Abstract
In patients with schizophrenia who do not have an optimal response to clozapine, it remains unclear if there is an evidence base to support a second antipsychotic in combination with clozapine. The present systematic review was therefore carried out to determine the efficacy of various clozapine combination strategies with antipsychotics. Relevant studies were located by searching the Cochrane Schizophrenia Group Trials Register, Medline, and Embase (up to November 2007). Only studies randomly allocating patients to clozapine plus another antipsychotic vs clozapine monotherapy were included. The search yielded 21 studies suitable for reanalysis. In 3 trials, clozapine was combined with a phenothiazine, in 8 trials with a benzamide, and in the remaining trials with risperidone. While the majority of randomized trials were not double blind, 6 studies were double-blind placebo-controlled trials. A total of 14 randomized open studies significantly favored clozapine combination strategy in terms of mean difference (random effect standardized mean difference [SMD] = −0.80, 95% confidence interval [CI] = −1.14 to −0.46); however, data extracted from 6 randomized double-blind studies did not show a statistically significant positive effect of this combination strategy in terms of mean difference (SMD = −0.12, 95% CI = −0.57 to 0.32). In terms of percentage of patients failing to show an improvement, a total of 10 randomized open studies significantly favored clozapine combination strategy (random effect relative risk [RR] = 0.64, 95% CI = 0.42 to 0.97), but data extracted from 6 randomized double-blind studies did not show a statistically significant positive effect of this combination strategy (RR = 0.91, 95% CI = 0.75 to 1.11). We conclude that the evidence base supporting a second antipsychotic in addition to clozapine in partially responsive patients with schizophrenia is weak. This weak evidence indicates modest to absent benefit.
Keywords: clozapine, combination strategies, schizophrenia
Introduction
One-fifth to one-third of people with schizophrenia are considered to have illnesses that are resistant to treatment. This usually means that these people have persistent psychotic symptoms and poor functioning despite adequate treatment with conventional or novel antipsychotic drugs.l For these people, clozapine has been shown to be the treatment of choice,2–4 with few adverse effects that result in movement problems and a beneficial effect in terms of mental state and suicide mortality.5 Clozapine is, however, only effective in producing clinically significant symptom improvement in 30%–50% of people receiving treatment. One-third to two-thirds of people still have persistent psychotic symptoms despite clozapine monotherapy of adequate dosage or have unwanted side effects that do not permit an adequate uptitration of clozapine.6 Under real-world circumstances, the need to provide effective therapeutic interventions to patients who do not have an optimal response to clozapine has been cited as the most common reason for simultaneously prescribing 2 or more antipsychotic drugs in combination treatment strategies.7 Similarly, European and American treatment guidelines recognize that the concurrent prescription of a second antipsychotic in addition to clozapine is a commonsense strategy in these partially responsive patients.8–11 However, it remains unclear if there is an evidence base to support a second antipsychotic drug in combination with clozapine. The present systematic review was therefore carried out to determine the efficacy of various clozapine combination strategies with antipsychotics in people who do not have an optimal response to clozapine.
Additionally, a methodological issue was investigated. Although systematic reviews assume that the inclusion of all available randomized evidence is crucial to limit the risk of random error or bias, typically systematic reviews include randomized trials only if study participants, those involved with their management and those collecting and analyzing clinical data, are blind to the assigned treatment. This approach is methodologically robust, but randomized evidence is inevitably lost. In this review, instead of focusing on double-blind trials only, losing this way a part of randomized evidence, we included all randomized trials irrespective of blindness, and we investigated whether lack of double blindness is associated with exaggeration of treatment effect estimates.
Methods
Types of Studies
Only studies randomly allocating patients to competitive treatment arms were included. Included and excluded studies were collected following the quality of reports of meta-analysis flow diagram.12 Considerable care was taken to exclude duplicate publications.
Types of Participants
Study participants were of either sex and any age (18+ y) with a diagnosis of schizophrenia or related disorders. Studies using any criteria to define schizophrenia and related disorders were included. Study participants had ongoing psychotic symptoms that had not responded adequately to optimal dosages of clozapine alone or had not responded adequately to dosages of clozapine that could not have been uptitrated because the size of the dose was limited by side effects. Inclusion criteria of “partial responders,” “failure to respond,” and “persistently psychotic” or description of poor functioning, side effects, and unwanted adverse reactions, as defined by the authors, were accepted.
Types of Intervention
Included trials compared clozapine plus another antipsychotic with clozapine plus placebo or clozapine alone. Included trials compared any dose and means of administration.
Outcome Measures
Efficacy outcomes were (a) group mean scores at the end of the trial, or group mean change from baseline to endpoint, on Positive and Negative Symptoms Scale or Brief Psychiatric Rating Scale or any other rating scale and (b) failure to respond to treatment: proportion of patients with no clinically significant response in global state, as defined by each of the studies.
Search Strategy for Identification of Studies
Relevant studies were located by searching the Cochrane Schizophrenia Group Trials Register using the phrase ([clozapin* or clozaril* or leponex* or denzapin* or zaponex*] in title, abstract, and index fields in REFERENCE) OR [clozapin* or clozaril* or leponex* or denzapin* or zaponex*] in interventions field in STUDY). This register is compiled by systematic searches of major databases, hand searches, and conference proceedings (see group module). Medline (1966 to November 2007) and Embase (1974 to November 2007) were additionally searched using the search term \clozapine and \randomised controlled trial or \random allocation or \double-blind method and \partial responders or \failure to respond or \persistently psychotic. Reference lists of relevant articles and previous systematic reviews were searched for other relevant studies. When outcome data were not reported, we attempted to contact the corresponding author for collecting supplemental data.
Study Selection
Material downloaded from electronic sources included details of author, institution, or journal of publication. Two reviewers (M.B. and A.C.) independently inspected all reports of identified studies. Any disagreement was solved by consensus; however, where doubt remained, we acquired the full article. Two reviewers (M.B. and A.C.) independently decided whether these then met the review criteria. No blinding to the names of authors, institutions, and journal of publication took place. We resolved any further disagreements by consensus with a third member of the review team (S.M.).
Data Extraction
Using a standardized form, 2 reviewers (A.S. and C.B.) independently extracted data on participant characteristics, intervention details, and outcome measures. Disagreements were resolved by discussion and consensus with a third member of the team (A.C.).
For dichotomous outcomes, the number of patients undergoing the randomization procedure and the number of patients who failed to respond to treatment were recorded. For continuous outcomes, the mean scores at endpoint or the mean change from baseline to endpoint, the SD or SE of these values, and the number of patients included in these analyses were extracted.13 When only the SE was reported, it was converted into SD according to Altman and Bland.14
Study Quality
The quality of reporting was assessed using the Jadad scale.15 The Jadad scale consists of 3 items pertaining to descriptions of randomization, masking, dropouts, and withdrawals. The scale ranges from 0 to 5, with higher scores indicating better reporting. A score of more than 2 of a maximum of 5 at the Jadad scale indicates high quality of reporting; a score of 2 or less indicates low quality of reporting. This threshold was derived from Moher et al.16
Data Handling
Data were initially entered and analyzed using the Cochrane Collaboration's Review Manager software (RevMan), version 4.2.10 (Cochrane Collaboration, Oxford, UK), and subsequently entered into a spreadsheet and reanalyzed using the “metan” command of STATA 9.0 (STATA Corp, College Station, TX). Outputs were cross-checked for internal consistency.
Antipsychotic Dosages
Clozapine and combination antipsychotic doses at study endpoint were converted into multiples of the defined daily dose (DDD) by dividing the prescribed daily dose (PDD) in milligrams by the DDD (PDD/DDD). The DDD is a theoretical unit of measurement defined as the assumed average maintenance daily dose for a drug, used for its main indication in adults.17 Expression of drug use in terms of multiples of DDDs allowed to calculate, for each treatment arm, a cumulative measure of drug exposure. This methodology was derived from Patten et al,18 who employed it in a study that examined international dosage differences in antidepressant clinical trials.
Statistical Analysis
For dichotomous outcomes, relative risk (RR) with a 95% confidence interval (CI) was calculated; continuous scores from different outcome scales were analyzed using SMDs with a 95% CI. A random-effects model was employed because this takes into account any differences between studies even if there is no statistically significant heterogeneity.19
Visual inspection of graphs was used to investigate the possibility of statistical heterogeneity. This was supplemented using, primarily, the I2 statistic. Where the I2 estimate was greater than or equal to 50%, we interpreted this as indicating the presence of high levels of heterogeneity.20 Visual inspection of funnel plots was additionally used to investigate the possibility of publication bias. However, this was not supplemented using asymmetry tests, considering that recent evidence warned that statistical conditions for employing asymmetry tests are absent from most meta-analyses.21
The effect of trial design on treatment estimates was investigated in an analysis that categorized trials in 2 groups: randomized, double-blind, placebo-controlled studies vs randomized nonblind (open) studies. In another subgroup analysis, we examined the effect of length of trials on treatment estimates. Studies were categorized in 2 groups: short follow-up studies (less than or equal to 10 wk) vs long follow-up studies (more than 10 wk). This threshold was derived from Paton et al,22 who suggested a positive relationship between length of trial and treatment estimates.
After data were collected, we planned a post hoc sensitivity analysis excluding the study carried out by Kreinin et al,23 who employed a crossover methodology and enrolled patients experiencing a specific unwanted adverse reaction associated with clozapine.
Results
Characteristics of Included Studies
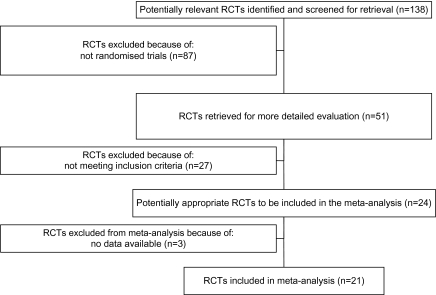
The original searches yielded 138 articles. Of these, 87 were excluded because a randomized design was not employed. The remaining 51 studies were retrieved for more detailed evaluation, 24 met the inclusion criteria, and 21 provided data suitable for reanalysis (figure 1).23–43 Only 2 randomized studies recruited 100 participants or more, and the mean length of follow-up was 13.8 weeks (SD = 19.6) (table 1). In 3 trials, clozapine was combined with a phenothiazine, in 8 trials with a benzamide, and in the remaining trials with risperidone (10 randomized controlled trials [RCTs]). The majority of randomized trials were conducted in China (15 RCTs), where patients were recruited employing diagnostic criteria not based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders or International Classification of Disorders, while 6 studies were carried out in Western countries.
Fig. 1.
Included and Excluded Studies with Reasons: The Quality Of Reports Of Meta-analysis Flow Diagram. RCT, randomized controlled trial.
Table 1.
Characteristics of Randomized Trials Assessing the Efficacy of Various Clozapine Combination Strategies With a Second Antipsychotic
| Second Antipsychotic Drug in Addition to Clozapine (WHO DDD) | Reference | Double Blind | Placebo Arm | Weeks of Follow-up | No. Allocated to Clozapine Plus AP | No. Allocated to Clozapine | DSM/ICD Criteria of Schizophrenia | Severity of Illness at Baseline (Mean) | Clozapine Dose >400 mg/d | Augmenting Drug dose (mg/d) | Efficacy Criteria—Continuous Outcome | Efficacy Criteria—Dichotomous Outcome | No Dropouts | Jadad Score |
| Amisulpride (DDD = 400) | Kreinin et al23 | Yes | Yes | 3 | 20 | 20 | Yes | Combination arm: mean PANSS = 29.80; clozapine arm: mean PANSS = 31.30 | — | 400 | PANSS | PANSS 20% or more reduction | Combination arm: 0/20; clozapine arm: 0/20 | 3 |
| Chlorpromazine (DDD = 300) | Cha et al. 199924 | No | No | 6 | 100 | 100 | No | Combination arm: mean BPRS = 56.7; clozapine arm: mean BPRS = 50.3 | — | — | BPRS | — | — | 1 |
| Pipothiazine (DDD = 10) | Jia et al27 | No | No | 12 | 26 | 24 | No | Combination arm: mean BPRS = 35.8; clozapine arm: mean BPRS = 35.9 | Yes | 100 | BPRS | Implicit | — | 1 |
| Zhu et al42 | No | No | 24 | 42 | 42 | No | Combination arm: mean BPRS = 36.2; clozapine arm: mean BPRS = 37.3 | Yes | 100 | BPRS | — | — | 1 | |
| Risperidone (DDD = 5) | Freudenreich et al 27 | Yes | Yes | 6 | 11 | 13 | Yes | Combination arm: mean PANSS = 72.4; clozapine arm: mean PANSS = 73.5 | Yes | 4.0 | PANSS | PANSS 20% or more reduction | Combination arm: 3/11; clozapine alone: 2/13 | 4 |
| Honer et al26 | Yes | Yes | 8 | 34 | 34 | Yes | Combination arm: mean PANSS = 102.5; clozapine arm: mean PANSS = 97.8 | Yes | 2.94 | PANSS | PANSS 20% or more reduction | Combination arm: 2/34; clozapine arm: 1/34 | 5 | |
| Josiassen et al28 | Yes | Yes | 12 | 20 | 20 | Yes | Combination arm: mean BPRS = 48.8; clozapine arm: mean BPRS = 41.1 | Yes | 4.43 | BPRS | BPRS 20% or more reduction | Combination arm: 0/20; clozapine arm: 0/20 | 4 | |
| Liu and Li30 | No | No | 10 | 32 | 32 | No | Combination arm: mean BPRS = 52.3; clozapine arm: mean BPRS = 52.0 | No | 4.5 | BPRS | Implicit | — | 1 | |
| Ni et al31 | No | No | 8 | 109 | 106 | No | Combination arm: mean PANSS = 83.4; clozapine arm: mean PANSS = 83.1 | No | 3.0 | PANSS | PANSS 30% or more reduction | — | 1 | |
| Peng et al32 | No | No | 8 | 32 | 34 | No | — | No | 6.0 | — | BPRS 20% or more reduction | — | 1 | |
| Wu36 | No | No | 12 | 33 | 34 | No | Combination arm: mean PANSS = 68.85; clozapine arm: mean PANSS = 68.54 | No | 4.0 | PANSS | — | — | 1 | |
| Xin et al38 | No | No | 12 | 32 | 32 | No | Combination arm: mean BPRS = 45.4; clozapine arm: mean BPRS = 45.8 | No | 4.0 | BPRS | Implicit | — | 1 | |
| Yagcioglu et al39 | Yes | Yes | 6 | 16 | 14 | Yes | Combination arm: mean PANSS = 77.4; clozapine arm: mean PANSS = 77.4 | Yes | 5.1 | PANSS | PANSS 20% or more reduction | Combination arm: 1/16; clozapine arm: 0/14 | 4 | |
| Yue et al40 | No | No | 96 | 19 | 27 | No | Combination arm: mean PANSS = 63.00; clozapine arm: mean PANSS = 62.29 | No | 2.1 | PANSS | — | — | 1 | |
| Sulpiride (DDD = 800) | Liu et al29 | No | No | 12 | 31 | 32 | Yes | Combination arm: mean BPRS = 36.0; clozapine arm: mean BPRS = 35.9 | Yes | 1127 | BPRS | Implicit | — | 1 |
| Si and Yuan33 | No | No | 12 | 50 | 50 | No | Combination arm: mean BPRS = 44.7; clozapine arm: mean BPRS = 45.8 | Yes | 1390 | BPRS | BPRS 20% or more reduction | — | 1 | |
| Shiloh et al34 | Yes | Yes | 10 | 16 | 12 | Yes | Combination arm: mean BPRS = 41.9; clozapine arm: mean BPRS = 43.5 | Yes | 600 | BPRS | BPRS 20% or more reduction | Combination arm: 0/16; clozapine arm: 0/12 | 3 | |
| Wang et al35 | No | No | 8 | 36 | 34 | No | Combination arm: mean PANSS = 17.62; clozapine arm: mean PANSS = 18.13 | No | 1000 | PANSS | Implicit | — | 1 | |
| Xao37 | No | No | 6 | 20 | 21 | No | Combination arm: mean BPRS = 49.84; clozapine arm: mean BPRS = 50.03 | No | 800 | BPRS | — | — | 1 | |
| Zhu41 | No | No | 12 | 29 | 30 | No | Combination arm: mean BPRS = 40.30; clozapine arm: mean BPRS = 40.20 | Yes | 100 | BPRS | Implicit | — | 1 | |
| Zou et al43 | No | No | 12 | 30 | 31 | No | Combination arm: mean BPRS = 44.3; clozapine arm: mean BPRS = 44.1 | — | 1200 | BPRS | BPRS 20% or more reduction | — | 1 |
Note: WHO DDD, World Health Organization–defined daily dose; AP, antipsychotics; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Classification of Disorders; PANSS, Positive and Negative Symptoms Scale; BPRS, Brief Psychiatric Rating Scale.
The 6 trials carried out in Western countries were double-blind placebo-controlled trials. Of these, 5 recruited patients who had ongoing psychotic symptoms who had not responded adequately to 400 mg/d or more of clozapine prescribed for more than 3 months at study entry.25,26,28,34,39 The sixth Western trial employed a crossover design and included inpatients with unwanted side effects associated with clozapine treatment.23 The Jadad rating indicated a high quality of reporting for the 6 Western trials only (table 1).
Efficacy on a continuous outcome was reported in 20 studies, while efficacy on a dichotomous outcome was reported in 16 studies. Unpublished outcome data were provided by Kreinin et al23 and Freudenreich et al.25 Visual inspection of funnel plots did not suggest the presence of publication bias.
Continuous Outcome Measures
While 14 randomized open studies significantly favored clozapine combination strategy in terms of mean difference (SMD = −0.80, 95% CI = −1.14 to −0.46, I2 = 85.1%), data extracted from 6 randomized double-blind studies did not show a statistically significant positive effect of this combination strategy in terms of mean difference (SMD = −0.12, 95% CI = −0.57 to 0.32, I2 = 63.1%) (figure 2). The exclusion of the study of Kreinin et al23 did not materially change the results (figure 2). In the group of open trials, the subgroup analysis by trial duration showed a statistically significant advantage for clozapine combination strategy both in short (SMD = −1.27, 95% CI = −2.20 to −0.34) and long (SMD = −0.67, 95% CI = −1.03 to −0.32) follow-up studies. In the group of double-blind trials, the subgroup analysis by trial duration showed no advantage for clozapine combination strategy both in short (SMD = 0.05, 95% CI = −0.51 to 0.63) and long (SMD = −0.49, 95% CI = −1.09 to 0.10) follow-up studies.
Fig. 2.
Random-Effects Meta-analysis of the Effect of Various Clozapine Combination Strategies on Standardized Outcome Measures.
Dichotomous Outcome Measures
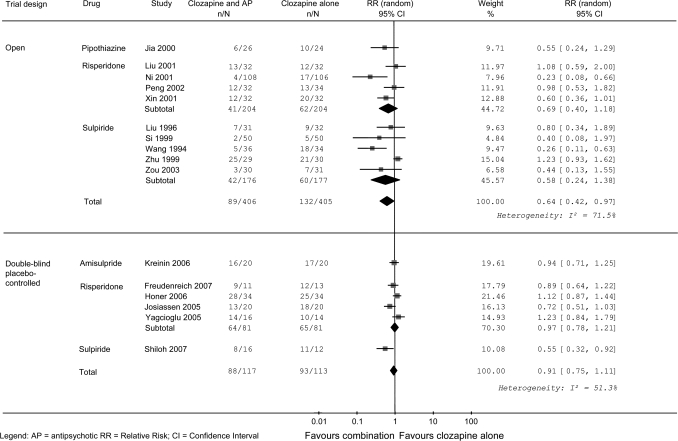
While 10 randomized open studies significantly favored clozapine combination strategy in terms of percentage of patients failing to show an improvement (RR = 0.64, 95% CI = 0.42 to 0.97, I2 = 71.5%), data extracted from 6 randomized double-blind studies did not show a statistically significant positive effect of this combination strategy (RR = 0.91, 95% CI = 0.75 to 1.11, I2 = 51.3%) (figure 3). The exclusion of the study of Kreinin et al23 did not materially change the results (figure 3). In the group of open trials, the subgroup analysis by trial duration failed to show a statistically significant advantage for clozapine combination strategy both in short (RR = 0.41, 95% CI = 0.14 to 1.16) and long (RR = 0.79, 95% CI = 0.53 to 1.16) follow-up studies. In the group of double-blind trials, the subgroup analysis by trial duration showed no statistically significant advantage for clozapine combination strategy in short follow-up studies (RR = 1.02, 95% CI = 0.88 to 1.19), while long follow-up studies significantly favored clozapine combination strategies (RR = 0.66, 95% CI = 0.49 to 0.88).
Fig. 3.
Random-Effects Meta-analysis of the Effect of Various Clozapine Combination Strategies on the Proportion of Patients Failing to Respond to Treatment.
Cumulative Measure of Drug Exposure
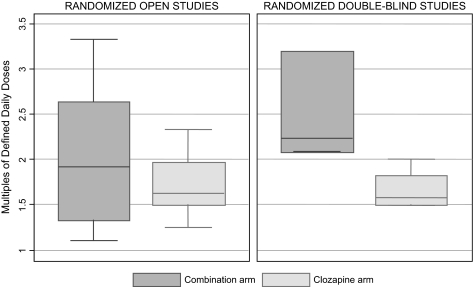
In randomized open studies, patients randomly allocated to clozapine plus a second antipsychotic received a similar total amount of antipsychotic drug compared with those allocated to clozapine alone (Spearman ρ = 0.305, P = .361) (figure 4). By contrast, in randomized double-blind studies, patients randomly allocated to clozapine plus a second antipsychotic received a higher amount of antipsychotic drug compared with those allocated to clozapine plus placebo (Spearman ρ = 1.000, P < .001) (figure 4). This different pattern of drug use was explained by a different management of clozapine dosages during the studies: while in randomized double-blind studies both treatment arms received a comparable amount of clozapine dose at study endpoint (clozapine mean dose, combination arm: 487.7 mg/d; clozapine plus placebo arm: 498.4 mg/d), in randomized open studies clozapine dose at study endpoint was significantly lower in the combination arm than in the clozapine-alone arm (clozapine mean dose, combination arm: 299.0 mg/d; clozapine alone arm: 488.1 mg/d).
Fig. 4.
Antipsychotic Drug Exposure in Patients Allocated to Clozapine Plus Another Antipsychotic (Combination Arm) Vs Clozapine Alone (Clozapine Arm).
Discussion
Although European and American treatment guidelines recommend the concurrent prescription of a second antipsychotic in addition to clozapine in partially responsive patients with schizophrenia, the present systematic review found that the evidence base supporting this treatment strategy is weak.
Systematic reviews rely on the assumption that the inclusion of all available randomized evidence limits the risk of random error or bias or both. In recent years, an ever-increasing number of randomized evidence is originating in China, and it has been emphasized that those undertaking systematic reviews should search the Chinese literature for relevant material.44 In the present systematic review of a total of 21 studies, we found 15 Chinese randomized trials. In comparison with Western trials, Chinese trials adopted different methodological standards: placebo and double blindness were never employed, the proportion of patients prematurely discontinuing treatment was never reported, and clozapine was administered at lower dosages in the combination than in the clozapine alone arm. There is evidence that lack of some methodological standards such as double blinding is associated with exaggeration of treatment effect estimates,45 and this analysis corroborated this by showing that open trials overemphasized the beneficial effect of clozapine combination strategies. It should be noted, however, that the relevance of blinding may vary according to study outcomes. It is particularly important when the response criteria are subjective, such as rating scale scores, but less important for objective criteria, such as treatment discontinuation or death.46
From a clinical viewpoint, the main message is that a second antipsychotic in addition to clozapine has modest to absent benefit. The small number of patients included in each trial and the employment of an open design in many studies make conclusions very difficult, and comparisons between individual drugs are hardly feasible. For example, 4 double-blind trials with 159 patients contributed to the negative findings for risperidone, while only 1 double-blind trial with only 28 patients contributed to the positive findings for sulpiride. For risperidone, initial randomized evidence was promising, but when the results of a large clinical trial were published26 the overall evidence base suggested lack of beneficial effect. Clearly, a similar course cannot be excluded for other drugs supported by few, albeit promising, data.
The meta-analytical approach employed in this review has limitations. First, we lumped together different antipsychotics with different mechanisms of actions and, theoretically, with potentially different effects when combined with clozapine. Clearly, this approach increased the statistical power to detect a signal of effect but required the underlying assumption that different antipsychotics have a similar pharmacological effect. A second limitation is that statistical heterogeneity was found in most analyses. Heterogeneity, ie, the extent to which different studies give similar or dissimilar results, may be the consequence of clinical, methodological, and biological variability. In the present analysis, heterogeneity may be the consequence of clinical variability, specifically in terms of patient population. We observed that no unique definition of partial responsiveness was available, and this might have led to the inclusion of slightly different patient populations across trials. In this sense, heterogeneity may be interpreted as a measure of whether a treatment is applicable to all or should be “individualized” because of variable benefits or harms in different types of patients.47
Very likely, additional randomized evidence assessing the benefit of various antipsychotics (including aripiprazole, benzamides, risperidone, pimozide, ziprasidone) will be available in the next few years (http://www.clinicaltrials.gov/). In some cases, comparative evidence will be produced to establish the relative efficacy and tolerability of combination treatment with clozapine plus one antipsychotic compared with combination treatment with clozapine plus another antipsychotic.48 Hopefully, these trials will guide clinicians in reaching a decision about optimal care in this difficult-to-treat patient population. In the meantime, clinicians prescribing a second antipsychotic to people who do not have an optimal response to clozapine should consider that the expected benefit, on the basis of available evidence, is at best modest.
Acknowledgments
We thank Lucia Muzio for translating and extracting data from the Chinese literature and the Cochrane Schizophrenia Group for providing editorial assistance. We are grateful to the Fondazione Cariverona for providing a 3-year Grant to the World Health Organization Collaborating Centre for Research and Training in Mental Health and Service Organization at the University of Verona, directed by Prof Michele Tansella. Disclosure: Corrado Barbui, Alessandra Signoretti, Serena Mulè, Marianna Boso, and Andrea Cipriani have no conflicts of interest.
References
- 1.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 2.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 3.Rosenheck R, Cramer J, Xu W, et al. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. N Engl J Med. 1997;337:809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- 4.Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990–999. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- 5.Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73(2–3):139–145. doi: 10.1016/j.schres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–526. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- 7.Sernyak MJ, Rosenheck R. Clinicians' reasons for antipsychotic coprescribing. J Clin Psychiatry. 2004;65:1597–1600. doi: 10.4088/jcp.v65n1203. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997;154(4 suppl):1–63. doi: 10.1176/ajp.154.4.1. [DOI] [PubMed] [Google Scholar]
- 9.National Collaborating Centre for Mental Health. Schizophrenia. Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. NICE Guidelines. London, UK: National Institute for Clinical Excellence; 2002. [Google Scholar]
- 10.Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry. 2004;65:500–508. doi: 10.4088/jcp.v65n0408. [DOI] [PubMed] [Google Scholar]
- 11.Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S–57S. [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Norman GR. Issues in the use of change scores in randomized trials. J Clin Epidemiol. 1989;42:1097–1105. doi: 10.1016/0895-4356(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 14.Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drug Statistic Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: WHO; 2003. [Google Scholar]
- 18.Patten S, Cipriani A, Brambilla P, Nose M, Barbui C. International dosage differences in fluoxetine clinical trials. Can J Psychiatry. 2005;50:31–38. doi: 10.1177/070674370505000107. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Chichester, UK: John Wiley & Sons, Ltd.; 2006. [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton C, Whittington C, Barnes TR. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacol. 2007;27:198–204. doi: 10.1097/JCP.0b013e318036bfbb. [DOI] [PubMed] [Google Scholar]
- 23.Kreinin A, Novitski D, Weizman A. Amisulpride treatment of clozapine-induced hypersalivation in schizophrenia patients: a randomized, double-blind, placebo-controlled cross-over study. Int Clin Psychopharmacol. 2006;21:99–103. doi: 10.1097/01.yic.0000188216.92408.69. [DOI] [PubMed] [Google Scholar]
- 24.Cha C, Hui G, Quing G. Evaluation of therapeutic effect with chlorpromazine and clozapine for treatment of schizophrenia. Xinxiang Med Stud. 1999;16:311–316. [Google Scholar]
- 25.Freudenreich O, Henderson DC, Walsh JP, Culhane MA, Goff DC. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92:90–94. doi: 10.1016/j.schres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 26.Honer WG, Thornton AE, Chen EY, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354:472–482. doi: 10.1056/NEJMoa053222. [DOI] [PubMed] [Google Scholar]
- 27.Jia Z, Zhang Z, Jin S. A controlled trial for comparing clozapine combined with pipothiazine palmitate to clozapine alone in the treatment of negative symptoms in schizophrenic patients. Herald Med. 2000;19:142–143. [Google Scholar]
- 28.Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162:130–136. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Li X, Zhang Y. A control study of clozapine in combination with sulpiride in alleviating the negative symptoms of schizophrenia. Chin J Psychiatry. 1996;29:87–90. [Google Scholar]
- 30.Liu Q, Li X. A comparative study on the efficacy of combining risperidone and clozapine in the treatment of schizophrenia. Shandong Arch Psychiatry. 2001;14:28–30. [Google Scholar]
- 31.Ni J, Jang L, Hong X. Therapeutic effects of clozapine, risperidone and their combination in the treatment of schizophrenia. Health Psychol J. 2001;3:181–182. [Google Scholar]
- 32.Peng H, Kuang Y, Huang X. A control study of risperidone in combination with clozapine in treating refractory schizophrenia. J Mod Clin Med Bioeng. 2002;7:100–102. [Google Scholar]
- 33.Si S, Yuan C. A comparative trial of the effects of sulpiride combined with clozapine in the treatment of schizophrenia. Shandong Arch Psychiatry. 1999;12:17–20. [Google Scholar]
- 34.Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry. 1997;171:569–573. doi: 10.1192/bjp.171.6.569. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Qin T, Lin Y, Zhao X. A clinical effect and following-up study about sulpiride and clozapine for 105 cases of the schizophrenia type. Xinxiang Med Stud. 1994;11:148–151. [Google Scholar]
- 36.Wu L. A control study of risperidone and clozapine combination for the treatment of refractory schizophrenia. Health Psychol J. 2002;10:135–137. [Google Scholar]
- 37.Xao H. A double-blind comparative study of the effects of sulpiride combined with clozapine in the treatment of schizophrenia. Sichuan Ment Health. 1999;12:250–251. [Google Scholar]
- 38.Xin X, Du B, Zeng Z. A controlled clinical study of risperidone and low dose of clozapine in treating schizophrenia. Herald Med. 2001;20:501–502. [Google Scholar]
- 39.Yagcioglu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66:63–72. doi: 10.4088/jcp.v66n0109. [DOI] [PubMed] [Google Scholar]
- 40.Yue H, Song L, Xu Y. A comparative trial of risperidone in the treatment of schizophrenia over two years. Shangai Arch Psychiatry. 2004;16:165–167. [Google Scholar]
- 41.Zhu Y, Zhang S, Zhang D. A controlled trial comparing chlorimipramine and sulpiride as adjunct to clozapine in the treatment of negative symptoms of schizophrenia. J Clin Psychol Med. 1999;9:204–205. [Google Scholar]
- 42.Zhu H, Deng D. A study of clozapine combined with or without pipotiazine palmitate in refractory schizophrenia. J Clin Psychol Med. 2002;12:15–17. [Google Scholar]
- 43.Zou G, Huang Y, Zou S, Yang Y. A comparative trial of the beneficial effects of sulpiride combined with clozapine in the treatment of refractory schizophrenia. J Yichun Univ. 2003;25:94–96. [Google Scholar]
- 44.Chakrabarti A, Adams CE, Rathbone J, et al. Schizophrenia trials in China: a survey. Acta Psychiatr Scand. 2007;116:6–9. doi: 10.1111/j.1600-0447.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 45.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 46.Day SJ, Altman DG. Statistics notes: blinding in clinical trials and other studies. BMJ. 2000;321:504. doi: 10.1136/bmj.321.7259.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulè S, Cipriani A, Barbui C. Aripiprazole in addition to clozapine in partially responsive patients with schizophrenia. A critical review of case series. Clin Schizophr Relat Psychoses. 2008;1:341–347. [Google Scholar]