Abstract
As well as catalyzing the conversion of cholesterol to pregnenolone for steroid synthesis, cytochrome P450scc (P450scc) can also metabolize vitamins D2 (D2) and D3 (D3). Two products of D2 metabolism by P450scc, 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2, have been identified and shown to exert biological activity on cultured keratinocytes. The aim of this study was to fully characterize the metabolism of D2 by P450scc, including identifying additional products and determining the kinetics of D2 metabolism. Two new products were isolated by reverse-phase high-performance liquid chromatography: a dihydroxy metabolite with a hydroxyl group at C20 plus another unidentified position, and a trihydroxy metabolite identified by NMR as 17,20,24-trihydroxyvitamin D2. Kinetics of D2 metabolism was determined with substrate solubilized by 2-hydroxypropyl-β-cyclodextrin or incorporated into phospholipid vesicles. In 2-hydroxypropyl-β-cyclodextrin, D2 was hydroxylated at C20 with a kcat/Km 5-fold lower than that for cholesterol metabolism. 20-Hydroxyvitamin D2 was hydroxylated with a similar kcat/Km to D2, whereas 17,20-dihydroxyvitamin D2 was hydroxylated with a lower kcat/Km than that for D2 in 2-hydroxypropyl-β-cyclodextrin. In vesicles, D2 displayed a high Km relative to that for cholesterol, but hydroxylation resulted in products that could be further hydroxylated with relatively low Km values. We conclude that P450scc catalyzes three sequential hydroxylations of D2 producing 20-hydroxyvitamin D2, 17,20-dihydroxyvitamin D2, and 17,20,24-trihydroxyvitamin D2, which dissociate from the active site of P450scc and accumulate in the reaction mixture. D2 metabolism occurs with lower efficiency (kcat/Km) than that observed for both cholesterol and D3 metabolism by P450scc.
Vitamin D2 (D2) is produced by the action of UVB irradiation on ergosterol, a 5,7-diene phytosterol, which is synthesized by fungi and phytoplankton but not in the animal kingdom (Holick, 2003). It is the major form of dietary vitamin D in humans (Holick, 2004). Like vitamin D3 (D3), it is converted to its hormonally active form, 1α,25-dihydroxyvitamin D2 [1,25(OH)2D2], by 25-hydroxylation in the liver, followed by 1-hydroxylation in the kidney (Holick, 2003; Prosser and Jones, 2004). 25-Hydroxyvitamin D2 is the major circulating form of D2. It can be converted to the active form in other tissues besides the kidney, such as skin, prostate, breast, and colon, which express CYP27B1, the enzyme catalyzing 1-hydroxylation (Holick, 2003, 2004). It has previously been reported that D2 is less effective in maintaining circulating levels of 25-hydroxyvitamin D than D3 (Armas et al., 2004; Brown et al., 2004), but a more recent report suggests that it is equally as effective (Holick et al., 2008). Besides regulation of calcium metabolism, 1,25(OH)2D2 and 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] also exert effects on the immune system and regulate cellular proliferation and differentiation of a range of cells, including keratinocytes (Holick, 2003, 2004; Bikle et al., 2004; Mitani et al., 2004).
Cytochrome P450scc (CYP11A1; P450scc) catalyzes the first step in steroid synthesis, the cleavage of the side chain of cholesterol to produce pregnenolone (Tuckey, 2005). This reaction involves hydroxylations of the cholesterol side chain at C22 and C20, followed by oxidative cleavage of the C-C bond between carbons 20 and 22 (Hume et al., 1984; Tuckey and Cameron, 1993; Tuckey, 2005). P450scc can also act on D2, producing 20-hydroxyvitamin D2 [20(OH)D2] and 17,20-dihydroxyvitamin D2 [17,20(OH)2D2], as well as on ergosterol (provitamin D2) generating 17,24-dihydroxyergosterol, but without the cleavage of the D2 or ergosterol side chain (Slominski et al., 2005a, 2006). We have recently shown that P450scc can hydroxylate D3 at C17, C20, and C23, producing eight identifiable products with one, two, or three hydroxyl groups (Tuckey et al., 2008a). The biological activity of the major product, 20-hydroxyvitamin D3, has been tested on human epidermal keratinocytes where it inhibits cell proliferation and stimulates differentiation with a potency similar to that of 1,25(OH)2D3 (Zbytek et al., 2008). 20,23-Dihydroxyvitamin D3 and 17,20,23-trihydroxyvitamin D3 [17,20,23(OH)3D3], other products of D3 metabolism by P450scc, similarly display biological activity on skin cells (Janjetovic et al., 2008). Preliminary studies on 20(OH)D2 and 17,20(OH)2D2 indicate that these metabolites of P450scc action on D2 also inhibit skin cell proliferation and promote differentiation (Slominski et al., 2006). Thus, these new metabolites of vitamin D produced by the action of P450scc are of interest because of their possible in vivo formation and their potential use as therapeutic agents for the treatment of hyperproliferative disorders, including cancer (Slominski et al., 2004, 2005b, 2006; Tuckey et al., 2008a,b; Zbytek et al., 2008).
The major products of D2 metabolism by P450scc were previously detected by the relatively insensitive technique of thin-layer chromatography (TLC) (Slominski et al., 2006). In the present study we have used reverse-phase high-performance liquid chromatography (HPLC) to isolate two further metabolites of P450scc action on D2, one of which was identified by NMR as 17,20,24-trihydroxyvitamin D2 [17,20,24(OH)3D2]. We also report the kinetics of the three hydroxylations catalyzed by P450scc leading to the production of 17,20,24(OH)3D2.
Materials and Methods
Materials. D2, 2-hydroxypropyl-β-cyclodextrin (cyclodextrin), dioleoyl phosphatidylcholine, bovine heart cardiolipin, and NADPH were from Sigma-Aldrich (Castle Hill, NSW, Australia). Adrenodoxin reductase, adrenodoxin, and P450scc were purified from bovine adrenal mitochondria (Tuckey and Stevenson, 1984a,b). The concentration of P450scc was determined from its CO-reduced minus reduced difference spectrum using an extinction coefficient of 91,000 M–1cm–1 for the absorbance difference between 450 and 490 nm (Omura and Sato, 1964).
Preparation of Hydroxyvitamin D2 Derivatives. 20(OH)D2 and 17,20(OH)2D2 were prepared enzymatically from 50-ml incubations of 50 μM D2 with 2.0 μM P450scc, 10 μM adrenodoxin, and 0.4 μM adrenodoxin reductase for 3 h in 0.9% cyclodextrin in a scaled-up version of the incubations described below for measuring P450scc activity. Products were extracted with dichloromethane and purified by preparative TLC as described before (Slominski et al., 2006). The purity of these samples was checked before use by HPLC, and if less than 98%, samples were further purified by preparative HPLC using a Brownlee Aquapore C18 column (25 cm × 10 mm, particle size 20 μm). Samples were applied in 64% methanol and eluted with a 64 to 100% methanol gradient in water at a flow rate of 1.5 ml/min (Tuckey et al., 2008c). Trihydroxyvitamin D2 [identified as 17,20,24(OH)3D2, see under Results] was produced from a 20-ml incubation of 50 μM 17,20(OH)2D2 with 2 μM P450scc for 3 h at 37°C in 0.9% cyclodextrin. The product was extracted with dichloromethane and purified by reverse-phase HPLC as described below for cytochrome P450 activity measurements, except that elution was performed isocratically using 68% methanol in water. This yielded 50 μg of product for NMR analysis. The concentrations of hydroxyvitamin D2 products were measured using an extinction coefficient of 18,000 M–1cm–1 at 263 nm (Hiwatashi et al., 1982).
Measurement of P450scc Activity. The measurement of P450scc activity with D2 in vesicles or cyclodextrin was carried out as previously described for D3 (Tuckey et al., 2008b). For vesicles, the incubation mixture comprised phospholipid vesicles (510 μM phospholipid), bovine P450scc (0.5–2 μM), 10 μM adrenodoxin, 0.4 μM adrenodoxin reductase, 2 mM glucose 6-phosphate, 2 U/ml glucose 6-phosphate dehydrogenase, and 50 μM NADPH. Substrates were present in the vesicles at concentrations ranging from 0.01 to 0.4 mol/mol phospholipid, depending on the experiment (see under Results). For the cyclodextrin system, 0.45% cyclodextrin containing the solubilized substrate at concentrations ranging from 2 to 80 μM replaced the vesicles. Samples were preincubated for 8 min, reactions initiated by the addition of NADPH, and incubations carried out at 37°C in a shaking water bath. Samples were extracted with dichloromethane and prepared for HPLC as before (Tuckey et al., 2008b). Incubation times were kept short (2–5 min) in experiments designed to measure the kinetic constants for P450scc catalysis to ensure initial rates were measured, as determined from time course experiments. Short incubation times also avoided the more complex kinetics that occurred later in the incubation as products from the initial hydroxylation became substrates for subsequent hydroxylations.
HPLC Analysis of Vitamin D Metabolites. Analytical reverse-phase HPLC for P450scc activity measurement was performed using a PerkinElmer Life and Analytical Sciences (Waltham, MA) HPLC equipped with a C18 column (Brownlee Aquapore, 22 cm × 4.6 mm, particle size 7 μm). Samples were applied in 64% methanol and eluted with a linear gradient of 64 to 100% methanol in water at a flow rate of 0.5 ml/min. Products were detected with a UV monitor at 265 nm and quantitated as before (Tuckey et al., 2008b,c).
NMR of Trihydroxyvitamin D2. Trihydroxyvitamin D2 and D2 were dissolved in 60 μl of methanol-d4 (99.8% d; Cambridge Isotope Laboratories, Inc., Andover, MA) and transferred to 3-mm Shigemi NMR tubes (Shigemi Inc., Allison Park, PA). NMR spectra were acquired on a Varian, Inc. (Palo Alto, CA) Inova 500-MHz NMR spectrometer equipped with a 3-mm inverse probe. Temperature was regulated at 296.5 K. All the NMR data were processed with standard parameters. Chemical shifts were referenced to the residue solvent peak (proton at 3.31 ppm and carbon at 49.15 ppm). Positions of the three hydroxyl groups in the trihydroxyvitamin D2 were determined by analysis of the acquired NMR spectra and comparison with those of parent D2.
Results
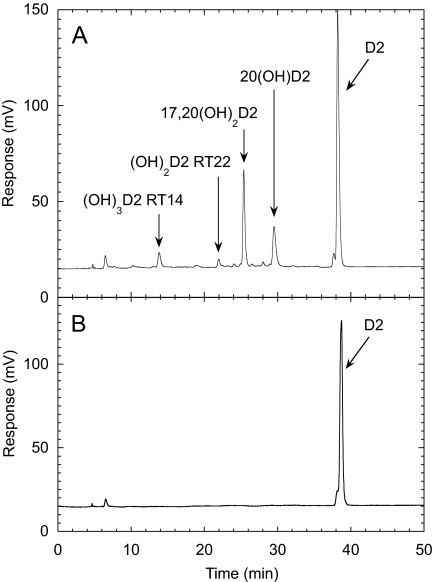
Pathways for the Metabolism of D2 by P450scc. Incubation of P450scc with D2 in 0.45% cyclodextrin and analysis of metabolites by reverse-phase HPLC gave four products in sufficient quantities for characterization, which were not present in the zero time control incubation (Fig. 1). The two major metabolites were 20(OH)D2 and 17,20(OH)2D2, which we have previously isolated by TLC and identified by NMR (Slominski et al., 2006). The additional two products detected by HPLC had retention times of 14 and 22 min and had UV spectra similar to D2. The electrospray mass spectrum of the product with retention time (RT) = 22 min in Fig. 1 showed the most abundant ion at m/z = 451.1 (428.1 + Na+), and ions with m/z = 467.1 (428.1 + K+) and m/z = 879.3 (2M + Na+) from which the sample was identified as dihydroxyvitamin D2. The electrospray mass spectrum of the RT = 14 min product showed the most abundant ion at m/z = 467.1 (444.1 + Na+) and ions with m/z = 483.1 (444.1 + K+) and 911.1 (2M + Na+), corresponding to trihydroxyvitamin D2. This product was subsequently identified as 17,20,24(OH)3D2 by NMR (see below). This name describing its full identification will be used throughout to avoid confusion.
Fig. 1.
D2 metabolites produced by P450scc. A, HPLC chromatogram showing products from the metabolism of D2 (50 μM) in 0.45% cyclodextrin by P450scc (1.0 μM) from a 1-h incubation in a reconstituted system containing adrenodoxin (15 μM) and adrenodoxin reductase (0.4 μM). B, chromatogram for a control incubation (zero time) showing the D2 substrate. RT, retention time in minutes. Abbreviations for products are (OH)D2, monohydroxyvitamin D2; (OH)2D2, dihydroxyvitamin D2; (OH)3D2, trihydroxyvitamin D2.
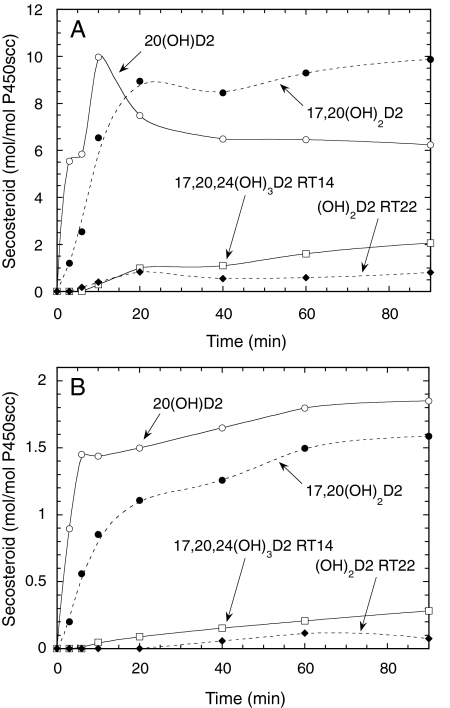
A time course for the metabolism of D2 in 0.45% cyclodextrin is shown in Fig. 2A. 20(OH)D2 was the major product for the first 10 min of incubation, but 17,20(OH)2D2 became the major product after this time. A lag in the time course for the production of 17,20(OH)2D2 was apparent, indicating that some accumulation of the immediate substrate, 20(OH)D2, was necessary for its synthesis. Likewise, lags were seen in the time courses for production of the dihydroxyvitamin D2 metabolite with RT = 22 min and 17,20,24(OH)3D2 (RT = 14 min).
Fig. 2.
Hydroxylation of D2 by P450scc in cyclodextrin and vesicles. A, time course for metabolism of D2 (50 μM) dissolved in 0.45% cyclodextrin and incubated with 1.0 μM P450scc. B, time course for metabolism of D2 in phospholipid vesicles containing 0.2 mol D2/mol phospholipid incubated with 2.0 μM P450scc. Samples were taken at the times indicated, and products were measured by HPLC. Abbreviations are as for Fig. 1.
Cyclodextrin provides a convenient but artificial means of holding D2 in solution for access by P450scc. Studies on D3 metabolism by P450scc in cyclodextrin have revealed that the cyclodextrin concentration used has a dramatic effect on both the Km and kcat for D3 consumption (Tuckey et al., 2008b). Therefore, we examined the metabolism of D2 in phospholipid vesicles made from phosphatidylcholine and cardiolipin, closely resembling the normal environment of the cytochrome in the inner mitochondrial membrane (Tuckey and Stevenson, 1985a; Headlam et al., 2003; Tuckey, 2005). Both D2 and D3 have been shown to partition quantitatively into the bilayer of phospholipid membranes (Merz and Sternberg, 1994; Kazanci et al., 2001; Tuckey et al., 2008a). The products of P450scc action on D2 observed in cyclodextrin, 20(OH)D2, 17,20(OH)2D2, 17,20,24(OH)3D2, and dihydroxyvitamin D2 (RT = 22 min) were also seen in vesicles (Fig. 2B). In contrast to cyclodextrin, 20(OH)D2 remained the major product throughout the 90-min incubation in vesicles.
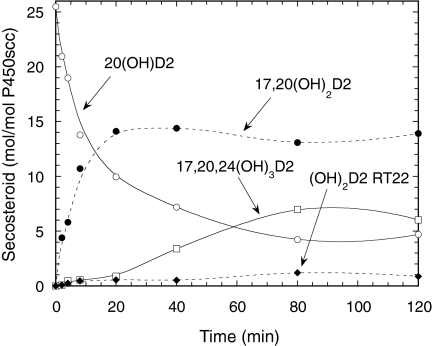
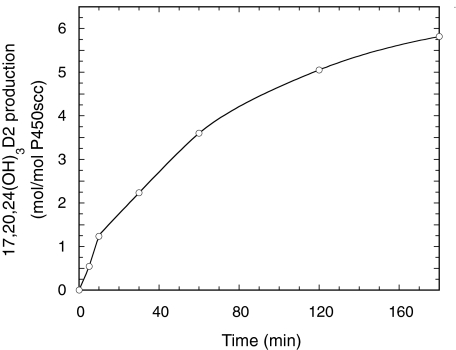
To elucidate the pathways leading to production of trihydroxyvitamin D2 by P450scc, we examined the products resulting from incubation of P450scc with purified 20(OH)D2, 17,20(OH)2D2, or dihydroxyvitamin D2 (RT = 22 min). Figure 3 shows the time course for metabolism of 20(OH)D2 by P450scc. Results show that the 20(OH)D2 serves as a precursor for production of 17,20(OH)2D2, dihydroxyvitamin D2 (RT = 22 min), and 17,20,24(OH)3D2. A lag was observed for the production of 17,20,24(OH)3D2, suggesting that accumulation of at least one of the dihydroxy metabolites was necessary to serve as its immediate substrate. Incubation of dihydroxyvitamin D2 (RT = 22 min) with P450scc in 0.45% cyclodextrin revealed that it is not further metabolized by P450scc (data not shown), indicating that it is a terminal product of the pathway and does not serve as a substrate for 17,20,24(OH)3D2. In contrast, 17,20(OH)2D2 was converted to 17,20,24(OH)3D2 (Fig. 4). This product was identical to the trihydroxyvitamin D2 product (RT = 14 min) made directly from D2 (Fig. 1) based on their mass spectra, HPLC RTs, and Rf values obtained by TLC. The position of the new hydroxyl groups in this metabolite, compared with its immediate substrate, was determined by NMR (see below). No other products were observed in Fig. 4, indicating that 17,20,24(OH)3D2 is not further metabolized.
Fig. 3.
Time course for metabolism of 20(OH)D2 in phospholipid vesicles. Vesicles containing 0.1 mol 20(OH)D2/mol phospholipid were incubated with 2.0 μM P450scc. Samples were taken at the times indicated, and products were measured by HPLC. Abbreviations are as for Fig. 1.
Fig. 4.
Time course for conversion of 17,20(OH)2D2 to 17,20,24(OH)3D2 in cyclodextrin. 17,20(OH)2D2 (50 μM) dissolved in 0.45% cyclodextrin was incubated with 1.0 μM P450scc. Samples were taken at the times indicated, and product was measured by HPLC.
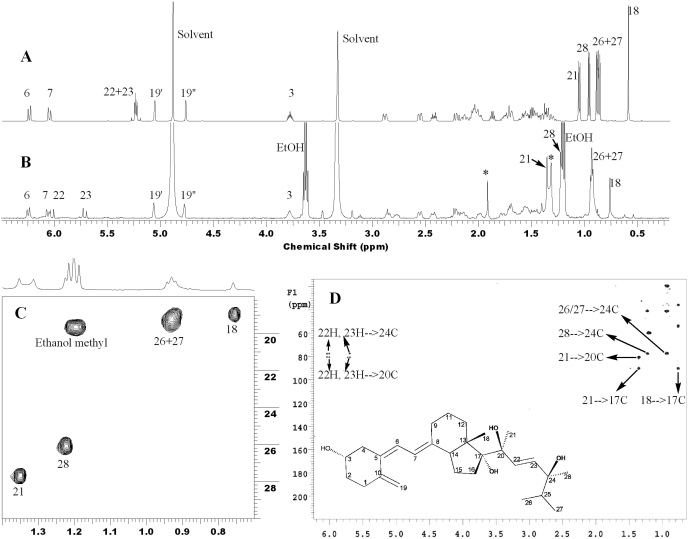
NMR of Trihydroxyvitamin D2. To identify the sites of hydroxylation of trihydroxyvitamin D2 by P450scc, 50 μg of this product was prepared from 17,20(OH)2D2 and analyzed by NMR (Fig. 5). Compared with the one-dimensional (1D) proton NMR spectrum of D2 (Fig. 5A), the proton NMR spectrum of trihydroxyvitamin D2 showed downfield shifts for several protons (Fig. 5B) as a result of the hydroxyl groups. Whereas protons in the methyl groups at positions 26 and 27 have a slight downfield shift of 0.05 ppm, protons in the methyl groups at positions 18, 28, and 21 have downfield shifts of 0.17, 0.26, and 0.29 ppm, respectively. More importantly, the methyl groups in both positions 28 and 21 became singlet as a result of the absence of vicinal coupling in this metabolite (Fig. 5, B and C). The downfield shift of the methyl group at C18 is caused by hydroxylation at C17, whereas the change of peak pattern and downfield shift for the methyl at C21 is caused by hydroxylation at C20, confirming the hydroxylation pattern of the starting material. The peak pattern change and downfield shift for the methyl at C28 strongly indicates hydroxylation at C24. This is consistent with the proton NMR spectrum in which both 22H and 23H show a substantial downfield shift (Fig. 5A). Further analysis of the proton-carbon heteronuclear multiple-bond correlation spectroscopy spectrum confirmed hydroxylation at C24 (Fig. 5D). The three quaternary carbons bearing hydroxyl groups (carbon chemical shifts at 77.5, 80.6, and 90.4 ppm) clearly display all the expected long-range correlations to the methyl protons as shown in Fig. 5D. In addition, 22H and 23H show the expected long-range coupling to C24. Hydroxylation at C25 is completely ruled out because first, there is no change in coupling patterns and negligible chemical shift changes for the methyl groups at positions 26 and 27, and second, there is long-range proton-carbon correlation from 22H, which is four bonds away from C25. Taken together, this metabolite is unambiguously identified as 17,20,24(OH)3D2. Based on the mechanism of action of P450s where a hydrogen is removed and replaced by a hydroxyl group (Meunier et al., 2004), the configurations at the three hydroxylation sites are likely to be preserved, giving the structure 17α,20β,24β(OH)3D2 as shown in Fig. 5D, although this remains to be confirmed.
Fig. 5.
NMR structure elucidation of trihydroxyvitamin D2. 1D proton NMR of parent D2 (A), 1D proton NMR of trihydroxyvitamin D2 (B), methyl region of proton-carbon HSQC spectrum of trihydroxyvitamin D2 (C), and proton-carbon heteronuclear multiple-bond correlation spectroscopy of trihydroxyvitamin D2 (D). The residue ethanol is carryover from a previous UV quantification measurement. An unknown impurity is indicated by the star in the 1D NMR spectrum. The identified structure is shown in D with the likely configurations for each hydroxy group, 17α,20β,24β(OH)3D2.
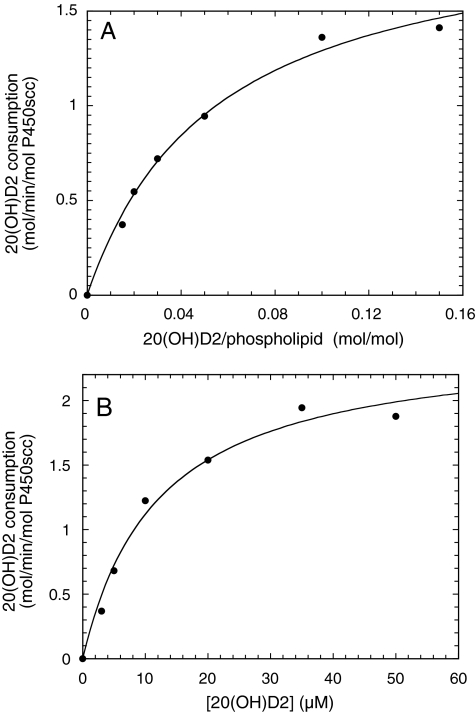
Kinetics of the Metabolism of D2 by P450scc. The kinetics of consumption of D2, 20(OH)D2, and 17,20(OH)2D2 by P450scc was determined in cyclodextrin and phospholipid vesicles and compared with the kinetics for cholesterol, the best characterized substrate for P450scc (Lambeth et al., 1982; Tuckey, 2005). These substrates permitted the rates of the first (C20), second (C17), and third (C24) hydroxylations, respectively, to be measured because for the short incubation times used, only the immediate product from a single hydroxylation was observed. Figure 6 shows typical hyperbolic-curve fits to obtain Km and Vmax for 20(OH)D2 in cyclodextrin and phospholipid vesicles, and data are summarized in Tables 1 and 2. In cyclodextrin, D2 displays a higher Km and lower kcat than cholesterol, resulting in the specificity constant, kcat/Km, being 5-fold lower than that for cholesterol (Table 1). Both 20(OH)D2 and 17,20(OH)2D2 showed similar Km values to that for D2, but 17,20(OH)2D2 displayed a much lower kcat and hence a lower kcat/Km than either D2 or 17,20(OH)2D2.
Fig. 6.
Michaelis-Menten plots for metabolism of 20(OH)D2 by P450scc in vesicles and cyclodextrin. P450scc was incorporated into phospholipid vesicles containing 20(OH)D2 (A) or 0.45% cyclodextrin containing 20(OH)D2 (B), and incubated at 37°C for 3 or 2 min, respectively. Products were extracted and analyzed by reverse-phase HPLC. Hyperbolic curves were fitted by nonlinear least-squares analysis using KaleidaGraph 3.6 (Synergy Software, Reading, PA). The correlation coefficients for the curve fits were 0.9965 and 0.9924 for 20(OH)D2 in vesicles and cyclodextrin, respectively.
TABLE 1.
Kinetics of D2 metabolism in 0.45% cyclodextrin
Values for Km and kcat are mean ± S.E. from the hyperbolic curve fitted to the data from a single experiment by least-squares nonlinear regression using KaleidaGraph 3.6 (Synergy Software).
| Substrates | Km | kcat | kcat/Km |
|---|---|---|---|
| μM | mol/min/mol P450scc | mM-1 min-1 | |
| Cholesterol | 9.1 ± 1.7 | 6.1 ± 0.4 | 670 |
| D2 | 17.5 ± 4.1 | 2.28 ± 0.20 | 130 |
| 20(OH)D2 | 12.0 ± 2.3 | 2.47 ± 0.17 | 206 |
| 17,20(OH)2D2 | 18.1 ± 3.2 | 0.33 ± 0.02 | 18.2 |
TABLE 2.
Kinetics of D2 metabolism in phosphatidylcholine vesicles
Kinetic constants were determined in vesicles prepared from dioleoyl phosphatidylcholine and cardiolipin. Values for Km and kcat are mean ± S.E. from the hyperbolic curve fitted to the data from a single experiment by least-squares nonlinear regression using KaleidaGraph 3.6 (Synergy Software).
| Substrates | Km | kcat | kcat/Km |
|---|---|---|---|
| mol/mol PL | mol/min/mol P450scc | (mol/mol PL)-1 min-1 | |
| Cholesterol | 0.21 ± 0.04 | 46 ± 5 | 219 |
| D2 | 1.65 ± 0.61 | 5.62 ± 1.73 | 3.4 |
| 20(OH)D2 | 0.055 ± 0.007 | 1.99 ± 0.11 | 36.2 |
| 17,20(OH)2D2 | 0.057 ± 0.022 | 0.21 ± 0.04 | 3.7 |
In vesicles, D2 displayed a Km 8-fold higher than for cholesterol and a kcat 8-fold lower (Table 2). Once formed, 20(OH)D2 is a better substrate (higher kcat/Km) than D2 itself, as indicated by a marked reduction in its Km, which is 30-fold lower than the value for D2. 17,20(OH)2D2 displays a low Km, similar to that for 20(OH)D2, but has a lower kcat, resulting in it having a similar kcat/Km to that for D2. Because of its low Km, 17,20(OH)2D2 will efficiently compete with D2 and 20(OH)D2 for binding to the active site of P450scc, but because of its very low kcat, will essentially act as a competitive inhibitor. This provides an explanation for the declining rates of D2 and 20(OH)D2 metabolism seen as 17,20(OH)2D2 accumulates in the time course in Fig. 2B. A similar effect, but less marked, is seen in cyclodextrin, where accumulation of 17,20(OH)2D2 is accompanied by decreases in production of both 20(OH)D2 and 17,20(OH)2D2, as it efficiently competes for binding to the active site of the P450scc but is only slowly metabolized (Figs. 2A and 3). It is also possible that the declining total hydroxylation rate that is observed as the incubation progresses is caused by either of the terminal metabolites, 17,20,24(OH)3D2 or dihydroxyvitamin D2 (RT = 22 min), acting as competitive inhibitors.
Discussion
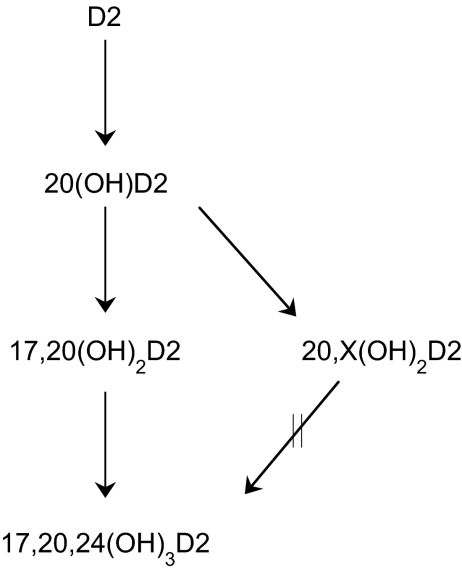
This study shows that there is a single pathway leading to the production of 17,20,24(OH)3D2 from D2 (Fig. 7), resulting from sequential hydroxylation at C20, C17, and C24. Intermediates accumulate at each step, indicating escape from the active site of P450scc, as we have also observed for intermediates in the metabolism of D3 and 1-hydroxyvitamin D3 by P450scc (Tuckey et al., 2008a,b,c). The only product detected that is not a direct intermediate in the synthesis of 17,20,24(OH)3D2 is dihydroxyvitamin D2, RT = 22 min (Fig. 7), which is produced in small amounts from both D2 and 20(OH)D2, and therefore must contain one hydroxyl group at C20. Although the position of the other hydroxyl group remains to be determined, it may be at C24, provided that hydroxylation at this position prevents subsequent hydroxylation at C17. This is supported by the observation that P450scc cannot metabolize 25-hydroxyvitmin D3, indicating that hydroxylation in this region prevents P450scc from acting on the side chain (Slominski et al., 2005b).
Fig. 7.
Pathway for the metabolism of D2 by P450scc. X represents a hydroxyl group in an unknown position. The arrow with hash marks indicates that reaction does not occur.
The order of hydroxylations of D2 leading to 17,20,24(OH)3D2 is more specific than the hydroxylation order by P450scc for producing 17,20,23(OH)3D3 from D3. Although initial hydroxylation at C20 is favored for both D2 and D3, 17,20,23(OH)3D3 can also be produced after initial hydroxylation of D3 at C17 or C23 (Tuckey et al., 2008a). The difference in side chain structures between D2 and D3, where D2 has a double bond between C22 and C23 and a methyl group at C24, clearly influences the hydroxylation pattern. Both substrates can undergo C20 and C17 hydroxylation, but C23 hydroxylation is specific to D3, whereas C24 hydroxylation is specific to D2. Ergosterol, the D2 precursor, is also hydroxylated at C24 by P450scc, as well as at C17 (Slominski et al., 2005a).
P450scc displays differences in the efficiency of metabolism of D2 and D3. Initial hydroxylation of D3 at C20 in 0.45% cyclodextrin occurs with a kcat/Km of 666 mM–1min–1 (Tuckey et al., 2008b), which is 5-fold higher than that reported here for D2 measured under comparable conditions. In vesicles, initial hydroxylation of D3 at C20 occurs with a kcat/Km approximately 2-fold higher than for C20 hydroxylation of D2 (Tuckey et al., 2008b). Both D2 and D3 display high Km values in vesicles, relative to that for cholesterol, making it difficult to achieve substrate saturation.
Because our initial studies show that the major products of D2 metabolism by P450scc are biologically active in inhibiting keratinocyte proliferation and promoting differentiation (Slominski et al., 2006), we were interested in optimizing their production to facilitate further biological testing. The relevance of this is highlighted by our detailed testing of some of the D3 metabolites produced by P450scc, which display potency on skin cells at least as good as the hormonally active form of D3, 1,25-dihydroxyvitamin D3, and may be of use pharmacologically (Janjetovic et al., 2008; Zbytek et al., 2008). Our kinetic study shows that the cyclodextrin system is superior to vesicles for large-scale production of D2 metabolites. Although the kcat for initial hydroxylation at C20 in 0.45% cyclodextrin is only 40% of that in vesicles, this is more than compensated for by the ability to solubilize the D2 substrate to a concentration equivalent to at least 3 times Km, which cannot be done in vesicles because of the high Km relative to the amount of vitamin D that can be incorporated into the phospholipid bilayer (Tuckey et al., 2008b).
It remains to be established whether dietary D2, or D2 administered to vitamin D-deficient patients, can be metabolized by P450scc in vivo. Little is known about the ability of tissues expressing P450scc, such as the adrenal cortex, gonads, placenta, skin, brain, and others, to take up vitamin D from the plasma (Slominski et al., 2005). We have shown that D3 exchanges between membranes more rapidly than cholesterol, plus the steroidogenic acute regulatory protein, which delivers cholesterol to the inner mitochondrial membrane for steroid synthesis, can also transport D3 (Tuckey et al., 2008b) and D2 (R. C. Tuckey, unpublished data). Thus, if D2 reaches steroidogenic tissues, transport into the mitochondria is likely. Effective competition of D2 with cholesterol would be required for D2 metabolism by P450scc, but given that P450scc is never saturated with cholesterol in the gonads or adrenals, even after tropic hormone stimulation, competition would be minimal (Jefcoate et al., 1973; Tuckey et al., 1985).
In conclusion, this study clearly defines the pathway of D2 metabolism by P450scc through to 17,20,24(OH)3D2, with characterization of the major kinetic constants for the three hydroxylations involved. The structure of the 17,20,24(OH)3D2 has been solved by NMR providing an additional product, besides the 20(OH)D2 and 17,20(OH)2D2, for future testing of biological activity.
Acknowledgments
We thank Dr. Tony Reeder for recording mass spectra.
This work was supported in part by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant R01-AR 052190]; and the University of Western Australia.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.025619.
ABBREVIATIONS: D2, vitamin D2; D3, vitamin D3; 1,25(OH)2D2, 1α,25-dihydroxyvitamin D2; 1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; P450scc, cytochrome P450scc; 20(OH)D2, 20-hydroxyvitamin D2; 17,20(OH)2D2, 17,20-dihydroxyvitamin D2; 17,20,23(OH)3D3, 17,20,23-trihydroxyvitamin D3; TLC, thin-layer chromatography; HPLC, high-performance liquid chromatography; 17,20,24(OH)3D2, 17,20,24-trihydroxyvitamin D2; cyclodextrin, 2-hydroxypropyl-β-cyclodextrin; RT, retention time; 1D, one-dimensional.
References
- Armas LA, Hollis BW, and Heaney RP (2004) Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89 5387–5391. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Oda Y, and Xie Z (2004) Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol 89–90 355–360. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Ritter CS, Holliday LS, Knutson JC, and Strugnell SA (2004) Tissue distribution and activity studies of 1,24-dihydroxyvitamin D2, a metabolite of vitamin D2 with low calcemic activity in vivo. Biochem Pharmacol 68 1289–1296. [DOI] [PubMed] [Google Scholar]
- Headlam MJ, Wilce MC, and Tuckey RC (2003) The F-G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. BBA Biomembranes 1617 96–108. [DOI] [PubMed] [Google Scholar]
- Hiwatashi A, Nishii Y, and Ichikawa Y (1982) Purification of cytochrome P-450D1a (25-hydroxyvitamin D3-1α-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun 105 320–327. [DOI] [PubMed] [Google Scholar]
- Holick MF (2003) Vitamin D: a millennium perspective. J Cell Biochem 88 296–307. [DOI] [PubMed] [Google Scholar]
- Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79 362–371. [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, and Tannenbaum AD (2008) Vitamin D2 is effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R, Kelly RW, Taylor PL, and Boyd GS (1984) The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone. Eur J Biochem 140 583–591. [DOI] [PubMed] [Google Scholar]
- Janjetovic Z, Zmijewski M, Tuckey RC, Nguyen MN, and Slominski A (2008) 20-Hydroxycholecalciferol and 20, 23-dihydroxycholecalciferol, products of vitamin D3 hydroxylation by P450-scc, decrease NFκB activity by increasing IκBα levels in normal and HaCaT-keratinocytes. J Invest Dermatol 128(S1): S20, 116. [Google Scholar]
- Jefcoate CR, Simpson ER, Boyd GS, Brownie AC, and Orme-Johnson WH (1973) The detection of different states of the P-450 cytochromes in adrenal mitochondria; changes induced by ACTH. Ann N Y Acad Sci 212 243–261. [DOI] [PubMed] [Google Scholar]
- Kazanci N, Toyran N, Haris PI, and Severcan F (2001) Vitamin D2 at high and low concentrations exert opposing effects on molecular order and dynamics of dipalmitoyl phosphatidylcholine membranes. Spectroscopy 15 47–55. [Google Scholar]
- Lambeth JD, Kitchen SE, Farooqui AA, Tuckey R, and Kamin H (1982) Cytochrome P-450scc-substrate interactions. Studies of binding and catalytic activity using hydroxycholesterols. J Biol Chem 257 1876–1884. [PubMed] [Google Scholar]
- Merz K and Sternberg B (1994) Incorporation of vitamin D3-derivatives into liposomes of different lipid types. J Drug Target 2 411–417. [DOI] [PubMed] [Google Scholar]
- Meunier B, de Visser SP, and Shaik S (2004) Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev 104 3947–3980. [DOI] [PubMed] [Google Scholar]
- Mitani H, Naru E, Yamashita M, Arakane K, Suzuki T, and Imanari T (2004) Ergocalciferol promotes in vivo differentiation of keratinocytes and reduces photodamage caused by ultraviolet irradiation in hairless mice. Photodermatol Photoimmunol Photomed 20 215–223. [DOI] [PubMed] [Google Scholar]
- Omura T and Sato R (1964) The carbon monoxide binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239 2370–2378. [PubMed] [Google Scholar]
- Prosser DE and Jones G (2004) Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29 664–673. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, and Tuckey RC (2006) An alternative pathway of vitamin D2 metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J 273 2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, and Tuckey RC (2005a) Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17a,24-dihydroxyergosterol. Chem Biol 12 931–939. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, and Tuckey RC (2005b) The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272 4080–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, and Tuckey RC (2004) A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271 4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC (2005) Progesterone synthesis by the human placenta. Placenta 26 273–281. [DOI] [PubMed] [Google Scholar]
- Tuckey RC and Cameron KJ (1993) Human placental cholesterol side-chain cleavage: enzymatic synthesis of (22R)-20α,22-dihydroxycholesterol. Steroids 58 230–233. [DOI] [PubMed] [Google Scholar]
- Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony JK, and Slominski A (2008c) Metabolism of 1α-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1α,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 112 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, and Slominski A (2008a) Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 275 2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Nguyen MN, and Slominski A (2008b) Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol 40 2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC and Stevenson PM (1984a) Properties of bovine luteal cytochrome P-450scc incorporated into artificial phospholipid vesicles. Int J Biochem 16 497–503. [DOI] [PubMed] [Google Scholar]
- Tuckey RC and Stevenson PM (1984b) Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem 16 489–495. [DOI] [PubMed] [Google Scholar]
- Tuckey RC and Stevenson PM (1985) Purification and analysis of phospholipids in the inner mitochondrial membrane fraction of the bovine corpus luteum, and properties of cytochrome P-450scc incorporated into vesicles prepared from these phospholipids. Eur J Biochem 148 379–384. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, and Slominski AT (2008) 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450sc, stimulates keratinocyte differentiation. J Invest Dermatol 128 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]