Abstract
Context: Despite lacking evidence for its safety and efficacy, antipsychotic cotreatment is common in schizophrenia.
Objective: To evaluate therapeutic and adverse effects of antipsychotic cotreatment vs monotherapy in schizophrenia.
Data Sources: Cochrane Schizophrenia Group register and hand searches of relevant journals/conference proceedings.
Study Selection: Randomized controlled trials comparing antipsychotic monotherapy to cotreatment with a second antipsychotic.
Data Extraction and Analysis: Two authors independently extracted data. For homogenous dichotomous data, we calculated random effects, relative risk (RR), 95% confidence intervals (CIs), and numbers needed to treat (NNT). For continuous data, weighted mean differences were calculated.
Results: In 19 studies (1229 patients) with 28 monotherapy and 19 cotreatment arms, antipsychotic cotreatment was superior to monotherapy regarding 2 a priori defined coprimary outcomes: less study-specific defined inefficacy (N = 22, n = 1202, RR = 0.76, CI = 0.63–0.90, P = .002, NNT = 7, CI = 4–17, P = .0008, I2 = 78.9%) and all-cause discontinuation (N = 20, n = 1052, RR = 0.65, CI = 0.54–0.78, P < .00001). Results were consistent using Clinical Global Impressions thresholds of less than much (P = .006) and less than minimally (P = .01) improved. Specific psychopathology and adverse event data were insufficient to yield meaningful results. In sensitivity analyses, 5 efficacy moderators emerged: concurrent polypharmacy initiation, clozapine combinations, trial duration >10 weeks, Chinese trials, and second-generation + first-generation antipsychotics. In a meta-regression, similar dose combinations, second-generation + first-generation antipsychotics and concurrent polypharmacy initiation remained significant.
Conclusions: In certain clinical situations, antipsychotic cotreatment may be superior to monotherapy. However, the database is subject to possible publication bias and too heterogeneous to derive firm clinical recommendations, underscoring the need for future research.
Keywords: antipsychotics, polypharmacy, combination, monotherapy, schizophrenia, efficacy, all-cause discontinuation, side effects, trial methodology
In schizophrenia, treatment resistance and unsatisfactory functional outcomes continue to be a significant clinical and public health problem.1–4 To date, clozapine has remained the only treatment that consistently resulted in significantly superior outcomes compared with other antipsychotics in patients unresponsive or partially responsive to antipsychotic monotherapies.5–7 A multitude of augmentation strategies have been tried in randomized controlled studies, including lithium,8 carbamazepine,9 valproate,10 benzodiazepines,11 beta-blockers,12 antidepressants,13 anti-inflammatory agents,14 glutamatergic agents,15 and electroconvulsive therapy.16 However, none of these strategies has reliably demonstrated efficacy for people whose psychosis did not respond to antipsychotic monotherapy.
In this context, antipsychotic combination treatment, also called antipsychotic polypharmacy, has been utilized frequently in clinical practice. Reports of the prevalence of antipsychotic polypharmacy in the United States vary from 7% to approximately 50%,3,17–21 with most studies finding prevalence rates of between 10% and 30%. Moreover, some studies have shown a trend toward the increasing use of polypharmacy in the same treatment settings over time,18,22 despite the fact that evidence-based treatment guidelines recommend antipsychotic cotreatment only after unsuccessful attempts of multiple monotherapies, including clozapine.23,24
While the lack of any pharmacologic rationale for combining antipsychotics with the same putative antipsychotic dopamine D2 receptor blockade has been criticized, there is also limited mechanistic understanding of antipsychotic efficacy and “polypharmacy” is an intrinsic characteristic of most antipsychotics due to multiple pharmacodynamic neurotransmitter effects. Nevertheless, antipsychotic cotreatment remains a controversial practice because of insufficient evidence supporting its efficacy,25,26 concerns about long-term safety,17,27 mortality,28,29 and increased cost.26 Moreover, all the few randomized controlled trials (RCTs) of antipsychotic polypharmacy cited in this context involve augmentation of clozapine with a high dopamine D2 affinity antipsychotic.30–33 This, however, is in contrast to clinical practice in the Western world, where clozapine is used relatively infrequently, both in monotherapy and as antipsychotic cotreatment,17–21 which is initiated mostly without a prior clozapine trial.
In view of the prevalence of this clinical practice and the paucity of evidence in its support, we conducted a comprehensive review of the available evidence regarding the efficacy, effectiveness, and safety of antipsychotic combinations compared with treatment with antipsychotic monotherapy in patients with schizophrenia.
Methods
Search
We searched the register of the Cochrane Schizophrenia Group (CSG) for published or unpublished RCTs that compared antipsychotic monotherapy with the combination of the same antipsychotic with another one in the treatment of schizophrenia or related disorders (schizoaffective-, schizophreniform-, or delusional disorder, any diagnostic criteria). The CSG register is compiled by regular methodical searches in numerous electronic databases (BIOSIS, CINAHL, Dissertation Abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsycINFO, RUSSMED, Sociofile), supplemented by hand searching of relevant journals and conference proceedings, and searches of several key literature sources (last search June 2006, updated since then by monthly MEDLINE searches until March 2007). For details of the register, see the description of the CSG.34
The following search terms were used: [((antipsychot* or neuroleptic* or drug*) and combin*)) or augmentation* or enhancement or add-on* or addition*or supplement*or cotreatment*or co-treatment*or adjunctive* or concurrent* or concomitant* or simultaneous* or parallel* or polypharmacy* in title terms) or (*add-on* or *addition*or *supplement*or *supplementation*or *cotreatment*or *co-treatment*or *adjunctive* or *concurrent* or *concomitant* or *simultaneous* or *parallel* or *polypharmacy* in abstract or index terms of REFERENCE]) or [*Polytherapy* or *Augmentation* or *Parallel* or *Combined* in interventions of STUDY].
Only studies meeting the quality criteria A (adequate randomization) and B (usually studies stated to be randomized without further details) according to the Cochrane handbook were included.35 There were no restrictions regarding language, sample size, or time period. In addition, the reference sections of included articles were screened, and the first authors of the included studies were asked whether they were aware of additional trials, as well as about missing data necessary for the meta-analysis. Two authors independently identified and extracted data from the trials.
Outcome Parameters
The primary outcomes of interest were a clinically significant response defined as at least 50% reduction of the Positive and Negative Syndrome Scale (PANSS) or the Brief Psychiatric Rating Scale (BPRS) or at least “much better” on the Clinical Global Impressions (CGI) scale,36,37 dropout rates, and relapse. If the response criteria mentioned above were not available, we used the authors’ definition. Secondary outcomes were general and specific aspects of the mental state (BPRS/PANSS total scores, positive and negative symptoms, depression, mania, aggression), service utilization, functioning, additional drug use, quality of life, general and specific side effects, and cost.
For dichotomous data, we applied a “once randomized—analyzed” end point analysis. Continuous data were reported as presented in the original studies without any assumptions about those lost to follow-up.
Meta-analytic Calculations
We applied standard meta-analytic procedures as used by the Cochrane Collaboration throughout. For dichotomous data, we calculated the relative risk (RR), and for continuous data we calculated Hedges's g as effect size measures, both along with their 95% confidence intervals (CIs). To combine studies, the random effects model by Der-Simonian and Laird38 was used in all cases, which is more conservative than fixed effects models. We explored study heterogeneity using the I2 statistic, a measure estimating how much of the variance is explained by study heterogeneity.39 I2 values of 50% or higher were considered to reflect considerable heterogeneity. In such cases, we sought reasons explaining the heterogeneity, conducting sensitivity analyses. In the case of significant differences between groups, the number of participants needed to treat (NNT) or the number of participants needed to harm was calculated as the inverse of the risk difference (RD). The possibility of publication bias was examined using the “funnel plot” method described by Egger and colleagues.40
In the primary analyses, we included all studies that compared monotherapy with one antipsychotic to polytherapy with the same antipsychotic combined with a different one. In the 9 studies that included 3 arms comparing the antipsychotic cotreatment with the monotherapy of each of the 2 antipsychotics that were part of the combination, we included the cotreatment condition twice in the analyses. This was done to be able to include each of the monotherapy arms separately in the analyses. Thus, the total number of study arms (N = 19 + 9 = 28) and patients (n = 1229 + 238 = 1454) included in the analyses was higher than the total number of studies (N = 19) and patients (n = 1216). However, in order to exclude that this methodology could have led to an alteration of the results, we repeated the meta-analysis counting the combination treatment only once, comparing it to the results of both monotherapy arms together in cases of 3 arm studies.
In addition to the primary analyses, we conducted 9 sensitivity analyses. This was done to examine if variations in design, clinical practice, settings, populations, and antipsychotics across studies conducted at different times and in different regions may have been responsible for the heterogeneity of the primary efficacy outcome (ie, inefficacy as defined by each study). These sensitivity analyses included: (1) double-blind vs single-blind/open study design; (2) Chinese vs non-Chinese studies; (3) enrollment of acutely exacerbated or chronically ill patients; (4) combined initiation vs delayed augmentation after nonresponse; (5) comparative vs reduced antipsychotic doses in the cotreatment arm; (6) treatment duration <10 weeks vs ≥10 weeks; (7) clozapine vs nonclozapine combinations; (8) cotreatment with 2 first-generation antipsychotics (FGAs) compared with 1 FGA, cotreatment with 2 second-generation antipsychotics (SGAs) compared with 1 SGA, cotreatment with an FGA plus an SGA compared with either an FGA or SGA, respectively; and (9) counting the cotreatment arm only once, comparing it to both monotherapy arms together in the 9 trials where 2 monotherapy treatment existed. All moderators tested in the sensitivity analyses were also included in an unrestricted maximum likelihood random effects meta-regression. Because these analyses were considered exploratory, we did not correct P values for multiple testing.
Finally, to examine in more detail if dose differences between the antipsychotic combination and monotherapy treatment arms could have influenced the results, we compared (a) the chlorpromazine equivalent doses between the antipsychotic monotherapy and polytherapy arms across all studies with efficacy data and (b) the ratio of the chlorpromazine equivalents between the monotherapy and polytherapy arms in studies finding superior efficacy of combination treatment vs studies that did not find such a difference, defined as either statistical superiority (ie, P < .05 indicated by no overlap of the 95% CI with the y-axis), or as the mean value being to the left of the y-axis (ie, favoring antipsychotic combinations), independent of whether or not the 95% CI overlapped with the y-axis (figure 2). Because efficacy as well as dose information for the mono- and polytherapy groups was available in only 20 of the 47 treatment arms (42.5%), inclusion of the chlorpromazine equivalent ratio into the meta-regression would have lead to the exclusion of the majority of treatment arms, potentially skewing the results of the other moderator variables for which information was available in all studies. Therefore, we repeated the primary end point analysis of efficacy as defined by each study in the subset of studies with both efficacy and dose information. This was done to ensure that the influence of dose was examined in a subset of studies that was representative of the primary efficacy result across all studies. Chlorpromazine equivalents were calculated based on proposed conversion factors for FGAs41,42 and SGAs.43
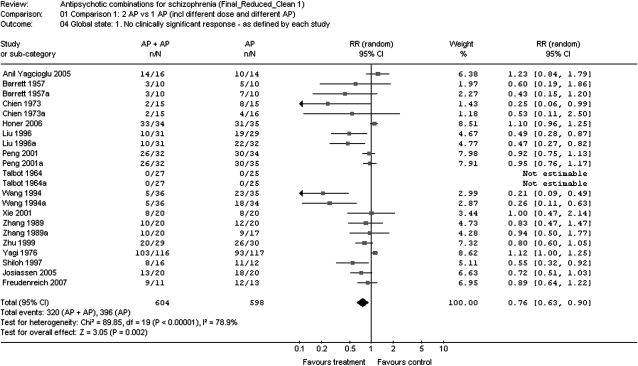
Fig. 2.
Lack of Efficacy as Defined in Each Study.
All meta-analytic calculations were performed with RevMan Analyses, a meta-analytic standard software used by the Cochrane collaboration,44 and STATA 8.0 for the meta-regression. All analyses were 2 tailed, with alpha set at 0.05.
Results
The Search
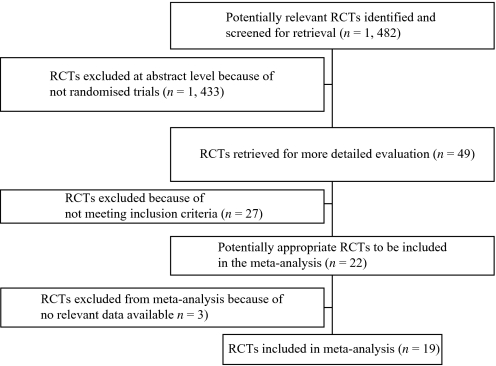
The original search yielded 1482 electronic records. A total of 1433 publications were excluded on the abstract level. For 49 hits, full reports were ordered for inspection. Of those, 30 studies were excluded, leaving 19 studies, because they were not randomized (N = 10) or only quasi-randomized (N = 7), did not analyze comparisons relevant for our review (N = 10), or reported no usable data (N = 3) (figure 1).
Fig. 1.
Included and Excluded Studies With Reasons: The Quality of Reports of Meta-analysis (QUOROM) Flow Diagram.
Characteristics of Included Studies and Participants
In total, the 19 studies included 1216 participants. Sample sizes per study varied widely, ranging from 17 to 233 (median 57; table 1). In 10 studies (52.6%), 1 antipsychotic combination treatment was compared with 1 monotherapy arm (n = 558, 45.9%). In 9 studies (47.4%), 1 antipsychotic combination treatment was compared with 2 antipsychotic monotherapy arms (n = 658, 54.1%). To allow for a comparison of each of the 2 monotherapy arms with the antipsychotic combination group, we entered the 238 patients in the combination arms from these 9 studies a second time in the primary analysis, which increased the total number of patients entered in the meta-analysis from 1216 to 1454.
Table 1.
Study and Patient Characteristics
| Study | N | Design:-Blinding-Timing of Cotreatment-Dosing-Setting | Trial Duration (weeks) | Country | Participants:-Diagnosis-Criteria-Illness Phase-Illness Duration | Agea: Years (Range) | Sex: Male (%) | Interventions: Antipsychotic Mean Dose (mg/day) Range (mg/day)Number of Patients | Comments |
| Antipsychotic combinations including clozapine (N = 12, n = 701) | |||||||||
| Clozapine plus a FGA (N = 2, n = 114) | |||||||||
| Potter et al. (1989)46 | 57 | -Double blind-Costart-Reduced dose combination-Inpatients | 8 | China | -Sz-Chronic-DSM-IV and CCMD-2-R-Illness duration: 5.9 years | 31.7 (16–58) | n = 35 (61%) | 1. CLZ (M = NR; R = 50–600); n = 17. 2. CPZ (M = NR; R = 100–600); n = 20. 3. CLZ (M = NR; R = 50–400) + CPZ (M = NR; R = 100–400); n = 20 | Data not shown for 4 BPRS positive and the depressive subscores that were reportedly significantly more improved in each clozapine arm vs the chlorpromazine monotherapy group. |
| Zhang and Xu (1989)48 | 57 | -Double blind-Costart-Reduced dose combination-Inpatients | 8 | China | -Sz-Acute-DSM-III and CCMD-2-R-Illness duration: 5.8 years | 31.7 (NR) | n = 36 (63%) | 1. CLZ (M = 363; R = 175–500); n = 17. 2. CPZ (M = 440; R = 350–800); n = 20. 3. CPZ (M = 355; R = 200–500) + CLZ (M = 256; R = 175–500); n = 20 | |
| Clozapine plus a SGA (N = 10, n = 587) | |||||||||
| Freudenreich (2007)49 | 24 | -Double blind-Augmentation-Comparative dose combination-Outpatients | 6 | United States | -Sz-DSM-IV-Chronic (refractory)-Illness duration: 20.6 years | 42.3 (27–55) | n = 21 (88%) | 1. CLZ (M = 456; R = 200–700); n = 11. 2. CLZ (M = 456; R = 200–700) + RIS (M = 4; R = NR); n = 13 | Four (14.3%) of 28 original patients were eliminated during 2-week placebo lead-in due to not reaching the symptom severity criterion anymore. |
| Anil Yagcioglu et al. (2005)32 | 30 | -Double blind-Augmentation-Comparative dose combination-Inpatients (n = 6) and outpatients (n = 24) | 6 | Turkey | -Sz-DSM-IV-Chronic(refractory)-Illness duration: 12.2 years | 33.4 (18–55) | n = 20 (67%) | 1. CLZ (M = 414.3; R = 300–900); n = 14. 2. CLZ (M = 515.6; R = 300–900) + RIS (M = 5.1; R = NR); n = 16 | Significantly greater number of hospitalizations (P = .01), clozapine dose (P = .05) and number of smokers (P = .03) in cotreatment group. |
| Honer et al. (2006)33 | 68 | -Double blind-Augmentation-Comparative dose combination-Inpatients: n = 26 + outpatients: n = 42 | 8 | Canada, FRG, China, United Kingdom | -Sz (92.6%); SzA (7.4%)-DSM-IV-Chronic (refractory)-Duration: 15.0 years | 37.2 (18-65) | n = 50 (74%) | 1. CLZ (M = 487; R = NR); n = 34. 2. CLZ (M = 494; R = NR) + RIS (M = 3; R = NR); n = 34 | Stability of PANSS score assessed after 1-week single-blind placebo augmentation run-in phase. |
| Josiassen et al. (2005)31 | 40 | -Double blind-Augmentation-Comparative dose combination-Inpatients (n = 32) and outpatients (n = 8) | 12 | United States | -Sz-DSM-IV-Chronic (refractory)-Illness duration: 22.1 years | 40.3 (20–65) | n = 35 (88%) | 1. CLZ (M = 403; R = NR); n = 20. 2. CLZ (M = 529; R = NR) + RIS (M = 4.4; R = NR); n = 20 | More males (P < .05) and higher clozapine dose (P < .006) in cotreatment than in monotherapy group; CGI data not reported. |
| Liu et al. (1996)50 | 92 | -Blindness “unclear”-Costart-Comparative dose combination-Inpatients | 12 | China | -Sz (negative symptom type)-Chronic-DSM-III-R-Illness duration: 6.9 years | 25.2 (16–54) | n = 60 (65%) | 1. CLZ (M = 466.8; R = 450–600); n = 32. 2. SUL (M = 1296.6; R = 900–1500); n = 29. 3. CLZ (M = 436.6; R = 450–600) + SUL (M = 1127.2; R = 900–1500); n = 31 | No information about the group assignment in the 5 patients who dropped out. |
| Peng et al. (2001)51 | 101 | -Double blind-Costart-Reduced dose combination-Inpatients | 8 | China | -Sz-Chronic (refractory)-CCMD-2-R-Illness duration: 7.1 years | 25.2 (16–60) | n = 67 (66%) | 1. CLZ (M = NR; R = 75–600 TID); n = 34. 2. RIS (M = NR; R = 1–8 QD); n = 35. 3. CLZ (M = NR; R = 25–200 QD) + RIS (M = NR; R = 1–6 QD); n = 32 | Flexible dosing. |
| Shiloh (1997)30 | 28 | -Double blind-Augmentation-Comparative dose combination-Inpatients | 10 | Israel | -Sz-Chronic (refractory)-DSM-IV-Illness duration: 20.0 years | 38.9 (NR) | n = 19 (68%) | 1. CLZ (M = 446; R = NR); n = 12. 2. CLZ (M = 403; R = NR) + SUL (M = NR; R = 100–600); n = 16 | All mental state data skewed. Significantly longer past hospitalization in PBO group (P < .05); CGI data not reported; CLZ serum levels not reported. |
| Wang et al. (1994)52 | 105 | -Double blind-Costart-Reduced dose combination-Inpatients (for acute 8-week trial) | 8 (plus follow-up at 12, 52, and 156 weeks) | China | -Sz (negative symptom type)-Acute-CCMD-2-Illness duration: 3.4 years | 30.3 (18–64) | n = 0 (0%) | 1. CLZ (M = 186.9; R = 50–300); n = 34. 2. SUL (M = 821.5; R = 600–1000); n = 35. 3. CLZ (M = 52.7; R = 25–100) + SUL (M = 738.5; R = 400–1000); n = 36 | No information about the group assignment in the 5 patients who dropped out during the 8-week inpatient trial, apparently only scores of completers included in the analyses at 3, 12, and 36 months. |
| Xie and Ni (2001)53 | 40 | -Double blind-Costart-Comparative dose combination-Setting: NR | 8 | China | -Sz-Acute-CCMD-2-R-Illness duration: 3.2 years | 32.3 (15–53) | n = 27 (68%) | 1. RIS (M = NR; R = 4–6); n = 20. 2. RIS (M = NR; R = 4–6) + CLZ (M = 141; R = 50–300); n = 20 | |
| Zhu et al. (1999)54 | 59 | -Double blind-Costart-Comparative dose combination-Setting: NR | 12 | China | -Sz (negative symptom type)-Chronic-CCMD-2-R-Illness duration: NR | 34.6 (NR) | n = 32 (54%) | 1. CLZ (M = NR; R = 50–500 BID); n = 302. CLZ (M = NR; R = 50–500 BID) + SUL (M = NR; R = 0.2–1); n = 29 | Third treatment group, consisting of CLZ + chlorimipramine (tricyclic antidepressant, n = 29), was excluded. |
| Nonclozapine antipsychotic combinations (N = 7, n = 528) | |||||||||
| FGA + FGA (N = 6, n = 511) | |||||||||
| Barrett et al. (1957)45 | 30 | -Triple blind(raters blind to active control)-Reduced dose combination-Costart-Inpatients | 12 | United States | -Sz (regressed)-Chronic- Illness duration: NR | 35.3 (27–47) | NR | 1. CPZ (M = 520; R = 200–1200); n = 10. 2. RES (M = 5.9; R = 4–8); n = 10. 3. CPZ (M = 230; R = 100–400) + RES (M = 2.3; R = 1–4); n = 10 | 2 months placebo run-in; raters told that study had placebo arm to reduce rater expectation bias. IP duration: 7–8 years. No diagnostic instrument. |
| Chien and Cole (1973)55 | 46 | -Single blind: (raters blind)-Costart-Comparative dose combination-Inpatients | 4 | United States | -Sz (76%), psychotic depression (19.6%), substance induced psychosis (4.4%)-Acute-Illness duration: NR | 36.8 (17–62) | NR | 1. CPZ (M = 388; R = NR); n = 15. 2. FLU ENAN (M = 28.5; R = 12.5–75); n = 16. 3. CPZ (M = 350; R = NR) + FLU (M = 26; R = 12.5–75); n = 15 | Data analyzed only for 10-day time point, due to “sequential” design patients who were much or very much improved or much or very much worse were removed from the study after day 10. No diagnostic instrument. |
| Higashima et al. (2004)56 | 19 | -Open label-Costart-Comparative dose combination-Inpatients | 8 | Japan | -Sz-DSM-IV-Acute-Illness duration: 3.8 years | 28.1 (NR) | n = 12 (63%) | 1. HAL (M = 5.4; R = NR); n = 10. 2. HAL (M = 5.4; R = NR) + LEV (M = 54; R = NR); n = 9 | Patients untreated at time of enrollment. Efficacy data only presented for week 1 and 2. Patients with subscores ≤3 excluded from the analyses to “reduce possibility of underestimation of therapeutic benefits.” |
| Nishikawa (1985)57 | 106 | -Double blind-Costart-Comparative dose combination-Outpatients | 52 | Japan | -Sz (residual)-Chronic-DSM-III-Illness duration: 12.3 years | 39.3 (NR) | n = 69 (75%) | 1. PIM (fixed dose at 2 or 6 mg; M = 3.8; R = 2–6); n = 24. 2. THI (fixed dose at 25 or 75 mg; M = 47.7; R = 25–75); n = 22. 3. PIM (fixed dose at 2 or 6 mg, M = 4.1; R = 2–6) + THI (fixed dose at 25 or 75 mg; M = 50.5; R = 25–75); n = 47 | Six patients who dropped out not included in final analyses, which were performed on 92 patients on active drug only. Outcome criteria of symptomatic relapse and adverse effect leading to study discontinuation were not defined. |
| Talbot (1964)58 | 77 | -Double blind-Costart-Comparative dose combination-Inpatients | 32 | United States | -Sz-Chronic-Illness duration: NR | NR | NR | 1. CPZ (150 × 2 months, 300 × 4 months); n = 25. 2. TRI (10 × 2 months, −20 × 4 months); n = 25. 3. CPZ (150 × 2 months, −300 × 4 months) + TRI (10 × 2 months, 20 × 4 months); n = 27 | Baseline characteristics of treatment groups not compared. No diagnostic instrument. |
| Yagi (1976)47 | 233 | -Double blind-Costart-Comparative dose combination-Inpatients | 8 | Japan | -Sz-Chronic-Illness duration: 3 years in 92% of patients | NR (18–50) | n = 126 (54%) | 1. CPZ (M = NR; R = 0–225); n = 117. 2. CPZ (M = NR; R = 0–225) + PER (M = NR; R = 100–599); n = 116 | Third treatment group, consisting of CPZ + carpipramine (tricyclic antidepressant, n = 118), was excluded. Diagnostic separation from depressive disorders unclear, primary outcome insensitive. Inpatient: 66% > 1 year. No diagnostic instrument. |
| SGA + SGA (N = 1, n = 17) | |||||||||
| Kotler et al. (2004)59 | 17 | -Open label-Augmentation-Comparative dose combination-Inpatients | 8 | Israel | -Sz-Chronic (refractory)-DSM-IV-Illness duration: 11.3 years | 31.2 (18–60) | n = 9 (53%) | 1. OLZ (M = 22.5; R = 20–30); n = 8. 2. OLZ (M = 22.2; R = 20–30) + SUL (M = 600; fixed dose); n = 9 | All outcome data skewed. Surprisingly low BMI (25.8 and 24.3) after a mean of 33.0 and 32.3 months on olanzapine in the 2 treatment groups and BMI increase of only 1.1 and 0.8 during 8-week trial. |
Weighted mean; M = mean; R = range; CLZ, clozapine; CPZ, chlorpromazine; FGA, first-generation antipsychotic; FLU, fluphenazine; ENAN, Enanthate given every 11 days; HAL: haloperidol, LEV, levomepromazine; OLZ, olanzapine; PIM, pimozide; RES, reserpine: RIS, risperidone; SGA, second-generation antipsychotic; SUL, sulpiride; THI, thioridazine; TRI, trifluoperazine.
The 19 individual antipsychotic combination arms (n = 518) consisted of cotreatment with 2 FGA (N = 6, n = 224), a first- plus a SGA (N = 7, n = 161), and 2 SGA (N = 6, n = 133). Mean number of inpatients and outpatients based on 1176 patients (96.7%) with available information. Age based on 906 patients (74.5%) with available information. Percent male based on 1109 patients (91.2%) with available information. Mean illness duration based on 830 patients (68.3%) with available information.
All included studies used a parallel group design. Fifteen studies (1042 patients) were double blind, 1 was single blind (n = 46), 2 were open (n = 36), and in one study (n = 92), the method of blinding was unclear. The mean trial duration was 12.1 ± 11.3 (range 4–52, median 8) weeks. Seven studies were conducted in China (n = 511), 5 in the United States (n = 217), 3 in Japan (n = 354), 2 in Israel (n = 45), 1 in Turkey (n = 30), and 1 in Canada, Germany, the United Kingdom, and China (n = 68). In 13 studies (n = 1009), the combination treatment was initiated at the start of the trial, while in 6 studies (n = 207), the second antipsychotic was added after nonresponse to an adequate dose and duration of antipsychotic monotherapy had been established. In 14 studies (n = 866), the monotherapy and polytherapy arms had comparable mean doses and dose ranges, while in 5 studies (n = 350), one or both of the antipsychotics in the combination arm were dosed considerably lower than in the monotherapy arm.
In the 17 studies with information, participants were 33.4 ± 5.1 (range 16–65, median 33.9) years old, and 517 (62.3%) of the 1109 patients with available data were male. In the 18 studies (n = 1176) with information, most participants (1038, 88.3%) were inpatients. Most participants were in the chronic illness phase (N = 15, n = 1054); only 4 studies (n = 162) were conducted in acutely exacerbated patients. The mean illness duration (N = 15, n = 830) was 10.2 ± 6.7 (median 6.8) years, and the mean number of psychiatric hospitalizations (N = 4, n = 245) was 3.9 ± 1.3 (median 3.6). Almost all patients (N = 19, n = 1200, 98.7%) suffered from schizophrenia. All but 4 studies used some form of standardized diagnostic criteria, but criteria varied across time and country of origin.
Regarding antipsychotic treatments and combinations, the 28 antipsychotic monotherapy arms (n = 698) included 14 FGA arms (N = 10, n = 378) and 14 SGA (N = 13, n = 320) arms (table 1). The 19 antipsychotic combination arms (n = 518) consisted of cotreatment with 2 FGAs (N = 6, n = 224), an FGA + SGA (N = 7, n = 161), and 2 SGAs (N = 6, n = 133). The antipsychotic used the most was clozapine (N = 11, n = 542). The second most used antipsychotic was chlorpromazine (N = 6, n = 375), followed by risperidone (N = 6, n = 188), and sulpiride (N = 5, n = 185). All other individual antipsychotics were used in only one study, each (pimozide, thioridazine, fluphenazine, trifluoperazine, reserpine, haloperidol, olanzapine, levomepromazine). Mean doses in the antipsychotic monotherapy and polytherapy groups were similar for clozapine (374.2 ± 167.9 vs 404.5 ± 96.9 mg/day, P = .65), sulpiride (1059 ± 335.9 vs 821.9 ± 273.3, P = .44), and chlorpromazine (412.0 ± 92.3 vs 282 ± 52.6 mg/day, P = .053). Doses of the other antipsychotics were reported too infrequently to enable a meaningful comparison.
Data Reporting and Study Quality
Most usable data existed regarding study discontinuation and lack of clinically relevant response. Very limited data were available regarding specific aspects of psychopathology. Data were mostly insufficient for adverse events.
Outcome Results
Antipsychotic Monotherapy vs Augmentation With a Second Antipsychotic Drug.
Concerning primary outcomes, antipsychotic polypharmacy was significantly superior regarding study-specific defined inefficacy (N = 22, n = 1202, RR = 0.76, CI = 0.63–0.90, P = .002, NNT = 7, CI = 4–17, P = .0008) (figure 2). However, the overall results were highly heterogeneous (I2 = 78.9%). When only those studies were considered that used the CGI to define response, the superiority was consistent when a cutoff of less than much improved (N = 13, n = 867, RR = 0.65, CI = 0.47–0.88, P = .006, NNT = 5, CI = 5–17, P = .004) or less than minimally improved was used (N = 13, n = 950, RR = 0.80, CI = 0.67–0.95, P = .01, NNT = 25, CI = 11 to unable to estimate, P = .07).
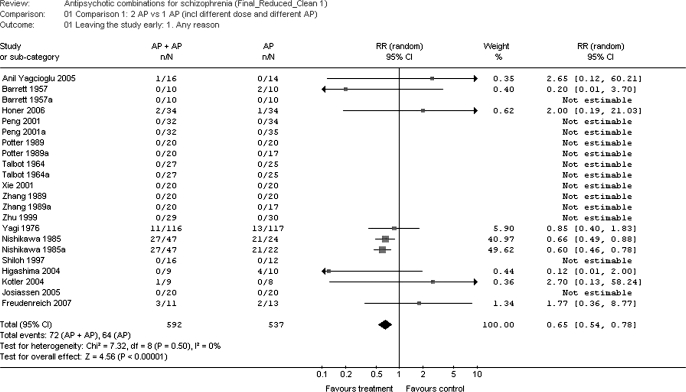
Overall, more participants dropped out due to any reason in the monotherapy groups than in the combination groups (N = 22, n = 1129, RR = 0.65, CI = 0.54–0.78, P = .00001) (figure 3). However, the RD/NNT result was not significant (P = .26), probably because 13 study arms had no dropouts in either group, excluding them from the RR analyses due to having a zero in the denominator. Analyzing specific dropout reasons, the superiority of the combination treatment was driven by less dropouts due to inefficacy of treatment (N = 18, n = 960, RR = 0.93, CI = 0.12–0.73, P = .003; NNT: again not estimable), rather than adverse events (N = 18, n = 960, RR = 1.69, CI = 0.73–3.92, P = .22).
Fig. 3.
Leaving the Study Early for any Reason.
Usable data on relapse, on secondary, scale-derived outcomes on overall efficacy (PANSS or BPRS total score) and on specific aspects of psychopathology (positive symptoms, negative symptoms, depression, agitation, functioning) were very sparse, involving no more than 4 studies. Because this low number of available study drastically limits the interpretability of the findings, these results are not shown here, but can be obtained from the authors upon request.
Limited results based on 1–11 studies found no group differences for the following adverse events: at least one adverse event, movement disorders, use of anticholinergic comedications, anticholinergic side effects, arousal, cardiovascular problems, central nervous system effects, endocrine disorders, gastrointestinal side effects, weight gain, hematology, and laboratory values including metabolic measures and drooling. The exception was prolactin levels that were significantly higher in the combination groups where sulpiride or risperidone was added to continued clozapine treatment (N = 2, n = 86, weighted mean difference: 65.1, CI = 51.1–79.1, P < 0.00001).
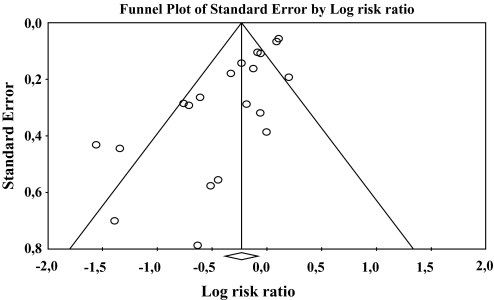
The funnel plot showed a statistically significant asymmetry (figure 4), suggesting the possibility that studies with negative results were not published (Egger's regression intercept: −2.11, df = 18, P < 0.0001).
Fig. 4.
Funnel Plot of Symmetry of Published Results.
Sensitivity Analyses.
Most of the sensitivity analyses confirmed the significant superiority for antipsychotic cotreatment vs monotherapy regarding the primary efficacy outcome, ie, lack of study-specific defined clinically significant response (table 2). Antipsychotic cotreatment was superior to monotherapy in studies that were double blind or open label (only 2 studies), conducted in China, lasted ≥10 weeks, included acutely exacerbated or chronically ill patients, started antipsychotic combinations at the beginning of treatment, used reduced or similar antipsychotic doses in the cotreatment compared with the monotherapy arms, included clozapine in the cotreatment arm, or used SGA + FGA combination, either compared with SGA or FGA monotherapy. Results were also confirmed when in the overall analyses the cotreatment arm was counted only once in the 9 trials where 2 monotherapy treatments existed. By contrast, 5 variables emerged as potential moderators of the superior efficacy of antipsychotic combinations (table 2). These included trials that were conducted outside of China, lasted <10 weeks, started antipsychotic combination treatment after nonresponse to monotherapy (mostly by prior history), or used combinations of 2 FGAs compared with 1 FGA, 2 SGAs compared with 1 SGA, or antipsychotics other than clozapine in the cotreatment arm.
Table 2.
Sensitivity Analyses of the Effect of Mediator Variables on the Outcome “Lack of Efficacy as Defined by Each Study”
| Variable | Study Arms | Patients | Risk Ratio | 95% CI | P Value | NNT | 95% CI | P Value |
| 1. Double-blind design | 20 | 1041 | 0.78 | 0.65–0.93 | 0.005 | 7 | 4–20 | 0.002 |
| Open design | 2 | 61 | 0.35 | 0.13–0.98 | 0.04 | — | — | 0.08 |
| 2. Chinese studiesa | 10 | 573 | 0.67 | 0.51–0.89 | 0.006 | 5 | 3–11 | 0.004 |
| Non-Chinese studiesa | 12 | 629 | 0.88 | 0.72–1.08 | 0.22 | — | — | 0.14 |
| 3. Acutely exacerbated patients | 7 | 319 | 0.52 | 0.30–0.90 | 0.02 | 4 | 2—12 | 0.003 |
| Chronically ill patients | 15 | 883 | 0.85 | 0.73–0.99 | 0.04 | 11 | 6—100 | 0.002 |
| 4. Costarting second antipsychotic | 17 | 1011 | 0.68 | 0.53–0.87 | 0.002 | 6 | 4—17 | 0.001 |
| Augmenting with second antipsychotic | 5 | 191 | 0.90 | 0.69–1.17 | 0.42 | — | — | 0.38 |
| 5. Comparative cotreatment doses | 12 | 707 | 0.81 | 0.67–1.00 | 0.05 | 7 | 4–100 | 0.03 |
| Reduced cotreatment doses | 10 | 495 | 0.64 | 0.43–0.93 | 0.02 | 6 | 3–50 | 0.03 |
| 6. Trial duration <10 weeks | 13 | 808 | 0.87 | 0.72–1.06 | 0.16 | - | - | 0.09 |
| Trial duration ≥10 weeks | 9 | 394 | 0.65 | 0.54–0.78 | <0.00001 | 5 | 3–20 | 0.01 |
| 7. Cotreatment including clozapine | 15 | 764 | 0.75 | 0.61–0.93 | 0.008 | 6 | 3–17 | 0.003 |
| Cotreatment not including clozapine | 7 | 438 | 0.59 | 0.28–1.25 | 0.17 | — | — | 0.37 |
| 8. FGA + FGA vs FGA | 7 | 438 | 0.59 | 0.28–1.25 | 0.17 | — | — | 0.37 |
| SGA + SGA vs SGA | 7 | 336 | 0.98 | 0.87–1.10 | 0.70 | ns | — | 0.65 |
| SGA + FGA vs FGA | 3 | 171 | 0.47 | 0.22–0.98 | 0.04 | 3 | 2–9 | 0.004 |
| SGA + FGA vs SGA | 5 | 257 | 0.59 | 0.40–0.88 | 0.009 | 3 | 2–6 | <0.0001 |
| 9. Counting cotreatment group once | 15 | 1031 | 0.81 | 0.67–0.98 | 0.03 | 8 | 4–33 | 0.01 |
Twelve patients with self-reported Asian ethnicity in the study by Honer et al. (2006)33 originated from the Chinese site.
CI, confidence interval; FGA, first-generation antipsychotic; NNT, number needed to treat; SGA, second-generation antipsychotic.
When grouping combinations by antipsychotic class, only SGA + FGA combinations, either compared with SGA or FGA monotherapy (N = 8, n = 428), were statistically superior to antipsychotic monotherapy (table 2). In the studies comparing SGA + FGA vs antipsychotic monotherapy, 351 patients (82.0%) received sulpiride, and 305 patients (71.3%) received clozapine either in monotherapy or combination treatment. In these trials, again, the majority of patients (400, 93.5%) came both from Chinese studies and had cotreatment initiated at the start of the trial. In addition, 218 patients (50.9%) had both an acute illness exacerbation and received reduced doses in the cotreatment arm.
In 13 studies and 23 treatment arms with available dose and efficacy information, the chlorpromazine equivalent dose was higher in the antipsychotic combination vs monotherapy treatment groups (N = 9, 1092.6 ± 366.7 mg/day vs N = 14, 670.0 ± 330.5 mg/day, F = 8.23, P = 0.0092). The mean chlorpromazine equivalent dose ratio in the monotherapy arms compared with the polytherapy arms was 0.64 ± 0.22 (range 0.19–0.91, 95% CI = 0.53–0.75). Like in the total sample, in the studies with available dose information, antipsychotic polytherapy was also associated with less inefficacy compared with monotherapy (N = 13, n = 669, RR = 0.63, CI = 0.43–0.92, P = 0.02, NNT = 6, CI = 3–33, P = 0.01). However, the monotherapy to polytherapy chlorpromazine equivalent dose ratio did not differ significantly in the studies with significant superiority for polytherapy (defined as P < 0.05, N = 5, see figure 2) compared with studies without significant superiority (N = 9) (0.67 ± 0.17 vs 0.61 ± 0.27, F = 0.28, P = 0.61). The same was true when superiority of combination treatment (N = 9) compared with monotherapy (N = 5) was defined as a favorable separation of the mean values independent of the 95% CI (see figure 2) (0.68 ± 0.23 vs 0.59 ± 0.17, F = 0.63, P = 0.44).
Meta-regression Analyses.
After subjecting all variables from the sensitivity analyses to a meta-regression that takes the interaction between variables into account, 3 moderators of superior efficacy of antipsychotic cotreatment emerged: similar doses in the mono- and polytherapy arm (P = 0.006, coeff = 0.48), SGA + FGA combinations (P = 0.027, coeff = 0.39) and concurrent polypharmacy initiation (P = 0.050, coeff = 0.35). Study duration (P = 0.057, coeff = 0.28), blinding (P = 0.067, coeff = 0.45), and clozapine combinations (P = 0.10, coeff = 0.39) trended toward significance.
Discussion
This first comprehensive meta-analysis of RCTs investigating antipsychotic cotreatment vs monotherapy in schizophrenia suggests superiority of antipsychotic cotreatment regarding the 2 a priori defined coprimary outcomes of all-cause discontinuation and inefficacy as defined by each study. The relevance of this finding is underscored by the fact that antipsychotic combinations were superior to monotherapy even when using a cutoff score of “much improved” on the CGI and by the relatively low NNT in the efficacy analyses that ranged mostly between 5 and 7. The interpretation of the results for specific psychopathology ratings and adverse effects was limited, however, by the low number of studies and participants with available data.
In 12 of 18 subgroup analyses, antipsychotic combinations were associated with significantly greater efficacy compared with monotherapy. Exceptions were studies with trial durations of <10 weeks and conducted outside of China, as well as those that used combinations after nonresponse to monotherapy rather than right from the start, nonclozapine antipsychotics, and combinations of 2 FGAs or 2 SGAs. However, Chinese studies used mostly designs that were also associated with increased efficacy of antipsychotic combinations. The reverse was true for non-Chinese studies, such as augmentation with a second antipsychotic after nonresponse to monotherapy and use of combinations not including clozapine.
The finding of superior efficacy of antipsychotic cotreatment compared with antipsychotic monotherapy is in contrast to the generally upheld notion that there is no support for combining 2 antipsychotics.17,25,26 However, all the studies discussed widely to date involved antipsychotic augmentation strategies of chronically ill patients refractory to clozapine.30–33 By contrast, positive effects for the antipsychotic polypharmacy were apparent the most in patients with acutely exacerbated schizophrenia and those who had 2 antipsychotics started at the same time, scenarios that have not been investigated except in studies conducted in China. Although, mean chlorpromazine equivalent doses were approximately one-third higher in the antipsychotic combination groups and although the use of therapeutic doses in both the mono- and polytherapy groups was a significant moderator variable, the mono- vs polytherapy chlorpromazine equivalent ratio did not differ between the studies finding superiority for the combination groups compared with those not finding such superiority.
In secondary sensitivity analyses, we confirmed and extended the finding of a relevant effect of study duration from a recent meta-analysis of the 4 well-known clozapine augmentation trials.60 Like in that meta-analysis where superiority of cotreatments was apparent only in the 2 studies lasting ≥10 weeks (n = 68), we also found antipsychotic cotreatments to be superior in the 6 studies (N = 9, n = 394) lasting 10 weeks or longer (NNT = 5, CI = 3–20), but not in the studies lasting <10 weeks. Obviously, this has relevant implications for the design of future studies. However, 4 of 6 studies lasting ≥10 weeks included a combination of clozapine plus either sulpiride (N = 3) or risperidone (N = 1). Therefore, it remains to be tested whether combinations that involve antipsychotics other than clozapine are significantly more effective than monotherapy and whether the time course of separation between antipsychotic cotreatment from monotherapy is similar or different if clozapine is not part of the cotreatment. Moreover, in the non-Chinese countries where clozapine is reserved for refractory patients, the 2 moderators of superior efficacy for antipsychotic cotreatment, ie, use of clozapine and simultaneous initiation of antipsychotics in acutely ill patients, present us with a dilemma. Thus, the latter finding needs to be replicated in trials of simultaneous initiation of 2 nonclozapine antipsychotics in acutely ill patients. Nevertheless, in acutely exacerbated patients with confirmed refractory schizophrenia, clozapine could be coinitiated with a second antipsychotic in patients who stopped their prior antipsychotic due to nonadherence or experienced a significant exacerbation despite treatment with a non-clozapine antipsychotic.
Unfortunately, insufficient data were available to evaluate the acute and even less data were available to evaluate the long-term safety of antipsychotic cotreatments. This is an important shortcoming as several cross-sectional and naturalistic studies reported an increased risk for diabetes27 and cardiovascular mortality28,29 associated with antipsychotic polypharmacy. However, it is unclear if these naturalistic findings are related to a direct toxic effect of specific or all antipsychotic combinations or whether it may be related to a cohort effect, in that patients who are selected for antipsychotic combination treatments are both psychiatrically and physically sicker than patients receiving monotherapy. The latter is suggested by a recent study in which the significant association between antipsychotic cotreatment and metabolic syndrome was solely explained by a significant greater prevalence of traditional risk factors compared with patients receiving antipsychotic monotherapy.17 On the other hand, controlled adverse event data in patients cotreated with a second antipsychotic are also required to assess the possibility that certain combinations may be associated with a decreased, rather than increased adverse effect burden because reduced weight gain and metabolic abnormalities have been reported after the addition of aripiprazole to clozapine.61,62
Several limitations need to be considered when interpreting these results. These include the fact that meta-analyses combine results from trials that differ in their methodology, study size and year, use of diagnostic instruments, patient and treatment selection, primary outcome variables, and study conduct. This fact was reflected by the significant heterogeneity of the results, suggesting the effect of relevant moderator and mediator variables. However, we sought to include all available data and were able to confirm the superiority of antipsychotic cotreatments in most of the secondary and sensitivity analyses that sought to disentangle relevant moderator variables, strengthening the primary finding. Importantly, although antipsychotic combination treatments did not separate statistically from monotherapy in all the sensitivity analyses, monotherapy was not superior in any of the analyses, and trends were almost all in the direction of antipsychotic cotreatment. Furthermore, despite extensive clinical utilization of antipsychotic cotreatment, data were restricted to 19 studies worldwide and limited regarding specific combinations, long-term outcomes, and psychopathology and adverse event domains. Moreover, there was a regional effect in that patients from Chinese studies predominated in many of the trials with characteristics that were also associated with superiority of the antipsychotic cotreatment. However, we only included randomized studies and the vast majority of them were double blind. Furthermore, explicit dose information was not available for all studies/treatment arms with data on similar vs reduced dose combination strategies. Besides the fact that the level of accuracy is higher regarding explicit dose information and that meta-regression results of heterogeneous studies have to be interpreted with caution, this partial lack of data could be an additional reason for the seeming disconnect between the fact that “similar dose” combination emerged as a significant moderator of the superior antipsychotic polytherapy efficacy, whereas the chlorpromazine ratio in the mono- vs polytherapy groups did not differ between studies favoring antipsychotic polypharmacy compared with those not finding an advantage of antipsychotic cotreatment. In addition, we cannot exclude a file drawer phenomenon because the funnel plot was significantly skewed toward positive studies. However, a significantly skewed funnel plot does not prove a publication bias because other reasons can lead to marked asymmetry, such as true heterogeneity or differences in the quality of assessments in smaller vs larger studies. In addition, data were insufficient to determine to what degree these results generalize to females, patients treated in the Western world, nonclozapine combinations, and whether antipsychotic cotreatment should be initiated concurrently in acutely exacerbated patients or whether it should be limited to sequential combinations in patients with insufficient response to antipsychotic monotherapy. Moreover, data were insufficient to determine the effect of specific combinations, long-term effects, and cost, further pointing to the need for more studies. Finally, the lack of conclusive adverse effect data make it difficult to weigh potential benefits against risks of antipsychotic polypharmacy because it is possible that certain relevant adverse effects may be additive some or most combinations.
Despite these limitations, this is the largest analysis of RCTs that have investigated the effect of antipsychotic cotreatment vs monotherapy in schizophrenia. Moreover, it is the first study to include all trials available without applying any language restrictions. This allowed the inclusion of previously unrecognized trials that utilized antipsychotic polypharmacy in acutely exacerbated patients and that initiated cotreatment from the beginning of the trial, rather than waiting for nonresponse to antipsychotic monotherapy, especially clozapine. In this sense, the analyzed data extend to some aspects of the general clinical practice where antipsychotic cotreatment strategies seem to be predominantly utilized in patients who have not failed clozapine. On the other hand, most combinations utilized in clinical practice also do not include clozapine, indicating the need to conduct studies with nonclozapine antipsychotic combinations that employ some of the design features that may have mediated the superior efficacy of antipsychotic combinations vs monotherapy in the examined trials. Such studies are necessary to determine whether the superiority of antipsychotic combinations generalizes to a variety of clinical settings and populations.
In summary, these data suggest that, at least, under certain circumstances, antipsychotic cotreatment may be superior to antipsychotic monotherapy regarding all-cause discontinuation and general measures of efficacy. Benefits may be apparent in acutely exacerbated patients in whom cotreatment is initiated at the beginning of treatment and when the cotreatment is administered for 10 weeks or more. Moreover, benefits of antipsychotic cotreatment did not seem to be simply a function of an increase in antipsychotic dose and resultant dopamine blockade in the polytherapy group. However, results were heterogeneous, suggesting the influence of relevant mediator and moderator variables, and there is the possibility of publication bias. Furthermore, the database was too limited to determine the effects of specific combinations, with the exception of the suggested benefits of including clozapine. It is also unclear whether potential benefits in acutely ill patients are restricted to combinations that include clozapine or that include clozapine augmented with an FGA and what the potential short-term and, particularly, long-term risks of antipsychotic combinations are. Thus, the results from this meta-analysis are insufficient to derive conclusive clinical recommendations. Rather, it provides relevant information regarding the need for specific studies and highlights methodological considerations that should guide the design of future controlled trials. Further, large-scale studies are needed, which compare antipsychotic monotherapy to antipsychotic combinations that do not necessarily involve clozapine. Further, studies combining nonclozapine SGAs with each other and with FGAs, utilized most in clinical practice, are required. Such studies should also explore the merits of combining antipsychotics in the acute phase, instead of waiting until nonresponse has occurred, last at least 10 week, and should be conducted in non-Asian countries too. To exclude the possibility of a dose effect in the cotreatment arm, one may need to consider including a “high dose” lead-in phase, high dose monotherapy arm, or both a similar dose plus a reduced dose cotreatment arm. Furthermore, recent data, suggesting that nonresponse at 1–4 weeks is highly predictive of future nonresponse,63,64 should also be taken into account when deciding at what time patients with unsatisfactory response should be randomized to antipsychotic combinations vs continued monotherapy. Until results from such studies are available, the use of antipsychotic cotreatment should most likely be reserved to severely ill patients with a documented lack of response to antipsychotic monotherapy during the acute or chronic illness phase.
Funding
The Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia, NIMH, Bethesda, MD (MH 074543-01) to J.M.K.; the Feinstein Institute for Medical Research, Manhasset, NY; the North Shore Long Island Jewish (NSLIJ) Research Institute National Institutes of Health General Clinical Research Center (MO1RR018535).
Acknowledgments
The authors would like to thank Drs Chengke Tang and Rong Xie for help with translation of studies published in Chinese. The authors would also like to thank Drs Oliver Freudenreich and Richard Josiassen for sharing unpublished data from their studies relevant for this review. The review was conducted under the auspices of the Cochrane Schizophrenia Group to which we are greatly indebted. Conflicts of interest: Christoph U. Correll has received speaker and/or consultancy honoraria from AstraZeneca, Bristol-Meyers Squibb, Eli Lilly, Intra-Cellular Therapies, Janssen, Organon, Otsuka, Pfizer, Solvay, Supernus, and Vanda. Christine Rummel-Kluge has received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen-Cilag, Eli Lilly, and Pfizer. Caroline Corves: nothing to declare. John M. Kane has received speaker and/or consultancy honoraria from Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Johnson & Johnson PRD, Otsuka, Pfizer, Inc., Wyeth, Lundbeck, Vanda, Astra-Zeneca, and PGxHealth. Stefan Leucht has received speaker and/or consultancy honoraria from SanofiAventis, BMS, Eli Lilly, Janssen/Johnson and Johnson, Lundbeck, and Pfizer; and he has received funding for research projects from EliLilly and SanofiAventis.
References
- 1.Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151(10):1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- 2.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63(10):1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(3):473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 5.Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull. 1988;24(1):62–67. [PubMed] [Google Scholar]
- 6.McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SW, Barnes TR, Davies L, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leucht S, McGrath J, Kissling W. Lithium for Schizophrenia (Cochrane Review). The Cochrane Library. 2003 doi: 10.1002/14651858.CD003834. Issue 3. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S, McGrath J, White P, Kissling W. The Cochrane Library. Issue 2 2003. Carbamazapine for Schizophrenia and Schizoaffective Psychoses (Cochrane Review) [Google Scholar]
- 10.Basan A, Kissling W, Leucht S. Valproate as an adjunct to antipsychotics for schizophrenia: a systematic review of randomized trials. Schizophr Res. 2004;70(1):33–37. doi: 10.1016/j.schres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Volz A, Khorsand V, Gillies D, Leucht S. Benzodiazepines for schizophrenia. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD006391. CD006391. [DOI] [PubMed] [Google Scholar]
- 12.Cheine M, Ahonen J, Wahlbeck K. Supplementing standard drug treatment of those with schizophrenia with beta-blocking medication (Cochrane Review) The Cochrane Library. 2001 Issue 2. [Google Scholar]
- 13.Rummel C, Kissling W, Leucht S. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD005581.pub2. CD005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedel M, Strassnig M, Schwarz MJ, Muller N. COX-2 inhibitors as adjunctive therapy in schizophrenia: rationale for use and evidence to date. CNS Drugs. 2005;19(10):805–819. doi: 10.2165/00023210-200519100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003730.pub2. Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia (Cochrane Review) The Cochrane Library. 2003 Issue 2. [Google Scholar]
- 17.Correll CU, Frederickson AM, Kane JM, Manu P. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1–3):91–100. doi: 10.1016/j.schres.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguly R, Kotzan JA, Miller LS, Kennedy K, Martin BC. Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000. J Clin Psychiatry. 2004;65(10):1377–1388. doi: 10.4088/jcp.v65n1013. [DOI] [PubMed] [Google Scholar]
- 19.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5(1):26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreyenbuhl J, Valenstein M, McCarthy JF, Ganoczy D, Blow FC. Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophr Res. 2006;84(1):90–99. doi: 10.1016/j.schres.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Tapp A, Wood AE, Secrest L, Erdmann J, Cubberley L, Kilzieh N. Combination antipsychotic therapy in clinical practice. Psychiatr Serv. 2003;54(1):55–59. doi: 10.1176/appi.ps.54.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Clark RE, Bartels SJ, Mellman TA, Peacock WJ. Recent trends in antipsychotic combination therapy of schizophrenia and schizoaffective disorder: implications for state mental health policy. Schizophr Bull. 2002;28:75–84. doi: 10.1093/oxfordjournals.schbul.a006928. [DOI] [PubMed] [Google Scholar]
- 23.Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry. 2004;65(4):500–508. doi: 10.4088/jcp.v65n0408. [DOI] [PubMed] [Google Scholar]
- 24.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 25.Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand. 2002;106(5):323–330. doi: 10.1034/j.1600-0447.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 26.Stahl SM. Antipsychotic polypharmacy: evidence based or eminence based? Acta Psychiatr Scand. 2002;106(5):321–322. doi: 10.1034/j.1600-0447.2002.2e011.x. [DOI] [PubMed] [Google Scholar]
- 27.Citrome L, Jaffe A, Levine J, Allingham B, Robinson J. Relationship between antipsychotic medication treatment and new cases of diabetes among psychiatric inpatients. Psychiatr Serv. 2004;55(9):1006–1013. doi: 10.1176/appi.ps.55.9.1006. [DOI] [PubMed] [Google Scholar]
- 28.Waddington JL, Youssef HA, Kinsella A. Mortality in schizophrenia. Antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry. 1998;173:325–329. doi: 10.1192/bjp.173.4.325. [DOI] [PubMed] [Google Scholar]
- 29.Joukamaa M, Heliovaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Schizophrenia, neuroleptic medication and mortality. Br J Psychiatry. 2006;188:122–127. doi: 10.1192/bjp.188.2.122. [DOI] [PubMed] [Google Scholar]
- 30.Shiloh R. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997:569–573. doi: 10.1192/bjp.171.6.569. [DOI] [PubMed] [Google Scholar]
- 31.Josiassen R, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162:130–136. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- 32.Anil Yagcioglu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63–72. doi: 10.4088/jcp.v66n0109. [DOI] [PubMed] [Google Scholar]
- 33.Honer WG, Thornton AE, Chen EY, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. New Engl J Med. 2006;354(5):472–482. doi: 10.1056/NEJMoa053222. [DOI] [PubMed] [Google Scholar]
- 34.Adams CE, Coutinho E, Davis JM, et al. The Cochrane Library. Chichester, UK: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 35.Higgins JPT, Green S. The Cochrane Library. Chichester, UK: Wiley and Sons; 2005. Cochrane handbook for systematic reviews of interventions 4.2.5. [Google Scholar]
- 36.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005;187:366–371. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- 37.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Der-Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M, Davey mith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 42.Haase HJ. Zur Dosierung von Neuroleptika. Ein Leitfaden für Klinik und Praxis unter besonderer Berücksichtigung psychotisch Kranker. Erlangen: Perimed Fachbuch-Verlagsbuchhandlung; 1983. [Google Scholar]
- 43.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 44.Review Manager. (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2003. RevMan Analyses [Computer program]. Version 1.0 for Windows. [Google Scholar]
- 45.Barrett WW, Elsworth RB, Clark LD, Ennis J. Study of the differential behavioral effects of reserpine, chlorpromazine, and a combination of these drugs in chronic schizophrenic patients. Dis Nerv Syst. 1957;18(6):209–215. [PubMed] [Google Scholar]
- 46.Potter WZ, Ko GN, Zhang LD, Yan W. Clozapine in China: a review and preview of US/PRC collaboration. Psychopharmacology. 1989;99:87–91. doi: 10.1007/BF00442568. [DOI] [PubMed] [Google Scholar]
- 47.Yagi G. A double-blind controlled study on the usefulness of carpipramine-chlorpromazine combination in the pharmacotherapy of chronic schizophrenic patients. Clin Eval. 1976;3:351–403. [Google Scholar]
- 48.Zhang L, Xu Y. A comparison study on treatment effect of clozapine, chlorpromazine and the combination of clozapine and chlorpromazine in schizophrenia. Chin J Nerv Ment Dis. 1989;5(5):306–308. [Google Scholar]
- 49.Freudenreich O. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1–3):90–94. doi: 10.1016/j.schres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Li X, Zhang Y, et al. The treatment effect of combined clozapine and sulpiride on negative symptoms of schizophrenia. Chin J Psychiatry. 1996;29:87–90. [Google Scholar]
- 51.Peng H, Kuang Y, Huang X. A control study of risperidone in combination with clozapine in treating refractory schizophrenia. J Mod Clin Med Bioeng. 2001;7(2):100–102. [Google Scholar]
- 52.Wang CH, Qin TF, Lin YL, Zhao XF. A clinical effect and following-up study about sulpiride and clozapine for 105 cases of the schizophrenia type. J Xinxiang Med Coll. 1994;11(2):148–151. [Google Scholar]
- 53.Xie C, Ni XL. The compared study of treating schizophrenia with risperidone combining clozapine. J Prev Med Inform. 2001;17(4):245. [Google Scholar]
- 54.Zhu Y, Zhang S, Zhang D. A controlled trial comparing chlorimipramine and sulpiride as adjunct to clozapine in the treatment of negative symptoms of schizophrenia. J Clin Psychol Med. 1999;9(4):204–205. [Google Scholar]
- 55.Chien CP, Cole JO. Depot phenothiazine treatment in acute psychosis: a sequential comparative clinical study. Am J Psychiatry. 1973;130(1):13–18. doi: 10.1176/ajp.130.1.13. [DOI] [PubMed] [Google Scholar]
- 56.Higashima M, Takeda T, Nagasawa T, et al. Combined therapy with low-potency neuroleptic levomepromazine as an adjunct to haloperidol for agitated patients with acute exacerbation of schizophrenia. Eur Psychiatry. 2004;19(6):380–381. doi: 10.1016/j.eurpsy.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa T. Prophylactic effects of neuroleptics in symptom-free schizophrenics: roles of dopaminergic and noradrenergic blockers. Biol Psychiatry. 1985;11:1161–1166. doi: 10.1016/0006-3223(85)90174-x. [DOI] [PubMed] [Google Scholar]
- 58.Talbot DR. Are tranquilizer combinations more effective than a single tranquilizer? Am J Psychiatry. 1964;121:597–600. doi: 10.1176/ajp.121.6.597. [DOI] [PubMed] [Google Scholar]
- 59.Kotler M, Strous RD, Reznik I, Shwartz S, Weizman A, Spivak B. Sulpiride augmentation of olanzapine in the management of treatment-resistant chronic schizophrenia: evidence for improvement of mood symptomatology. Int Clin Psychopharmacol. 2004;19(1):23–26. doi: 10.1097/00004850-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Paton C, Whittington C, Barnes TR. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacology. 2007;27(2):198–204. doi: 10.1097/JCP.0b013e318036bfbb. [DOI] [PubMed] [Google Scholar]
- 61.Henderson DC, Kunkel L, Nguyen DD, et al. An exploratory open-label trial of aripiprazole as an adjuvant to clozapine therapy in chronic schizophrenia. Acta Psychiatr Scand. 2006;113(2):142–147. doi: 10.1111/j.1600-0447.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 62.Karunakaran K, Tungaraza TE, Harborne GC. Is clozapine-aripiprazole combination a useful regime in the management of treatment-resistant schizophrenia? J Psychopharmacol. 2007;21(4):453–456. doi: 10.1177/0269881106068289. [DOI] [PubMed] [Google Scholar]
- 63.Correll CU, Malhotra AK, Kaushik S, McMeniman M, Kane JM. Early prediction of antipsychotic response in schizophrenia. Am J Psychiatry. 2003;160(11):2063–2065. doi: 10.1176/appi.ajp.160.11.2063. Erratum in: Am J Psychiatry 2005;162(9):1774. [DOI] [PubMed] [Google Scholar]
- 64.Leucht S, Busch R, Kissling W, Kane JM. Early prediction of antipsychotic nonresponse among patients with schizophrenia. J Clin Psychiatry. 2007;68(3):352–360. doi: 10.4088/jcp.v68n0301. [DOI] [PubMed] [Google Scholar]