–4852 [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Hafler DA 2006 Human regulatory T cells and their role in autoimmune disease. Immunol Rev 212:203–216 [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM 2007 FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 178:2458–2468 [DOI] [PubMed] [Google Scholar]
- Cambos M, Belanger B, Jacques A, Roulet A, Scorza T 2008 Natural regulatory CD4(+)CD25(+)FOXP3(+) T cells control the production of pro-inflammatory cytokines during plasmodium chabaudi adami infection and do not contribute to immune evasion. Int J Parasitol 38:229–238 [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Ziegler SF 2007 FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 7:305–310 [DOI] [PubMed] [Google Scholar]
- Bettelli E, Dastrange M, Oukka M 2005 Foxp3 interacts with nuclear factor of activated T cells and NF-κB to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA 102:5138–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Battaglia M 2007 Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol 7:585–598 [DOI] [PubMed] [Google Scholar]
- Suri-Payer E, Fritzsching B 2006 Regulatory T cells in experimental autoimmune disease. Semin Immunopathol Springer 28:3–16 [DOI] [PubMed] [Google Scholar]
- Cretney E, McQualter JL, Kayagaki N, Yagita H, Bernard CC, Grewal IS, Ashkenazi A, Smyth MJ 2005 TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol 83:511–519 [DOI] [PubMed] [Google Scholar]
- Yao Q, Wang S, Gambotto A, Glorioso JC, Evans CH, Robbins PD, Ghivizzani SC, Oligino TJ 2003 Intra-articular adenoviral-mediated gene transfer of trail induces apoptosis of arthritic rabbit synovium. Gene Ther 10:1055–1060 [DOI] [PubMed] [Google Scholar]
- Hirata S, Matsuyoshi H, Fukuma D, Kurisaki A, Uemura Y, Nishimura Y, Senju S 2007 Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL. J Immunol 178:918–925 [DOI] [PubMed] [Google Scholar]
- Ren X, Ye F, Jiang Z, ChuY, Xiong S, Wang Y 2007 Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ 14:2076–2084 [DOI] [PubMed] [Google Scholar]
- Mi QS, Ly D, Lamhamedi-Cherradi SE, Salojin KV, Zhou L, Grattan M, Meagher C, Zucker P, Chen YH, Nagle J, Taub D, Delovitch TL 2003 Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes 52:1967–1975 [DOI] [PubMed] [Google Scholar]
- Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE 2006 Proinflammatory CD4+CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res 66:57–61 [DOI] [PubMed] [Google Scholar]
- Luke PP, Deng JP, O'Brien CA, Everest M, Hall AV, Chakrabarti S, O'Connell PJ, Zhong R, Jevnikar AM 2003 Alteration in CD45RBhi/CD45RBlo T-cell ratio following CD45RB monoclonal-antibody therapy occurs by selective deletion of CD45RBhi effector cells. Transplantation 76:400–409 [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Cross SJ, Heinly CS, Scearce RM, Haynes BF 2004 CD7 and CD28 are required for murine CD4+CD25+ regulatory T cell homeostasis and prevention of thyroiditis. J Immunol 172:787–794 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N 2007 CD4(+)CD25(+) naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2(h4) mice. J Autoimmun 29:195–202 [DOI] [PubMed] [Google Scholar]
- Morris GP, Kong YC 2006 Tolerance to autoimmune thyroiditis: (CD4+)CD25+ regulatory T cells influence susceptibility but do not supersede MHC class II restriction. Front Biosci 11:1234–1243 [DOI] [PubMed] [Google Scholar]
- McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B 2007 The link between Graves' disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology 148:5724–5733 [DOI] [PubMed] [Google Scholar]
- Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YC 2005 Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res 65:8471–8478 [DOI] [PubMed] [Google Scholar]
- Verginis P, Li HS, Carayanniotis G 2005 Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. J Immunol 174:7433–7439 [DOI] [PubMed] [Google Scholar]
- Gangi E, Vasu C, Cheatem D, Prabhakar BS 2005 IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol 174:7006–7013 [DOI] [PubMed] [Google Scholar]
- Flynn JC, Meroueh C, Snower DP, David CS, Kong YC 2007 Depletion of CD4+CD25+ regulatory T cells exacerbates sodium iodide-induced experimental autoimmune thyroiditis in human leucocyte antigen DR3 (DRB1*0301) transgenic class II-knockout non-obese diabetic mice. Clin Exp Immunol 147:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotti G, Sorvillo F, Naclerio C, Farzati A, Cioffi M, Perna R, Valentini G, Farzati B, Amato G, Carella C 2003 Type-1 response in peripheral CD4+ and CD8+ T cells from patients with Hashimoto's thyroiditis. Eur J Endocrinol 148:383–388 [DOI] [PubMed] [Google Scholar]
- Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, Thompson NW, Baker Jr JR 2004 Interleukin-1β and tumor necrosis factor (TNF)-α sensitize human thyroid epithelial cells to TNF-related apoptosis-inducing ligand-induced apoptosis through increases in procaspase-7 and bid, and the down-regulation of p44/42 mitogen-activated protein kinase activity. J Clin Endocrinol Metab 89:250–257 [DOI] [PubMed] [Google Scholar]
- Wang SH, Cao Z, Wolf JM, Van Antwerp M, Baker Jr JR 2005 Death ligand tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis. Endocrinology 146:4721–4726 [DOI] [PubMed] [Google Scholar]
- Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ 2001 IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol 166:3789–3796 [DOI] [PubMed] [Google Scholar]
- Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E 2007 Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-β, and various inhibitors of TCR signaling. J Immunol 179:3578–3587 [DOI] [PubMed] [Google Scholar]
- Huang YM, Pirskanen R, Giscombe R, Link H, Lefvert AK 2004 Circulating CD4+CD25+ and CD4+CD25+ T cells in myasthenia gravis and in relation to thymectomy. Scand J Immunol 59:408–414 [DOI] [PubMed] [Google Scholar]
- Marazuela M, García-López MA, Figueroa-Vega N, de la Fuente H, Alvarado-Sánchez B, Monsiváis-Urenda A, Sánchez-Madrid F, González-Amaro R 2006 Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab 91:3639–3646 [DOI] [PubMed] [Google Scholar]
- Ban Y, Tozaki T, Tobe T, Ban Y, Jacobson EM, Concepcion ES, Tomer Y 2007 The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: an association analysis in Caucasian and Japanese cohorts. J Autoimmun 28:201–207 [DOI] [PubMed] [Google Scholar]
- Vasu C, Gorla SR, Prabhakar BS, Holterman MJ 2003 Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int Immunol 15:641–654 [DOI] [PubMed] [Google Scholar]
- Vasu C, Dogan RN, Holterman MJ, Prabhakar BS 2003 Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol 170:5511–5522 [DOI] [PubMed] [Google Scholar]
- Fowell D, Mason D 1993 Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med 177:627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL 1994 Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1:553–562 [DOI] [PubMed] [Google Scholar]
- Davies JD, O'Connor E, Hall D, Krahl T, Trotter J, Sarvetnick N 1999 CD4+ CD45RB low-density cells from untreated mice prevent acute allograft rejection. J Immunol 163:5353–5357 [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY 2003 Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S 2003 Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD 2007 CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med 204:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Fiocchi C 2004 Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol 11:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Sutherland RM, Zhan Y, Deliyannis G, Brown LE, Lew AM 2006 Targeting CD45RB alters T cell migration and delays viral clearance. Int Immunol 18:291–300 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Varela N, O'Valle F, Delgado M 2007 Therapeutic effect of urocortin on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of regulatory T cells. Arthritis Rheum 56:531–543 [DOI] [PubMed] [Google Scholar]
- Behrens F, Himsel A, Rehart S, Stanczyk J, Beutel B, Zimmermann SY, Koehl U, Moller B, Gay S, Kaltwasser JP, Pfeilschifter JM, Radeke HH 2007 Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann Rheum Dis 66:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA 2006 Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg 244:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M 2008 Regulatory T cells and immune tolerance. Cell 133:755–787 [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ 2004 CD25-T cells generate CD25+ Foxp3+ regulatory T cells by peripheral expansion. J Immunol 173:7259–7268 [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H 2005 Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 6:1219–1227 [DOI] [PubMed] [Google Scholar]