Abstract
Carcinogenesis is a multistage process consisting of initiation, promotion and progression phases. Thus, the multistage sequence of events has many phases for prevention and intervention. Chemoprevention, a novel approach for controlling cancer, involves the use of specific natural products or synthetic chemical agents to reverse, suppress or prevent premalignancy before the development of invasive cancer. Several natural products, such as, grains, nuts, cereals, spices, fruits, vegetables, beverages, medicinal plants and herbs and their various phytochemical constituents including, phenolics, flavonoids, carotenoids, alkaloids, nitrogen containing as well as organosulfur compounds confer protective effects against wide range of cancers including colon cancer. Since diet has an important role in the etiology of colon cancer, dietary chemoprevention received attention for colon cancer prevention. However, identification of an agent with chemopreventive potential requires in vitro studies, efficacy and toxicity studies in animal models before embarking on human clinical trials. A brief introduction about colon cancer and the role of some recent natural products in colon cancer chemoprevention with respect to multiple molecular mechanisms in various in vitro, in vivo and clinical studies are described in this review.
Keywords: Colon cancer, chemoprevention, natural products, in vitro studies, in vivo studies
Introduction
Carcinogenesis is a multistage process consisting of initiation, promotion and progression phases involving sequential generations of cells that exhibit continuous disturbance of cellular and molecular signal cascades [Vincent and Gatenby, 2008]. Thus, the multistage sequence of events has many phases for intervention to inhibit, reverse and/or delay each process of carcinogenesis before the development of invasive malignancy. Agents that can suppress these multiple pathways have great potential for chemoprevention [Krzystyniak, 2002]. An ideal chemopreventive agent should have (i) little or no toxicity, (ii) high efficacy in multiple sites, (iii) capability of oral consumption, (iv) known mechanisms of action, (v) low cost, and (vi) human acceptance. In recent years, natural products have received great attention for cancer prevention owing to their various health benefits, noticeable lack of toxicity and side effects, and the limitations of chemotherapeutic agents [Manson et al., 2005].
Overall, natural products have been used worldwide as traditional medicines for thousands of years to treat various forms of diseases including cancer. Several studies have revealed that natural products exhibit an extensive spectrum of biological activities such as, stimulation of the immune system, antibacterial, antiviral, anti-hepatotoxic, anti-ulcer, anti-inflammatory, antioxidant, anti-mutagenic, and anti-cancer effects [Miyata, 2007; Espín et al., 2007]. A variety of grains, cereals, nuts, soy products, olives, beverages such as tea and coffee, and spices including turmeric, garlic, ginger, black pepper, cumin and caraway confer a protective effect against cancer [Lila, 2007; Williams and Hord, 2005]. Several studies have also documented the relationship between decreased cancer risk and high consumption of vegetables, including cabbage, cauliflower, broccoli, brussels sprout, tomatoes, and fruits such as, apples, grapes, and berries [Vainio and Weiderpass, 2006; Gordaliza, 2007]. In addition, a number of medicinal plants and herbs such as milk thistle have also been reported to reduce the risk of cancer in multiple sites [Park and Pezzuto, 2002; Kroll et al., 2007]. In particular, natural products consist of a wide variety of biologically active phytochemicals including phenolics, flavonoids, carotenoids, alkaloids and nitrogen containing as well as organosulfur compounds, which have been shown to suppress early and late stages of carcinogenesis [Nishino et al., 2007]. Several epidemiological studies have validated the inverse relation between the consumption of natural products and the risk of wide range of human cancers including colon cancer [Lila, 2007; Williams and Hord, 2005; Vainio and Weiderpass, 2006; Giovannucci, 2003; Satia-About a et al., 2004]. A brief introduction about colon cancer and the role of some recent natural products in colon cancer chemoprevention with respect to multiple molecular mechanisms in various in vitro, in vivo and clinical studies are described in following sub-sections.
Colon Cancer: Pathology, Risk Factors and Genetics
Colon cancer is defined as any malignant neoplasm arising from the inner lining of the colonic epithelium, and is the third most common cancer and the third leading cause of cancer related deaths for both men and women in United States [American Cancer Society, 2008]. Although the mortality rate has fallen dramatically over the last two decades, 108,070 new colon cancer cases and 49,960 deaths from colon cancer are estimated for 2008 [American Cancer Society, 2008]. The 5-year survival rate of colon cancer after diagnosis at an early and localized stage is 90 per cent; however, when distant metastasis has occurred, the 5-year survival rate drops to 10 per cent [American Cancer Society, 2008].
The occurrence of colon cancer is mainly associated with the incidence of aberrant crypt foci (ACF), an earliest neoplastic lesion, which are clusters of mucosal cells with an enlarged and thicker layer of epithelia than the surrounding normal crypts that progress in to polyps followed by adenomas and adenocarcinomas [Cappell, 2007]. These sequences of events are considered to be a consequence of the accumulation of multiple genetic alterations in colonic epithelium [Humphries and Wright, 2008]. Though all ACF do not progress in to colon cancer, several studies have reported that all colon cancer arises from ACF [Cappell, 2007].
The occurrence of colon cancer is strongly related to age, with 90% of the cases arising in people who are 50 years or older; until age 50, both men and women have equal risk for colon cancer, but in later life males predominate with this malignancy [American Cancer Society, 2008]. Epidemiological studies have suggested that colon cancer is a manifestation of a number of inherited cancer predisposition syndromes, including familial adenomatous polyposis, hereditary non-polyposis colorectal cancer, and personal or family history of colorectal cancer and/or polyps and inflammatory bowel disease [Rowley, 2005]. Furthermore, other factors such as obesity, lack of exercise, smoking, alcohol consumption, diet rich in high fat, red and processed meats and inadequate intake of dietary fiber, fruits and vegetables are also associated with increased colon cancer risk [Cappell; 2007; Papapolychroniadis. 2004; Kim and Milner, 2007].
Vogelstein et al. [1988] first described the time dependent accumulation of genetic mutations and sequential phenotypic correlation in the colonic epithelium. Mutations due to loss of heterozygosity as well as chromosal instability in oncogenes such as k-ras, c-erb2 or c-myc, or tumor suppressor genes such as adenomatous polyposis coli (APC), delete in colon cancer (DDC) or p53 have been implicated in 80% of sporadic colon cancer [Mutch, 2007]. Microsatellite instability associated mutations in mismatch repair genes resulting in replication errors have also been suggested to play a key role in colon cancer development [Niv, 2007]. Gene abnormalities and aberrant expression of cell cycle regulators and cell-cell adhesion molecules are the other genetic events which have been involved in the pathogenesis of colon cancer [Mutch, 2007].
Colon Cancer Chemoprevention: Biological Endpoints and Associated Mechanisms
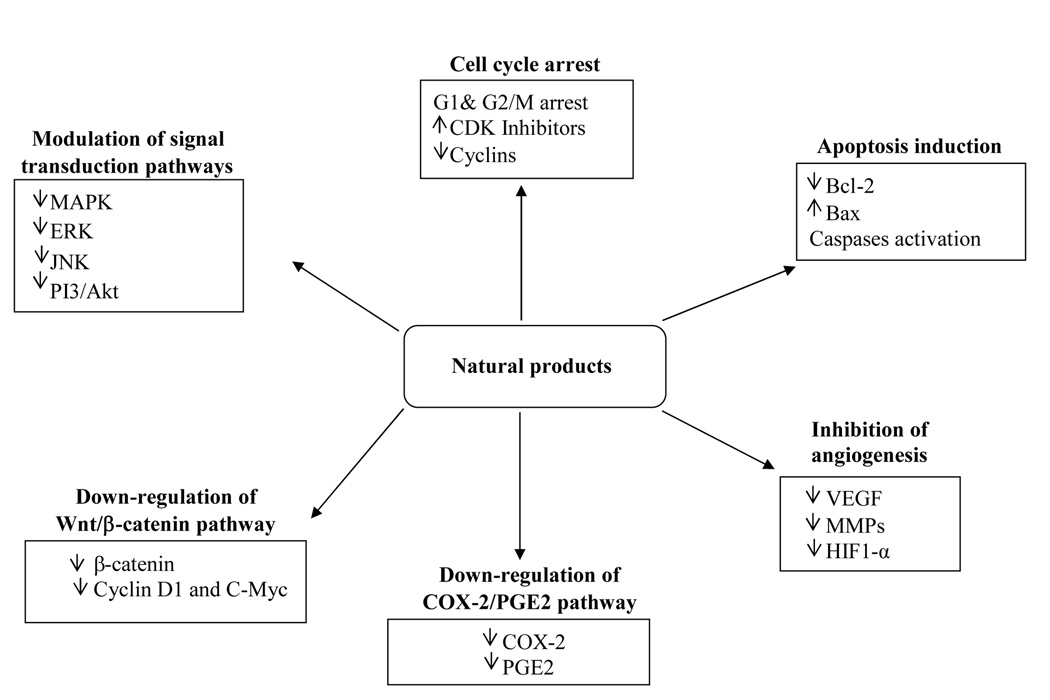
Several epidemiological studies have reported inverse correlation between a high intake of fruits, vegetables, and phytochemicals such as carotenoids and flavonoids and reduced risk of colon cancer [Lila, 2007; Williams and Hord, 2005; Vainio and Weiderpass, 2006; Giovannucci, 2003; Satia-About a et al., 2004; Nishino et al., 2007]. Plant-based chemopreventive agents and their constituent phytochemicals have been known to interfere with various molecular pathways involved in colon cancer initiation and progression [Gustin and Brenner, 2004]. Colon cancer development is associated with an excessive cell proliferation and a dysregulation of both cell cycle progression and apoptosis [Mutch, 2007]. Moreover, “neo angiogenesis” is an essential process in the development, growth and metastasis of colon tumor [Kumar, 2005]. Cyclooxygenase -2 (COX-2), an inducible prostaglandin G/H synthase, is know to play a major role in prostaglandin (PG) synthesis. Over expression of COX-2 and subsequent prostaglandin (PG) production from free arachidonic acid, have also been implicated in colon carcinogenesis [Spychalski et al., 2007]. In particular, COX-2-mediated increased PGE2 levels have been believed to enhance tumor promotion by promoting cell proliferation, angiogenesis and apoptotic evasion, stimulating tumor metastasis, and decreasing immune surveillance [Eisinger et al., 2007]. An abnormal activation of the Wnt/β-catenin pathway has also been implicated in the development of human colon cancer, and therefore is considered as a hallmark for this malignancy [Spychalski et al., 2007]. Over expression of Wnt ligand and/or mutations in the downstream molecules in the Wnt signaling cascade have been implicated in the activation of Wnt pathway [Luu et al., 2004]. Increased phosphorylation of extra cellular regulated kinases (ERK1/2) is required for PGE2 to stimulate cell proliferation of human colon cancer cells [Gustin and Brenner, 2004]. Deregulation of phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway and its downstream transcription factors are the other molecular events that have been implicated in cancer development [Bode and Dong, 2004]. A number of natural products and their active phytochemicals have been found to exert their chemopreventive effects by inducing cell cycle arrest and apoptosis, decreasing cell proliferation and angiogenesis, inhibiting tumor cell invasion and metastasis, and modulating various signal transduction as well as COX-2/PGE2 and Wnt/β-catenin pathways, involved in colon cancer development [Dragnev et al., 2007]. Herein, the potentials of several natural products targeting the above mentioned molecular pathways leading to colon cancer prevention and/or intervention are described. Figure 1 shows mechanisms of actions of various natural products and their constituent phytochemicals in colon cancer chemoprevention.
Figure 1.
Mechanisms of action of natural products and their constituent phytochemicals in colon cancer chemoprevention.
Colon Cancer Chemoprevention: Effects on Molecular Events Associated with Proliferation and Apoptosis
Studies have shown the antiproliferative as well as apoptosis inducing ability of natural products in colon cancer chemoprevention. For example, blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts have been shown to inhibit the growth and stimulate apoptosis in HT-29 and HCT-116 cells [Seeram et al., 2006]. In particular, black raspberry and strawberry extracts exhibit significant pro-apoptotic effects in HT-29 cells [Seeram et al., 2006]. Procyanidine enriched fraction of apple is shown to inhibit cell growth through G2/M phase arrest and induction of apoptosis as evidenced by caspase 3 activation [Gossé et al., 2005]. Previously, we have shown that grape seed extract treatment inhibits HT-29 and LoVo cell growth by inducing G1 phase cell cycle arrest and caspase 3-dependent apoptotic cell death, which was associated with an increase in Cip1/p21 protein expression and a decrease in G1 phase-associated cyclins and cyclin-dependent kinases [Kaur et al., 2006]. Resveratrol (3,4',5 tri-hydroxystilbene), a naturally occurring polyphenolic compound highly enriched in grapes and red wine, has been shown to induce apoptosis in HT-29 cells [Park et al., 2007]. Resveratrol has also been shown to down-regulate telomerase activity in HT-29 and WiDr human colon cancer cell lines together with inhibition of cell proliferation [Fuggetta et al., 2006]. Engelbrecht et al. [2007] have reported that grape seed proanthocyanidin extract inhibits cell viability and induces apoptosis by suppressing PI3-K pathway in CaCo2 cells.
Silibinin, an active constituent of milk thistle, has been reported to inhibit proliferation and induce cell-cycle arrest of human colon cancer cells, Fet, Geo, and HCT116 [Hogan et al., 2007]. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and induces cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells [Agarwal et al., 2003]. Also in HT-29 cells, beta-escin, a principle component of horse chestnut, treatment induces growth arrest at the G1-S phase together with an induction of Cip1/p21 and an associated reduction in the phosphorylation of retinoblastoma protein [Patlolla et al., 2006]. Wheat bran and its phytosterols fraction, phytosterol ferulates and 5-alk(en)yl-resorcinols exhibit significant inhibitory effect on the growth of HCT-116 human colon cancer cells [Sang et al., 2006]. Wheat bran and its phytic acid administration have also been shown to significantly induce apoptosis and differentiation in azoxymethane (AOM) – induced colonic preneoplastic lesions [Jenab and Thompson, 2000].
Tian and Song [2006] have documented that inositol hexaphosphate (IP6, also known as phytic acid) has potent inhibitory effect on proliferation of HT-29 cells by modulating proliferating cell nuclear antigen (PCNA) and Cip1/p21 expression. Treatment with chokeberry juice inhibits CaCo2 cell proliferation by promoting G2/M cell cycle arrest [Bermúdez-Soto et al., 2007]. Hong et al. [2007] have reported that Chinese red yeast rice, a food herb, inhibits cell growth and induces apoptosis in HCT-116 cells.
Diallyl sulfide (DAS), an organosulfur component of garlic has been reported to induce cell cycle arrest at G2/M phase as well as apoptosis in Colo 320 DM colon cancer cells by increasing caspase 3 expression and decreasing ERK-2 activity [Sriram et al., 2008]. Treatment of HT-29 and CaCo2 cells with diallyl disulfide (DADS), a garlic constituent, results in the inhibition of histonedeacetylase activity and histone hyperacetylation together with an increase in Cip1/p21 expression [Druesne et al., 2004]. Thiacremonone, another sulfur compound isolated from garlic, is found to induce apoptotic cell death in colon cancer SW620 and HCT-116 cells by suppressing NF-kappaB (NF-κB)-mediated anti-apoptotic genes, Bcl-2, cIAP1/2, and XIAP and inducing Bax, cleaved caspase 3 and cleaved PARP [Ban et al., 2007].
Curcumin, the yellow pigment in turmeric, also induces apoptosis in human colon cancer colo205 cells through the production of reactive oxygen species, Ca2+ and the activation of caspase 3; it also enhanced the expression of bax, cytochrome C, p53 and Cip1/p21 but inhibited the expression of Bcl-2 [Su et al., 2006]. Studies have shown the anti-proliferative and apoptosis inducing effects of curcuminoids, a mixture of demethoxycurcumin and bisdemethoxycurcumin, against primary colon cancer cells isolated from Taiwanese patients [Hsu et al., 2007]. Recently, Watson et al. [2008] have reported a p21-independent inhibition of cell proliferation and induction of apoptosis by curcumin in both p21(+/+) and p21(−/−) HCT-116 cells. β-Ionone, a precursor for carotenoids present in many fruits and vegetables, also induces cytotoxicity, G1 phase cell cycle arrest and apoptosis in HCT-116 cells [Janakiram et al., 2008]. Together, the above summarized studies clearly and convincingly show that there are a vast range of phytochemicals from different sources, which exert potent cell growth inhibitory, cell cycle arrest inducing and apoptosis causing effects is a wide panel of human colon cancer cell lines with varying degree of genetic alterations/defects by targeting various molecular pathways.
Colon Cancer Chemoprevention: Effects on Molecular Events Associated with Angiogenesis
Numerous natural products with “angiopreventive” effects have been considered as chemopreventive agents [Dragnev et al., 2007]. Under serum starved condition in HT-29 cells, epigallocatechin gallate (EGCG) treatment is shown to inhibit the increase in the expression of vascular endothelial growth factor (VEGF), which is a potent angiogenic factor [Jung et al., 2001]. Lu et al. [2006] have reported that down-regulation of VEGF expression is one of the key mechanisms of chemoprevention by black raspberries. Liposomal curcumin preparation exhibits anti-angiogenic effects in Colo205 and LoVo xeografts by decreasing VEGF, CD31, and interleukin-8 [Li et al., 2007]. Treatment with ethanolic extract of Ka-mi-kae-kyuk-tang a formula of ten Chinese oriental herbs has been shown to inhibit the invasiveness of the mouse colon 26-L5 cancer cells in vitro [Lee et al., 2006]. Aged garlic extract has been reported to inhibit invasive activities of SW480 and SW620 cells [Matsuura et al., 2006].
Recently, it has been reported that, soybean saponin significantly inhibits the invasion of HT-29 cells through a Matrigel-coated membrane [Kang et al., 2008]. In addition, two weeks pretreatment with dietary soybean saponin decreased the incidence of CT-26 colon metastatic tumor colonization in lungs of mice which receive tail vein injection of CT-26 cells [Kang et al., 2008]. Recent studies in our laboratory clearly showed that silibinin decreases hypoxia inducible facter-1 alpha and VEGF expression thereby exerting its angiopreventive effects in HT-29 xenografts [Singh et al., 2008]. Curcumin has been found to inhibit cell migration of human colon cancer colo205 cells by decreasing matrix metalloproteinase (MMP)-2 expression [Su et al., 2006]. Together, these reports suggest that various phytochemicals, which exert anti-proliferative and pro-apoptotic effects in colon cancer cells, are also effective as anti-angiogenic agents. This is an important observation because angiogenesis is the most important event for cancer growth including colon cancer beyond a restricted size in to a full-blown malignancy [Jain, 2002].
Colon Cancer Chemoprevention: Effects on Cyclooxygenase Pathway
Plant based chemopreventive agents and their active constituents are reported to interfere with COX-2 activity and PGE2 synthesis. Hong et al. [2004] have documented the modulation of arachidonic acid metabolism by curcumin and its related beta-diketone derivatives, tetrahydrocurcumin and dibenzoylmethane, in HT-29 cells. EGCG showed dose-dependent inhibition of PGE2 synthesis and down-regulation of genes involved in inflammatory pathways in tumor necrosis factor (TNF)-alpha-stimulated HT-29 and T84 cells [Porath et al., 2005]. Moreover, EGCG treatment significantly decreased PGE2 synthesis and cellular levels of both COX-2 protein and mRNA as well as transcriptional activation of COX-2 by inhibiting epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases (RTKs) in HT-29 cells [Shimizu et al., 2005a]. EGCG has also been shown to modulate AMP-activated protein kinase followed by the reduction in COX-2 expression and PGE2 secretion in HT-29 colon cancer cells [Hwang et al., 2007].
Thiacremonone, a garlic sulfur compound, has been reported to suppress inflammatory genes such as inducible nitric oxide synthase (iNOS) and COX-2 in SW620 and HCT-116 cells [Ban et al., 2007]. More recently, Zykova et al. [2008] have demonstrated that resveratrol and its analogues, 3,3',4',5',5-pentahydroxy-trans-stilbene inhibit COX-2 mediated PGE2 production in HT-29 cells. Dietary administration of Perilla oil significantly decreased AOM-induced ACF formation and PGE2 levels in colonic mucosa indicating its protective effect in the early stage of colon carcinogenesis [Onogi et al., 1996]. Modulatory effects of green tea on arachidonic acid metabolism during AOM-induced colon carcinogenesis have been documented [Ju et al., 2003]. Sengupta et al. [2005] have reported that dietary cardamom inhibits the formation of AOM-induced colonic aberrant crypt foci and reduces COX-2 and iNOS expression in mice model. Soybean saponin has been found to inhibit both protein and mRNA levels of COX-2 and PGE2 secretion via NF-κB dependent pathway [Kang et al., 2008].
Treatment with tricin, a rice bran flavone reduces PGE2 production in both HCEC and HCA-7 cells. Moreover, Dietary supplementation of 0.2% tricin reduced number of intestinal adenomas by 33% by decreasing PGE2 production [Cai et al., 2005]. Interestingly, wheat bran fractions are also reported to decrease iNOS and COX-2 expression during experimental colon carcinogenesis [Reddy et al., 2000]. Anthocyanin-rich extracts of bilberry and grape are also shown to down-regulate COX-2 mRNA expression in AOM-treated rat colon [Lala et al., 2006].
Colon Cancer Chemoprevention: Effects on Wnt/β-catenin Pathway
The Wnt/β-catenin pathway has been considered as an attractive target for colon cancer chemoprevention. In HT-29 cells, treatment with EGCG increases protein levels of E-cadherin by 27% to 58%, induces the translocation of β-catenin from nucleus to cytoplasm and plasma membrane, and decreases c-Myc and cyclin D1 [Ju et al., 2005]. EGCG has also been shown to effectively inhibit intestinal tumorigenesis in Apc min mice, possibly through the attenuation of the carcinogenic events, which include aberrant nuclear β-catenin and activated Akt and ERK signaling [Ju et al., 2005]. Rajakangas et al. [2008] have reported that dietary administration of white currant, a family of berries suppresses intestinal tumors in min mice by decreasing nuclear β-catenin and NF-κB proteins. In SW480 cells, methanolic extract of Polysiphonia japonica attenuates Wnt/β-catenin signaling without altering the levels of β-catenin protein, and reduces the expression of cyclin D1 [Gwak et al., 2006].
Thearubigins, the most abundant polymeric black tea polyphenols, inhibit dimethylhydrazine (DMH)-induced cell proliferation by suppressing Wnt/β-catenin pathway. Treatments with polymeric black tea polyphenols (PBP) extract showed decreased levels of COX-2, c-MYC and cyclin D1 proteins which aid cell proliferation probably by regulating β-catenin and thereby maintaining expression of APC and decreasing inactivation of GSK3β [Patel et al., 2008]. In APC min mice, treatments with Polyphenon E and EGCG are shown to significantly reduce the number of polyps by decreasing β-catenin nuclear expression [Hao et al., 2007]. Mahmoud et al. [2000] have shown that both curcumin and caffeic acid phenethyl ester (CAPE) significantly decrease tumor formation and decrease β-catenin expression in APC Min mice.
Colon Cancer Chemoprevention: Effects on Signal Transduction Pathways
Natural products and their active phytochemicals have also been known to modulate mitogen activated protein kinases (MAPKs) and PI3K/Akt pathways. DMH-induced activation of MAPKs such as ERK and c-Jun N-terminal kinase (JNK) was found to be inhibited by treatments with thearubigins extract [Patel et al., 2008]. Sulforaphane (SFN), an isothiocyanate which is present abundantly in broccoli and cauliflower, has been shown to reduce small intestinal polyps and proliferative index and induce apoptosis, together with a down-regulation in the phosphorylation of JNK, ERK and Akt, which were found to be highly expressed in the adenomas of Apc Min mice [Hu et al., 2006]. Other studies have shown that EGCG and Polyphenon E decrease the phosphorylated forms of EGFR, HER2, ERK and Akt proteins thereby inhibiting the growth of CaCo2, HCT-116, HT-29, SW480, and SW837 colon cancer cells [Shimizu et al., 2005b]. Furthermore, polyphenon E and EGCG reduced phospho-Akt levels thereby decreasing cell proliferation and inducing apoptosis in APC min mice [Hao et al., 2007].
Collett and Campbell [2004] have reported that curcumin treatment induces JNK-dependent apoptosis by enhancing sustained phosphorylation of c-jun and stimulation of activator protein-1 transcriptional activity. It has been known that curcumin inhibits CaCo-2 and HT-29 human colon cancer cells growth by suppressing gene expression of EGFR through reducing the trans-activation activity of Egr-1 [Chen et al., 2006]. DAS decreases ERK activity thereby suppressing the growth of Colo 320 DM colon cancer cells in vitro [Sriram et al., 2008]. Grape seed proanthocyanidin extract is shown to attenuate PI3-kinase (p110 and p85 subunits) and decrease Akt phosphorylation at Ser473 thereby inducing apoptosis in CaCo2 cells [Engelbrecht et al., 2007]. More recently we have shown that silibinin treatment inhibits both ERK1/2 and Akt signaling in HT-29 xenograft [Sigh et al., 2008]
Colon Cancer Chemoprevention: In Vitro Studies
Natural products and their active constituents have been reported to exert their chemopreventive effects in a wide range of colon cancer cell lines, namely HT-29, LoVo, CaCo2, SW480 and 620, HCT-116 [Table 1]. Various in vitro studies showed that, extracts from various fruits including black raspberry, strawberry, apple, grape seeds induce apoptosis in HT-29 cells [Seeram et al., 2006; Gossé et al., 2005; Kaur et al, 2006]. Aged garlic extract as well as number of organo sulfur compounds from garlic, DAS, DADS and thiacremonone have been documented to exert anti-cancer effects by modulating various carcinogenic mechanisms in Colo 320 DM, HT-29, CaCo-2, SW620 and HCT-116 cell lines [Sriram et al., 2008; Druesne et al., 2004; Ban et al., 2007; Matsuura et al., 2006]. Curcumin has been shown to inhibit colon cancer cells growth by arresting them at various phases of cell cycle and inducing apoptosis [Su et al., 2008; Hsu et al., 2007; Watson et al., 2008]. Treatment with EGCG has been found to modulate VEGF expression, PGE2/COX-2 as well as beta-catenin pathways in colon cancer cells under in vitro conditions [Jung et al., 2001; Shimizu et al., 2005; Ju et al., 2005]. In particular, EGCG treatment decreased phospho EGFR, HER2, ERK and Akt levels in various colon cancer CaCo2, HCT-116, HT-29, SW480, and SW837 cells [Shimizu et al., 2005]. In vitro chemopreventive potential of various natural products has also been summarized above in previous sections.
Table 1.
In vitro studies showing chemopreventive potential of natural products.
| Reference | Natural product | Cells | Mechanisms of action |
|---|---|---|---|
| Seeram et al., 2006 | Blackberry, black Raspberry, Blueberry, Cranberry, red Raspberry, and Strawberry | HT-29 and HCT-116 | Growth inhibition Apoptosis induction |
| Kaur et al., 2006 | Grape seed extract | HT-29 and LoVo | G1 phase cell cycle arrest |
| ↑Caspase 3 dependent apoptotic cell death. | |||
| ↓Decrease in G1 phase-associated cyclins and cyclin-dependent kinases | |||
| Park et al., 2007 | Resveratrol | HT-29 | Apoptosis induction |
| Engelbrecht et al., 2007 | CaCo2 | ↓PI3-K pathway | |
| Zykova et al., 2008 | HT-29 | ↓Cox-2 & PGE2 | |
| Agarwal et al., 2003 | Silibinin | HT-29 | Cell cycle arrest; CDKIs and Apoptosis induction |
| Hogan et al., 2007 | Silibinin | Fet, Geo, and HCT116 | Cell cycle arrest |
| Sang et al., 2006 | Wheat bran and its phytosterols | HCT-116 | Growth inhibition ↓PCNA |
| Tian and Song 2006 | IP6 | HT-29 | ↑p21 expression |
| Su et al., 2006 | Curcumin | Colo205 | Activation of caspase 3 ↑Bax, cytochrome C, p53 and p21 ↓Bcl-2 and MMP-2 |
| Sriram et al., 2008 | DAS | Colo 320 DM | G2/M phase arrest, ↑Caspase 3 activation ↓ERK-2 activity, ↑p21 expression |
| Druesne et al., 2004 | DADS | HT-29 and CaCo2 | Apoptosis induction,↓Cox-2 and iNOS |
| Ban et al., 2007 | Thiacremonone | SW620 and HCT-116 | ↓Invasion |
| Matsuura et al., 2006 | Aged garlic extract | SW480 and SW620 | |
| Jung et al., 2001 | EGCG | HT-29 | ↓VEGF expression |
| Porath et al., 2005 | HT-29 and T84 | ↓PGE2 synthesis | |
| Shimizu et al., 2005a | HT-29 | ↓EGFR,↓COX-2 | |
| Shimizu et al., 2005b | CaCo2, HCT-116, HT-29, SW480, and SW837 | ↓p-HER2, -ERK and -Akt | |
| Ju et al., 2005 | HT-29 | ↓c-Myc and cyclin D1 | |
| Kang et al., 2008 | Soybean saponin | HT-29 | ↓Invasion |
Colon Cancer Chemoprevention: In Vivo Studies
Experimental carcinogenesis models are useful to understand multistage nature of carcinogenesis and different ways to interfere with the process under in vivo conditions [Reddy, 1998]. In this regard DMH- or AOM- induced experimental colon carcinogenesis models in rodents are ideal experimental models for colon cancer and have been extensively used for colon cancer chemoprevention research. Besides these models, genetic models of intestinal carcinogenesis (APC min) as well as xenograft models have also been widely used to test the chemopreventive efficacy of natural products.
Sengupta et al. [2008] have reported synergistic chemoprotective effects of garlic and tomato on AOM-induced colon carcinogenesis in Sprague-Dawley rats. Similarly, a 5% dried onion dietary feeding is shown to significantly reduce AOM-induced ACF formation [Taché et al., 2007]. Protective effects of wheat bran have been documented against AOM-induced experimental carcinogenesis [Reddy et al., 2000]. Soy protein consumption has been demonstrated to reduce the risk of developing colon tumors in animal models [Hakkak et al., 2001]. Chemoprotective effects of cooked navy beans have been reported during AOM-induced colon carcinogenesis in obese ob/ob mice [Bobe et al., 2008]. Dietary administration of 0.025% or 0.05% β-escin has shown dose dependent inhibition of AOM-induced ACF formation in F344 rats [Patlolla et al., 2006]. Raju et al., [2005] have shown that low doses of β-carotene and lutein inhibit AOM-induced ACF formation whereas high doses enhance ACF incidence. Three weeks treatment with phenethyl isothiocyanate (PEITC), a constituent of cruciferous vegetables significantly reduced the number of polys in APC min mice by modulating cyclins D1, A and E as well as P21 expression [Khor et al., 2008]. Chemopreventive effects of SFN as well as PEITC have been documented against AOM-induced ACF formation in Fischer rats [Chung et al., 2000].
Several other studies have revealed chemopreventive effects of various spices including cumin, caraway, black pepper, fenugreek seeds, ginger on DMH-induced colon carcinogenesis [Nalini et al., 2006; Kamaleeswari et al., 2006; Manju and Nalini, 2005; Devasena et al., 2007]. Cloudy apple juice has been demonstrated to reduce DMH-induced colonic crypt proliferation, ACF formation and DNA damage in rats [Barth et al., 2005]. Resveratrol has been shown to decrease AOM-induced ACF formation by increasing bax and Cip1/p21 expression [Tessitore et al., 2000]. Recently, white currant, a family of berries was found to reduce intestinal tumors in min mice [Rajakangas et al., 2008]. Dietary feeding of anthocyanin-rich tart cherry extract in combination with suboptimal levels of the nonsteroidal anti-inflammatory drug sulindac to APC Min mice for 19 weeks is shown to inhibit intestinal tumorigenesis in APC(Min) mice [Bobe etal., 2006].
Beneficial effects of green tea on ACF formation in AOM-induced colon carcinogenesis have also been documented [Ju et al., 2003]. Treatment with green tea selectively decreases initial stages of intestinal carcinogenesis in the AOM-APC Min mouse model [Issa et al., 2007]. Similarly, EGCG administration is shown to effectively reduce the number of small intestinal tumors, increased E-cadherin expression and to decrease nuclear β-catenin, c-Myc, phospho Akt and phospho-ERK1/2 expression in APC min mice [Ju et al., 2005]. Dietary supplementation of green tea extract has also been demonstrated to inhibit HCT-116 cancer cells growth in athymic male nude mice. Inhibition by green tea is evidenced by the inhibition of mitotic index, MMP-9 and VEGF secretion [Roomi et al., 2005].
Our xenograft studies showed that eight weeks of grape seed extract treatment significantly decreases HT-29 tumor xenograft volume by 44% by decreasing cell proliferation and inducing apoptosis as evidenced by decreased PCNA expression, increased Cip1/p21 protein levels as well as TUNEL positive cells and poly(ADP-ribose) polymerase cleavage [Kaur et al., 2006]. Recent studies from our laboratory have also demonstrated in vivo anticancer efficacy of silibinin on HT-29 human colon cancer xenograft growth in nude mice. These findings suggested that antiproliferative, proapoptotic, and antiangiogenic activities of silibinin may be responsible for its in vivo antitumor efficacy [Singh et al., 2008]. Chemopreventive effects of various natural products and their active constituents in various in vivo models are summarized in Table 2.
Table 2.
Chemopreventive effect of natural products and their active constituents against colorectal cancer in various in vivo models.
| References | Natural product | Animal models |
|---|---|---|
| Sengupta et al.,2004 | Garlic and tomato | AOM |
| Taché et al., 2007 | Onion | AOM |
| Tessitore et al., 2000 | Resveratrol | AOM |
|
Reddy et al., 2000 and Jenab and Thompson, 2000 |
wheat bran | AOM |
| Hakkak et al., 2001 | Soy protein | AOM |
| Bobe et al., 2008 | Navy beans | AOM |
| Lala et al., 2006 | Bilberry and grape | AOM |
| Barth et al., 2005 | Cloudy apple juice | DMH |
| Nalini et al., 2006 and | Cumin, and black pepper | DMH |
| Kamaleeswari et al, 2006 | Caraway | DMH |
| Manju and Nalini, 2005 | Ginger | DMH |
| Sengottuvelan and Nalini,2006 | Resveratrol | DMH |
| Devasena et al. 2003 and 2007 | Fenugreek seeds | DMH |
| Perkins et al., 2002 and Mahmoud et al.,2000 |
Curcumin | APC min mice |
| Li et al., 2007 | Curcumin | HT-29 and LoVo xenografts |
| Kaur et al., 2006 | Grape seed extract | HT-29 xenograft |
| Singh et al., 2008 | Silibinin | HT-29 xenograft |
| Bobe et al., 2006 | Tart cherry extract | APC min mice |
| Rajakangas et al., 2008 | White currant | Min mice |
| Issa et al., 2007 | Green tea | AOM-APC min mice |
| Roomi et al., 2005 | Green tea | HCT-116 xenograft |
| Ju et al., 2005 | EGCG | APC min mice |
| Hao et al., 2007 | EGCG and polyphenon B | APC min mice |
| Patel et al., 2008 | Thearubigins | DMH |
| Chung et al., 2000 | Sulforaphane and PEITC | AOM |
| Hu et al, 2006 | Sulforaphane | APC min mice |
| Khor et al., 2008 | PEITC | APC min mice |
Colon Cancer Chemoprevention: Clinical Trials
Insulin-like growth factor-I (IGF-1) has been considered as a risk factor for various types of cancer including colon cancer. The plasma concentration of IGF-1 decreased significantly by 25% after tomato lycopene extracts [Walfisch et al., 2007]. Results from a preliminary double-blind, randomized clinical trial showed that 12 months treatment with aged garlic extract significantly reduced size and number of colonic adenomas in patients with colorectal adenomas [Tanaka et al., 2006]. Phase I clinical trial has been completed to determine the curcumin dose that can be tolerated to help in preventing colon cancer in healthy men and women [Cheng et al., 2001]. Grubben et al. [2000] have reported in their randomized trial in healthy volunteers that unfiltered coffee intake increase the detoxification capacity and anti-mutagenic properties in the colorectal mucosa through an increase in glutathione concentration but does not influence proliferation in colonic epithelium.
Conclusion and Future Prospective
This review summarized chemopreventive efficacy of natural products and their constituent phytochemicals in various in vitro and in vivo colon cancer models. All these results strengthen the fact that natural products can modulate various molecular pathways involved in cancer initiation and progression. Studies described here and elsewhere clearly highlight the use of natural products as novel chemopreventive agents for colon cancer intervention. It is expected that future studies with natural products will define various molecular mechanisms and targets for tumor growth inhibition, apoptosis, and especially angioprevention. To date, chemoprevention clinical trials with natural products conducted in colon cancer are very limited. Extensive clinical research is warranted to evaluate further safety and chemopreventive efficacy of natural products either alone or in combination with chemotherapeutic agents against colon cancer.
Acknowledgements
This work was supported by USPHS grant RO1 CA112304 from the National Cancer Institute, NIH.
References
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts and figures 2007–2008. American Cancer Society; 2007–2008. [Google Scholar]
- Ban JO, Yuk DY, Woo KS, Kim TM, Lee US, Jeong HS, Kim DJ, Chung YB, Hwang BY, Oh KW, Hong JT. Inhibition of cell growth and induction of apoptosis via inactivation of NF-kappaB by a sulfur compound isolated from garlic in human colon cancer cells. J Pharmacol Sci. 2007;104:374–383. doi: 10.1254/jphs.fp0070789. [DOI] [PubMed] [Google Scholar]
- Barth SW, Fändrich C, Bub A, Dietrich H, Watzl B, Will F, Briviba K, Rechkemmer G. Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis. 2005;26:1414–1421. doi: 10.1093/carcin/bgi082. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Soto MJ, Larrosa M, Garcia-Cantalejo JM, Espìn JC, Tomá-Barberan FA, Garcìa-Conesa MT. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J Nutr Biochem. 2007;18:259–271. doi: 10.1016/j.jnutbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bobe G, Barrett KG, Mentor-Marcel RA, Saffiotti U, Young MR, Colburn NH, Albert PS, Bennink MR, Lanza E. Dietary cooked navy beans and their fractions attenuate colon carcinogenesis in azoxymethane-induced ob/ob mice. Nutr Cancer. 2008;60:373–381. doi: 10.1080/01635580701775142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe G, Wang B, Seeram NP, Nair MG, Bourquin LD. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J Agric Food Chem. 2006;54:9322–9328. doi: 10.1021/jf0612169. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Cai H, Al-Fayez M, Tunstall RG, Platton S, Greaves P, Steward WP, Gescher AJ. The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice. Mol Cancer Ther. 2005;4:1287–1292. doi: 10.1158/1535-7163.MCT-05-0165. [DOI] [PubMed] [Google Scholar]
- Cappell MS. From colonic polyps to colon cancer: pathophysiology, clinical presentation, screening and colonoscopic therapy. Minerva Gastroenterol Dietol. 2007;53:351–373. [PubMed] [Google Scholar]
- Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–2189. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- Devasena T, Venugopal Menon P. Fenugreek seeds modulate 1,2-dimethylhydrazineinduced hepatic oxidative stress during colon carcinogenesis. Ital J Biochem. 2007;56:28–34. [PubMed] [Google Scholar]
- Dragnev KH, Feng Q, Ma Y, Shah SJ, Black C, Memoli V, Nugent W, Rigas JR, Kitareewan S, Freemantle S, Dmitrovsky E. Uncovering novel targets for cancer chemoprevention. Recent Results Cancer Res. 2007;174:235–243. doi: 10.1007/978-3-540-37696-5_21. [DOI] [PubMed] [Google Scholar]
- Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, Martel P, Chaumontet C. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis. 2004;25:1227–1236. doi: 10.1093/carcin/bgh123. [DOI] [PubMed] [Google Scholar]
- Eisinger AL, Prescott SM, Jones DA, Stafforini DM. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007;82:147–154. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Engelbrecht AM, Mattheyse M, Ellis B, Loos B, Thomas M, Smith R, Peters S, Smith C, Myburgh K. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007;258:144–153. doi: 10.1016/j.canlet.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Espìn JC, Garcìa-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Fuggetta MP, Lanzilli G, Tricarico M, Cottarelli A, Falchetti R, Ravagnan G, Bonmassar E. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp Clin Cancer Res. 2006;25:189–193. [PubMed] [Google Scholar]
- Giovannucci E. Diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health (Larchmt) 2003;12:173–182. doi: 10.1089/154099903321576574. [DOI] [PubMed] [Google Scholar]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, Raul F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26:1291–1295. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- Grubben MJ, Van Den Braak CC, Broekhuizen R, De Jong R, Van Rijt L, De Ruijter E, Peters WH, Katan MB, Nagengast FM. The effect of unfiltered coffee on potential biomarkers for colonic cancer risk in healthy volunteers: a randomized trial. Department of Aliment Pharmacol Ther. 2000;14:1181–1190. doi: 10.1046/j.1365-2036.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- Gustin DM, Brenner DE. Chemoprevention of colon cancer: current status and future prospects. Cancer Metastasis Rev. 2004;21:323–348. doi: 10.1023/a:1021271229476. [DOI] [PubMed] [Google Scholar]
- Gwak J, Park S, Cho M, Song T, Cha SH, Kim DE, Jeon YJ, Shin JG, Oh S. Polysiphonia japonica extract suppresses the Wnt/beta-catenin pathway in colon cancer cells by activation of NF-kappaB. Int J Mol Med. 2006;17:1005–1010. [PubMed] [Google Scholar]
- Hakkak R, Korourian S, Ronis MJ, Johnston JM, Badger TM. Soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats. Cancer Lett. 2001;166:27–32. doi: 10.1016/s0304-3835(01)00441-4. [DOI] [PubMed] [Google Scholar]
- Hao X, Sun Y, Yang CS, Bose M, Lambert JD, Ju J, Lu G, Lee MJ, Park S, Husain A, Wang S. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–69. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- Hong MY, Seeram NP, Zhang Y, Heber D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J Nutr Biochem. 2008;19:448–458. doi: 10.1016/j.jnutbio.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Weng HC, Lin S, Chien YW. Curcuminoids-cellular uptake by human primary colon cancer cells as quantitated by a sensitive HPLC assay and its relation with the inhibition of proliferation and apoptosis. J Agric Food Chem. 2007;55:8213–8222. doi: 10.1021/jf070684v. [DOI] [PubMed] [Google Scholar]
- Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, Xu C, Reddy B, Chada K, Kong AN. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Issa AY, Volate SR, Muga SJ, Nitcheva D, Smith T, Wargovich MJ. Green tea selectively targets initial stages of intestinal carcinogenesis in the AOM-ApcMin mouse model. Carcinogenesis. 2007;28:1978–1984. doi: 10.1093/carcin/bgm161. [DOI] [PubMed] [Google Scholar]
- Janakiram NB, Cooma I, Mohammed A, Steele VE, Rao CV. Beta-ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid X receptor-alpha in human colon cancer cells. Mol Cancer Ther. 2008;7:181–190. doi: 10.1158/1535-7163.MCT-07-0529. [DOI] [PubMed] [Google Scholar]
- Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- Jenab M, Thompson LU. Phytic acid in wheat bran affects colon morphology, cell differentiation and apoptosis. Carcinogenesis. 2000;21:1547–1552. [PubMed] [Google Scholar]
- Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, Yang GY, Liu YY, Hou Z, Lin Y, Ma J, Shih WJ, Carothers AM, Yang CS. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- Ju J, Liu Y, Hong J, Huang MT, Conney AH, Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–178. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaleeswari M, Deeptha K, Sengottuvelan M, Nalini N. Effect of dietary caraway (Carum carvi L.) on aberrant crypt foci development, fecal steroids, and intestinal alkaline phosphatase activities in 1,2-dimethylhydrazine-induced colon carcinogenesis. Toxicol Appl Pharmacol. 2006;214:290–296. doi: 10.1016/j.taap.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kang JH, Han IH, Sung MK, Yoo H, Kim YG, Kim JS, Kawada T, Yu R. Soybean saponin inhibits tumor cell metastasis by modulating expressions of MMP-2, MMP-9 and TIMP- 2. Cancer Lett. 2008;261:84–92. doi: 10.1016/j.canlet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN. Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC) Mol Carcinog. 2008;47:321–325. doi: 10.1002/mc.20390. [DOI] [PubMed] [Google Scholar]
- Kumar R. Commentary: targeting colorectal cancer through molecular biology. Semin Oncol. 2005;32:S37–S39. doi: 10.1053/j.seminoncol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:2576S–2579S. doi: 10.1093/jn/137.11.2576S. [DOI] [PubMed] [Google Scholar]
- Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- Krzystyniak KL. Current strategies for anticancer chemoprevention and chemoprotection. Acta Pol Pharm. 2002;59:473–478. [PubMed] [Google Scholar]
- Lala G, Malik M, Zhao C, He J, Kwon Y, Giusti MM, Magnuson BA. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer. 2006;54:84–93. doi: 10.1207/s15327914nc5401_10. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee EO, Rhee YH, Ahn KS, Li GX, Jiang C, Lű J, Kim SH. An oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer activities by targeting angiogenesis, apoptosis and metastasis. Carcinogenesis. 2006;27:2455–2463. doi: 10.1093/carcin/bgl104. [DOI] [PubMed] [Google Scholar]
- Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- Lila MA. From beans to berries and beyond: teamwork between plant chemicals for protection of optimal human health. Ann N Y Acad Sci. 2007;1114:372–380. doi: 10.1196/annals.1396.047. [DOI] [PubMed] [Google Scholar]
- Lu H, Li J, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr Cancer. 2006;54:69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–927. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Manson MM, Farmer PB, Gescher A, Steward WP. Innovative agents in cancer prevention. Recent Results Cancer Res. 2005;166:257–275. doi: 10.1007/3-540-26980-0_17. [DOI] [PubMed] [Google Scholar]
- Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, Katsuki T, Hirata K, Sumi S, Ishikawa H. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136:842S–846S. doi: 10.1093/jn/136.3.842S. [DOI] [PubMed] [Google Scholar]
- Miyata T. Pharmacological basis of traditional medicines and health supplements as curatives. J Pharmacol Sci. 2007;103:127–131. doi: 10.1254/jphs.cpj06016x. [DOI] [PubMed] [Google Scholar]
- Mutch MG. Molecular profiling and risk stratification of adenocarcinoma of the colon. J Surg Oncol. 2007;96:693–703. doi: 10.1002/jso.20915. [DOI] [PubMed] [Google Scholar]
- Nalini N, Manju V, Menon VP. Effect of spices on lipid metabolism in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Med Food. 2006;9:237–245. doi: 10.1089/jmf.2006.9.237. [DOI] [PubMed] [Google Scholar]
- Nishino H, Satomi Y, Tokuda H, Masuda M. Cancer control by phytochemicals. Curr Pharm Des. 2007;13:3394–3399. [PubMed] [Google Scholar]
- Niv Y. Microsatellite instability and MLH1 promoter hypermethylation in colorectal cancer. World J Gastroenterol. 2007;13:1767–1769. doi: 10.3748/wjg.v13.i12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onogi N, Okuno M, Komaki C, Moriwaki H, Kawamori T, Tanaka T, Mori H, Muto Y. Suppressing effect of perilla oil on azoxymethane-induced foci of colonic aberrant crypts in rats. Carcinogenesis. 1996;17:1291–1296. doi: 10.1093/carcin/17.6.1291. [DOI] [PubMed] [Google Scholar]
- Papapolychroniadis C. Environmental and other risk factors for colorectal carcinogenesis. Tech Coloproctol. 2004;8 Suppl 1:s7–s9. doi: 10.1007/s10151-004-0097-x. [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, Choi YH, Kwon TK. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- Patel R, Ingle A, Maru GB. Polymeric black tea polyphenols inhibit 1,2- dimethylhydrazine induced colorectal carcinogenesis by inhibiting cell proliferation via Wnt/beta-catenin pathway. Toxicol Appl Pharmacol. 2008;227:136–146. doi: 10.1016/j.taap.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Patlolla JM, Raju J, Swamy MV, Rao CV. Beta-escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21(waf1/cip1) in colon cancer cells. Mol Cancer Ther. 2006;5:1459–1466. doi: 10.1158/1535-7163.MCT-05-0495. [DOI] [PubMed] [Google Scholar]
- Porath D, Riegger C, Drewe J, Schwager J. Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J Pharmacol Exp Ther. 2005;315:1172–1180. doi: 10.1124/jpet.105.090167. [DOI] [PubMed] [Google Scholar]
- Rajakangas J, Misikangas M, Päivärinta E, Mutanen M. Chemoprevention by white currant is mediated by the reduction of nuclear beta-catenin and NF-kappaB levels in Min mice adenomas. Eur J Nutr. 2008;47:115–122. doi: 10.1007/s00394-008-0704-0. [DOI] [PubMed] [Google Scholar]
- Raju J, Swamy MV, Cooma I, Patlolla JM, Pittman B, Reddy BS, Steele VE, Rao CV. Low doses of beta-carotene and lutein inhibit A``xOM-induced rat colonic ACF formation but high doses augment ACF incidence. Int J Cancer. 2005;113:798–802. doi: 10.1002/ijc.20640. [DOI] [PubMed] [Google Scholar]
- Reddy BS. Colon carcinogenesis models for chemoprevention studies. Hematol Oncol Clin North Am. 1998;12:963–973. doi: 10.1016/s0889-8588(05)70036-8. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Hirose Y, Cohen LA, Simi B, Cooma I, Rao CV. Preventive potential of wheat bran fractions against experimental colon carcinogenesis: implications for human colon cancer prevention. Cancer Res. 2000;60:4792–4797. [PubMed] [Google Scholar]
- Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human colon cancer cell HCT 116 xenografts in nude mice: evaluation of tumor growth and immunohistochemistry. Oncol Rep. 2005;13:421–425. [PubMed] [Google Scholar]
- Rowley PT. Inherited susceptibility to colorectal cancer. Annu Rev Med. 2005;56:539–554. doi: 10.1146/annurev.med.56.061704.135235. [DOI] [PubMed] [Google Scholar]
- Sang S, Ju J, Lambert JD, Lin Y, Hong J, Bose M, Wang S, Bai N, He K, Reddy BS, Ho CT, Li F, Yang CS. Wheat bran oil and its fractions inhibit human colon cancer cell growth and intestinal tumorigenesis in Apc(min/+) mice. J Agric Food Chem. 2006;54:9792–9797. doi: 10.1021/jf0620665. [DOI] [PubMed] [Google Scholar]
- Satia-Abouta J, Galanko JA, Martin CF, Ammerman A, Sandler RS. Food groups and colon cancer risk in African-Americans and Caucasians. Int J Cancer. 2004;109:728–736. doi: 10.1002/ijc.20044. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Ghosh S, Bhattacharjee S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac J Cancer Prev. 2005;6:118–122. [PubMed] [Google Scholar]
- Sengupta A, Ghosh S, Das S. Modulatory influence of garlic and tomato on cyclooxygenase-2 activity, cell proliferation and apoptosis during azoxymethane induced colon carcinogenesis in rat. Cancer Lett. 2008;208:127–136. doi: 10.1016/j.canlet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Deguchi A, Joe AK, Mckoy JF, Moriwaki H, Weinstein IB. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J Exp Ther Oncol. 2005a;5:69–78. [PubMed] [Google Scholar]
- Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005b;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- Spychalski M, Dziki L, Dziki A. Chemoprevention of colorectal cancer - a new target needed? Colorectal Dis. 2007;9:397–401. doi: 10.1111/j.1463-1318.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- Sriram N, Kalayarasan S, Ashokkumar P, Sureshkumar A, Sudhandiran G. Diallyl sulfide induces apoptosis in Colo 320 DM human colon cancer cells: involvement of caspase-3, NF-kappaB, and ERK-2. Mol Cell Biochem. 2008;311:157–165. doi: 10.1007/s11010-008-9706-8. [DOI] [PubMed] [Google Scholar]
- Su CC, Chen GW, Lin JG, Wu LT, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B /p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006;26:1281–1288. [PubMed] [Google Scholar]
- Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, Lin WC, Chen GW. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4389. [PubMed] [Google Scholar]
- Taché S, Ladam A, Corpet DE. Chemoprevention of aberrant crypt foci in the colon of rats by dietary onion. Eur J Cancer. 2007;43:454–458. doi: 10.1016/j.ejca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, Chayama K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr. 2006;136:821S–826S. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis. 2000;21:1619–1622. [PubMed] [Google Scholar]
- Tian Y, Song Y. Effects of inositol hexaphosphate on proliferation of HT-29 human colon carcinoma cell line. World J Gastroenterol. 2006;12:4137–4142. doi: 10.3748/wjg.v12.i26.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54:111–142. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- Vincent TL, Gatenby RA. An evolutionary model for initiation, promotion, and progression in carcinogenesis. Int J Oncol. 2008;32:729–737. [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Walfisch S, Walfisch Y, Kirilov E, Linde N, Mnitentag H, Agbaria R, Sharoni Y, Levy J. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. Eur J Cancer Prev. 2007;16:298–303. doi: 10.1097/01.cej.0000236251.09232.7b. [DOI] [PubMed] [Google Scholar]
- Watson JL, Hill R, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Exp Mol Pathol. 2008 doi: 10.1016/j.yexmp.2008.02.002. (Ahead of print) [DOI] [PubMed] [Google Scholar]
- Williams MT, Hord NG. The role of dietary factors in cancer prevention: beyond fruits and vegetables. Nutr Clin Pract. 2005;20:451–459. doi: 10.1177/0115426505020004451. [DOI] [PubMed] [Google Scholar]
- Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog. 2008 doi: 10.1002/mc.20437. (Ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]