Abstract
LY573636-sodium is a promising anti-tumour agent, which causes growth arrest and apoptosis of a variety of human solid tumours in vitro and in vivo. Moreover, studies have shown that the compound is selectively toxic towards tumour cells over their normal counterparts. This targeted effect makes LY573636 a candidate for combined therapy regimens in patients with advanced or resistant cancers. Here, we studied for the first time, the anti-tumour properties of LY573636 against a variety of human hematopoietic malignancies, including AML, B-ALL, large B-cell and mantle cell lymphoma cell lines. Cells were treated with the compound in vitro and its effect on cell proliferation, apoptosis and differentiation was determined. All cell lines underwent growth arrest in response to treatment with LY573636 in a dose-dependent manner. This antiproliferative activity was associated with induction of apoptosis, loss of mitochondrial membrane potential and induction of ROS. Furthermore, we show that LY573636 was able to induce granulocytic/monocytic differentiation of HL60 and U937 cells. LY573636, as shown before in solid tumors, is effective in hematopoietic cell lines as well. These data suggest the use of LY573636 alone or in combination with conventional chemotherapeutic regimens in hematopoietic malignancies.
Keywords: small-molecule drugs, acylsulfonamides, LY573636, hematopoietic malignancies
Introduction
LY573636-sodium (hereafter referred to as LY573636), a benzamide, N-[(5-bromo-2-thienyl)sulfonyl]-2,4-dichloro-sodium salt (Lilly Research Laboratories [LRL]), was initially identified for its selective activity against solid tumors using an in vitro soft agar disk diffusion assay. This compound has demonstrated significant dose-dependent antitumor activity against xenograft models of HCT116 and SW620 human colon cancers, as well as A375 melanoma (1) (LRL, unpublished data). Other acyl sulfonamides analogues identified during the development of LY573636 have been reported in recent years to exhibit considerable antitumor activity, particularly against human colon, lung, prostate, ovarian, and breast xenografts growing in immunodeficient mice (2, 3). In addition, toxicity of LY573636 has been evaluated in vitro and in vivo in different species including rats and beagle dogs (LRL, unpublished data). Currently, Phase 1 study of LY573636 has been completed, with bone marrow suppression, most commonly thrombocytopenia, identified as the dose-limiting toxicity. Phase 2 studies in non-small cell lung cancer, malignant metastatic melanoma, ovarian cancer, and soft tissue sarcoma are currently in progress (4). Importantly, National Cancer Institute’s COMPARE analysis did not identify any similarities between LY573636 and any known cytotoxic compound suggesting a unique mechanism of action. These findings are consistent with recent pre-clinical mechanism of action studies indicating that LY573636-induced apoptosis occurs by a mitochondrial-targeted mechanism (LRL, unpublished data). To date, no one has evaluated the effects of LY573636 against malignant hematopoietic cells; in this study, we examined the antileukemic and antilymphoma activity of the compound using a large variety of cell lines.
Materials and methods
Cell culture
B-cell acute lymphocytic leukemia (RCH, kindly provided by Dr. Janet Rowley, University of Chicago; Reh, a generous gift from Dr. Gary Gilliland, Harvard University; BALL-1, kindly provided by Dr. Sven de Vos, University of California, Los Angeles), Burkitt’s lymphoma (Daudi, American Type Culture Collection [ATCC], Manassas, VA), diffuse large B-cell lymphoma (MD901, kindly provided by Dr. Miki, Tokyo Medical and Dental University, Japan; LY4, generously donated by Ari Melnick, Albert Einstein College of Medicine), and myeloid leukemia cell lines (HL60 and U937, ATCC) were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal bovine serum (FBS), 10 U/ml penicillin and 10 mg/ml streptomycin (P/S) (Invitrogen) at 37 °C in 5 % CO2. Mantle cell lymphoma cell lines (NCEB1, Jeko1, and SP49) (5) were maintained under similar conditions in RPMI 1640 supplemented with 20 % FBS and P/S.
MTT proliferation assays
Cells were treated with various concentrations of LY573636 (kindly provided by Lilly Research Laboratories, Indianapolis, IN). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO) was performed as previously described (6). Briefly, MTT was dissolved in phosphate-buffered saline (PBS) at 5 mg/mL. Approximately 1,000 cells per well were incubated in culture medium for 96 hours in 96-well plates; and then, 10 μL of the MTT solution was added. After a 4-hour incubation, 100 μL of solubilization solution (20 % sodium dodecyl sulfate [SDS]) was added, and the mixture was incubated at 37 °C for 16 hours. In this assay, MTT was cleaved to an orange formazan dye by metabolically active cells; and the absorbance of the formazan product was measured with an enzyme-linked immunoabsorbent assay (ELISA) reader at 540 nm.
Apoptosis analyses
Annexin V staining was performed as previously described (7). Briefly, after a 72-hour treatment period with 40 μM LY573636, cells were washed with cold PBS, resuspended in 1X binding buffer (BD Biosciences, Pharmingen, San Jose, CA), and incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V and 2.5 μg/ml propidium iodide (PI) at room temperature for 15 minutes in the dark. Prior to analysis, 80 % 1X binding buffer was added and analysis was performed within one hour. All studies were performed using the flow cytometry (FACScan, Becton Dickinson, Franklin Lakes, NJ).
Mitochondrial membrane potential
Cells were incubated with either 1, 10 or 40 μM LY573636 for 72 hours at 37 °C. Loss of mitochondrial membrane potential was analyzed using the lipophilic cationic dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide), according to the manufacturer’s protocol (Cell Technology, Mountain View, CA). Briefly, cells were pelleted and resuspended in 1X JC-1 reagent and incubated for 15 minutes at 37 °C. Cells were washed twice with culture media and resuspended in complete media, and analyses were immediately performed by flow cytometry (FACScan).
ROS determination
Cells were incubated with 40 μM LY573636 for either 24, 48 or 72 hours at 37 °C. They were washed once with complete media, incubated for 30 minutes at 37 °C with 10 μM DCFDA (2′,7′-dichlorofluorescein diacetate) (Sigma-Aldrich) in complete media, and analyses were immediately performed by flow cytometry (FACScan).
Study of differentiation
Cells were incubated with various concentrations of LY573636 for four days at 37 °C. Cells were washed with phosphate-buffered saline (PBS) and incubated for 30 minutes with either R-phycoerythrin (RPE)-conjugated murine anti-human CD11b or negative control RPE-conjugated murine IgG1 antibody (DAKO, Carpinteria, CA). Expression of cell surface antigens was determined by flow cytometry (FACScan).
Results
To determine if LY573636 inhibits the proliferation of malignant hematopoietic cells, different cell lines were cultured in various concentrations of LY573636 (0.5 – 40 μM) for 96 hours and growth inhibition was measured by MTT. The mean ±standard deviation of duplicate experiments performed in triplicate were plotted and the concentration inhibiting 50 % growth (ED50) was calculated (Table 1, with representative dose-response curves in Figure 1). In agreement with the antiproliferative effects observed in solid tumors (LRL, unpublished data), our results demonstrate that LY573636 interfered with the growth potential of a wide panel of human malignant hematopoietic cell lines, including AML (ED50, 7 and > 40 μM for HL60 and U937 cells, respectively), B-ALL (mean ED50, 13 μM), as well as Burkitt’s lymphoma, diffuse large B-cell lymphomas, and mantle cell lymphomas (mean ED50, 21 μM) (Table 1). Following this initial screen, we chose to limit the remainder of our studies to a few select cell lines from each subtype: two AML cell lines (HL60 and U937) with disparate ED50’s, as well as Reh (B-ALL), MD901 (diffuse large B-cell lymphoma), and Jeko1 (mantle cell lymphoma) (Figure 1).
Table I. LY573636 inhibits growth of various human leukemia and lymphoma cell lines.
Cells were cultured for 96 hours with varying concentrations of LY573636 (0.5 – 40 μM), and cell growth was measured by MTT assay. Data were plotted, and the effective dose which inhibited 50 % growth (ED50) was calculated for each cell line. Results represent the mean ED50 of duplicate experiments done with triplicate wells per point.
| Cell lines | Cell type | ED50 (μM LY573636) |
|---|---|---|
| HL60 | Acute myeloid leukemia | 7 |
| U937 | Acute myeloid leukemia | > 40 |
| Reh | B-cell acute lymphocytic leukemia | 23 |
| RCH | B-cell acute lymphocytic leukemia | 12 |
| BALL1 | B-cell acute lymphocytic leukemia | 5 |
| MD901 | Diffuse large B-cell lymphoma | 19 |
| LY4 | Diffuse large B-cell lymphoma | 25 |
| Daudi | Burkitt’s lymphoma | 31 |
| Jeko1 | Mantle cell lymphoma | 16 |
| SP49 | Mantle cell lymphoma | 21 |
| NCEB1 | Mantle cell lymphoma | 13 |
Figure 1. Growth inhibitory activity of LY573636-sodium against HL60, U937, Reh, MD901, and Jeko1 cells.
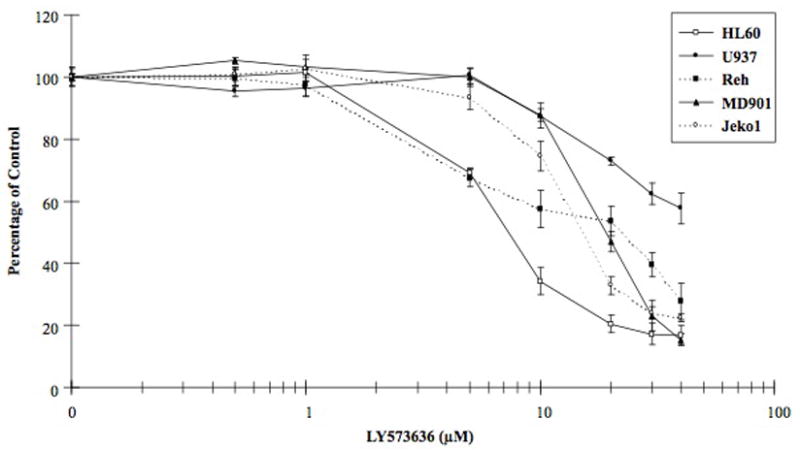
MTT assay measuring proliferation of HL60 (□), U937(●), Reh (■), MD901 (▲), and Jeko1 (○) human hematopoietic cell lines in the presence of varying doses (0.5 – 40 μM) of LY573636 for 96 hours. Results for untreated control cells were set at 100 %; experimental data are shown as a percentage of control. Data represent mean ±standard error of duplicate experiments done with triplicate samples per point.
Previous results had demonstrated that LY573636 induced apoptosis in colorectal and ovarian cancer and melanoma cell lines (LRL, unpublished data). To assess whether the antiproliferative effects of LY573636 observed by MTT assay were attributed to an induction of cell death, HL60, U937, Reh, MD901, and Jeko1 cells were cultured in 40 μM LY573636 or control for 24, 48 and 72 hours. While no significant effects were evident after 24 and 48 hours (data not shown), HL60, Reh, and MD901 cells demonstrated between a 3.3- to 4.5-fold increase in the percentage of Annexin V- and PI-double positive cells following a 72-hour incubation period. Nevertheless, none of the cell lines exhibited greater than 10 % apoptotic cells (Table 2). U937 cells which were the least sensitive to the growth inhibitory action of LY573636 (Figure 1, as measured by MTT), still showed an almost 3-fold increase in apoptotic cells in the presence of LY573636 compared to diluent control cells. In contrast, Jeko1 cells did not exhibit any significant induction of apoptosis.
Table II. LY573636 induces apoptosis in HL60, Reh, and MD901 cells.
Cells were cultured for 72 hours with 40 μM LY573636, and the percentage of Annexin V- and PI-double positive cells was determined by flow cytometry. Data represent mean of triplicate dishes.
| Cell lines | Percentage of AnnexinV+/PI+ cells | |
|---|---|---|
| control | LY573636 | |
| HL60 | 3 | 10 |
| U937 | 2 | 6 |
| MD901 | 3 | 10 |
| Reh | 2 | 9 |
| Jeko1 | 2 | 2 |
Mitochondrial membrane permeability and the consequential collapse of the electrochemical potential across the mitochondrial membrane are early key steps in the induction of apoptosis, and occur through the formation of membrane pores via activation of pro-apoptotic proteins and release of cytochrome-C into the cytoplasm (8). LY573636-induced apoptosis in solid tumor cell lines was found to be mediated by the intrinsic, mitochondrial-mediated cell death pathways, associated with a reduction in cellular ATP levels (LRL, unpublished data). To determine whether this mechanism also applied to hematopoietic malignant cell lines used in this study, we looked for changes in mitochondrial membrane potential as a function of mitochondrial damage using the JC-1 dye. In healthy cells, positively charged JC-1 molecules accumulate in aggregates within the negatively charged mitochondria, and emit red fluorescence at the 590 nm spectrum. In apoptotic cells, however, collapse of the mitochondrial membrane potential interferes with the accumulation of the JC-1 molecules, which remain in the cytoplasm in monomeric form, emitting green fluorescence at the 527 nm spectrum. Hence, healthy cells can easily be distinguished from apoptotic cells by their loss of mitochondrial membrane potential.
HL60, U937, Reh, MD901, and Jeko1 cells were cultured with increasing concentrations of LY573636 (1, 10 and 40 μM) for 72 hours (time point at which induction of apoptosis was observed), and changes in the levels of monomeric JC-1 (green fluorescence) were assessed (Figure 2). In HL60, Reh, and MD901 cells, a dose-dependent increase in green fluorescence, corresponding to a decrease in mitochondrial membrane potential was observed following treatment with LY573636. HL60 cells demonstrated a mean 6.6-fold increase in green fluorescence compared to control cells (Figure 2A), while Reh and MD901 exhibited a mean 77- and 81-fold increase, respectively, after culture with 40 μM LY573636 (Figures 2C–D). Thus, LY573636-induced apoptosis observed in HL60, Reh, and MD901 cells was coincident with a decrease in their mitochondrial membrane potential. Moreover, U937 cells, which exhibited a lower sensitivity to the antiproliferative (MTT) and pro-apoptotic (Annexin V positive) activities of LY573636, likewise had a smaller shift toward green fluorescence (only ~2.7-fold) (Figure 2B). Remarkably, although Jeko1 cells did not reveal a significant increase in the percentage of cells undergoing apoptosis, they did, however, demonstrate a 15-fold increase in their levels of green fluorescence (Figure 2E).
Figure 2. LY573636-sodium induces mitochondrial membrane collapse of HL60, Reh, MD901, and Jeko1 cells.
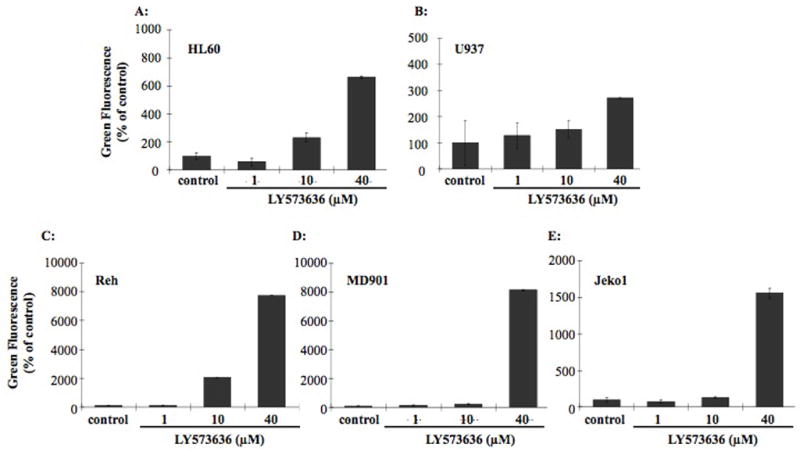
HL60 (A), U937 (B), Reh (C), MD901 (D), and Jeko1 (E) cells were incubated for 72 hours with increasing amounts of LY573636 (1, 10 or 40 μM). Loss of mitochondrial membrane potential was determined by measuring changes in the levels of green fluorescence (monomeric JC-1) at the 527 nm spectrum. Data represent the mean ±SE of three separate experiments.
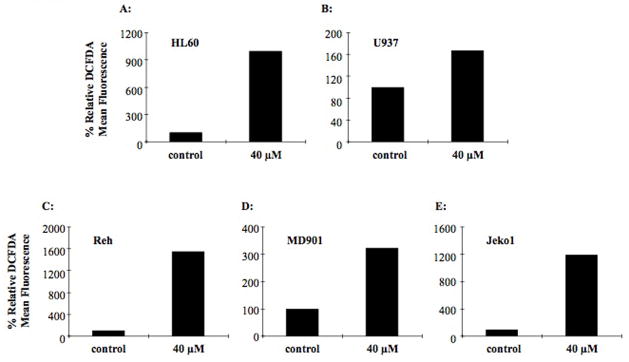
Generation of significant levels of reactive oxygen species (ROS) in a cell occurs as a late event in induction of apoptosis after the activation of caspases (8). Given our observations of enhanced apoptosis, coupled with the loss of mitochondrial membrane potential in various cell lines, we assessed the levels of ROS production after exposure to LY573636. Following treatment with LY573636 (40 μM; 24, 48, or 72 hours), HL60, U937, Reh, MD901, and Jeko1 cells were incubated with DCFDA, which generates fluorescent diclorofluroescein upon oxidation. We observed a significant time-dependent increase in levels of ROS production (measured by relative DCFDA mean fluorescence) in HL60, Reh, and Jeko1 cells (>10-fold) (Figures 3A, C, E). The MD901 cells generated an approximate 3-fold rise in ROS levels after 72 hours of treatment (Figure 3D). Once again, the least sensitive cell line, U937, did not have a significant enhancement in DCFDA fluorescence, with relative levels comparable to control even after 72 hours of exposure (Figure 3B). This is consistent with previous findings in HCT116 colon cancer cells that showed a dose-dependent, congruent induction of apoptosis and ROS upon LY573636 treatment (LRL, unpublished data).
Figure 3. LY573636-sodium induces ROS generation in HL60, Reh, MD901, and Jeko1 cells.
HL60 (A), U937 (B), Reh (C), MD901 (D), and Jeko1 (E) cells were cultured for 72 hours with 40 μM LY573636. ROS production was determined by relative mean fluorescence levels of DCFDA. The figure shows data of one representative experiment out of three independent experiments. Results for untreated control cells were set at 100 %, with remaining data shown as a percentage of control.
Because induction of differentiation represents a potential therapeutic strategy in AML, we investigated the ability of LY573636 to induce myeloid differentiation. Myeloid leukemic cells, such as HL60 and U937 used in this study, express specific cell surface antigens as they undergo differentiation (9). Accordingly, we evaluated by flow cytometry the ability of LY573636 to induce differentiation as measured by expression of the monocyte- and granulocyte-marker, CD11b. Following a 4-day exposure to a non-apoptotic dose of LY573636 (20 μM), both HL60 and U937 exhibited a dose-dependent increase in the percentage of CD11b expressing cells, with greater than 40 % and 20 % of the cells becoming positive, respectively (Figure 4).
Figure 4. LY573636-sodium treatment induces CD11b expression in AML cell lines.
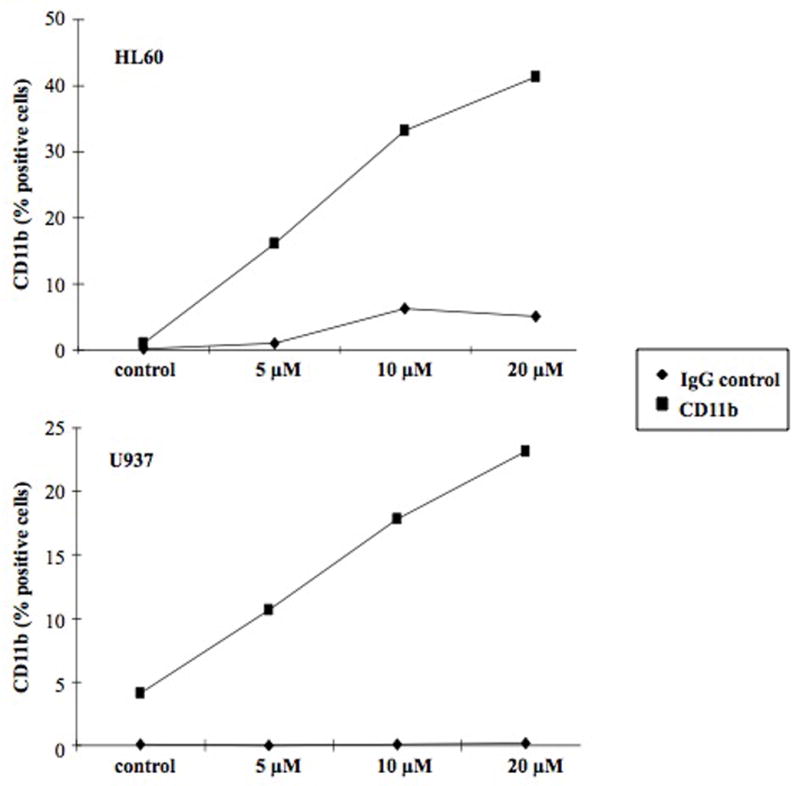
Following a 96-hour treatment with 20 μM LY573636, HL60 (top) and U937 (bottom) cells were incubated with RPE-conjugated CD11b-specific antibody (square) or RPE-conjugated IgG1 control antibody (diamond), and flow cytometry was performed. Results of each figure are representative of two independent experiments.
Discussion
Acyl sulfonamides, such as LY573636, were initially recognized for their significant antiproliferative activities in solid tumor cell lines, but their mechanism of action was unknown (1). Subsequent studies have revealed that LY573636 induces apoptosis via a mitochondrial-mediated mechanism that appears unique among other anti-cancer compounds. Little is known of the effects of LY573636 on hematopoietic malignancies, however, which prompted our study of these cells.
Consistent with findings in solid tumors (LRL, unpublished data), the antiproliferative effects of LY573636 observed in our studies demonstrate that LY573636 inhibits growth and induces apoptosis in diverse human malignant hematopoietic cell lines. In most cell lines, a dose-dependent decrease of membrane potential and elevated levels of ROS upon treatment of sensitive cell lines was associated with apoptosis. Importantly, these effects were achieved at LY573636 concentrations that ranged clearly below the maximum tolerated targeted Cmax concentration in patients (420 μg/ml or 960 μM) identified in the phase I study JZAA and taken forward into Phase 2 (4).
Surprisingly, apoptosis rates upon a 72-hour treatment with LY573636 did not exceed 10 % of the cells, but still this demonstrates a 3 to 4.5-fold increase of apoptotic cells. Compared to the 8-fold increase in HCT-116 colon cancer cells upon treatment with lower concentrations of LY573636 (LRL, unpublished data), the hematopoietic cells seem to be less sensitive to this compound. Nevertheless, LY57636 alone or in combination might be useful as therapy for leukemia or lymphoma given the relatively long half-life in humans (approximately 11 days), which could provide prolonged exposure to potentially active drug concentrations (4) (LRL, unpublished data). Furthermore, the compound appears to have a novel mechanism of action supporting the further exploration of LY573636 as a therapeutic agent for acute leukemias and lymphomas.
Acknowledgments
This work was supported in part by the Sheryl Weissberg Lymphoma Research Foundation (HPK), the Tower Cancer Research Foundation Fellowship (TH, SG), the Deutsche Krebshilfe (SG) and a gift from Lilly Research Laboratories. HPK holds the Mark Goodson Chair of Oncology Research at Cedars-Sinai Medical Center and is a member of the Molecular Biology Institute and Jonsson Comprehensive Cancer Center at UCLA.
References
- 1.Mader MM. Novel antiproliferative antitumor agents. Curr Opin Drug Discov Devel. 2005;8:613–618. [PubMed] [Google Scholar]
- 2.Corbett TH, White K, Polin L, Kushner J, Paluch J, Shih C, Grossman CS. Discovery and preclinical antitumor efficacy evaluations of LY32262 and LY33169. Invest New Drugs. 2003;21:33–45. doi: 10.1023/a:1022912208877. [DOI] [PubMed] [Google Scholar]
- 3.Lobb KL, Hipskind PA, Aikins JA, et al. Acyl sulfonamide anti-proliferatives: benzene substituent structure-activity relationships for a novel class of antitumor agents. J Med Chem. 2004;47:5367–5380. doi: 10.1021/jm030594r. [DOI] [PubMed] [Google Scholar]
- 4.Ilaria RL, Jr, Simon GR, Sovak M, et al. Phase I study of LY573636-sodium, an acylsulfonamide anti-cancer compound with a novel mechanism of action, administered as 2-hour IV infusion in patients with advanced solid tumors; Journal of Clinical Oncology; 2007. ASCO Annual Meeting Proceedings Part I. [Google Scholar]
- 5.Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai T, O’Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95:896–905. doi: 10.1093/jnci/95.12.896. [DOI] [PubMed] [Google Scholar]
- 7.Hisatake J, O’Kelly J, Uskokovic MR, Tomoyasu S, Koeffler HP. Novel vitamin D(3) analog, 21-(3-methyl-3-hydroxy-butyl)-19-nor D(3), that modulates cell growth, differentiation, apoptosis, cell cycle, and induction of PTEN in leukemic cells. Blood. 2001;97:2427–2433. doi: 10.1182/blood.v97.8.2427. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 9.Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]