Abstract
We utilized single-voxel 1H magnetic resonance spectroscopy (MRS) to examine for biochemical abnormalities related to late-life depression in the medial prefrontal cortex and medial temporal lobe. Fourteen elderly subjects whose depression responded to treatment and 12 nondepressed subjects were enrolled. Subjects were scanned using a GE 3.0 Tesla whole body MR scanner. Metabolite concentrations were quantified using the LC Model software and adjusted for CSF and ratio of gray to white matter. ANCOVA models tested for group differences while controlling for age and sex. Older previously depressed individuals showed significantly reduced concentrations of total N-Acetyl aspartate, choline, and creatine in the prefrontal cortex and significantly elevated left medial temporal lobe concentrations of NAA and myo-inositol. There were no significant group differences in right temporal metabolite concentrations. The prefrontal cortex observations suggest that reduced neuronal, phospolipid, and energy metabolism is present even in clinically improved depression. In contrast, elevated NAA and myo-inositol concentrations in the left medial temporal lobe could be associated with neuronal and glial cell changes in the amygdala.
Keywords: Geriatrics, magnetic resonance spectroscopy, prefrontal cortex, temporal lobe, metabolite concentration
1. Introduction
Structural abnormalities have been widely reported in geriatric depression (Vaishnavi and Taylor, 2006), but corresponding biochemical abnormalities are not as well understood. Magnetic resonance spectroscopy (MRS) has been used to study depression in young- to midlife-adult populations (Yildiz-Yesiloglu and Ankerst, 2006), but has not been as extensively used in older depressed samples despite its potential to provide important information on the pathophysiology of late-life depression.
Although many brain regions may be interesting targets in MRS studies of geriatric depression, the prefrontal cortex and amygdala may be of particular interest given their interconnections and involvement in mood regulation (Phillips et al., 2003b). Structural differences in the prefrontal cortex have been reported in geriatric depression (Ballmaier et al., 2004; Bae et al., 2006; Taylor et al., 2007b) while the amygdala plays an important role in the regulation of emotional expression (Phillips et al., 2003a). More recently, depression-related differences in left hemispheric white matter connections between these regions have been reported (Taylor et al., 2007a), supporting theories of fronto-limbic dysregulation in depression. Postmortem studies of these regions support the hypothesis that these broader changes identified on structural neuroimaging may be secondary to microstructural changes (Ongur et al., 1998; Rajkowska et al., 1999; Rajkowska et al., 2005) as depressed individuals show neuronal and glial alterations in these regions. In turn, these neuronal and glial changes are themselves ssociated with metabolite changes such as N-acetyl aspartate (NAA, a neuronal marker, also related to myelin), choline (Cho, a marker of membrane phospholipid metabolism), myo-Inositol (mI, a glial cell marker), creatine (Cre), and may affect glutamate regulation (Rajkowska and Miguel-Hidalgo, 2007).
Although 1H MRS has been used to study major depression, there are differences in results and conclusions across published studies. MRS studies of the amygdala have shown changes in Cho (Mervaala et al., 2000; Kusumakar et al., 2001) and glutamate (Michael et al., 2003b), although none of these studies examined exclusively older subjects. Previous studies of the frontal lobe have reported lower glutamate plus glutamine (Glx) concentrations in depression (Hasler et al., 2007), and these levels may increase with successful antidepressant treatment (Auer et al., 2000; Michael et al., 2003a; Pfeiderer et al., 2003; Hasler et al., 2005). This finding may not be seen in other metabolites; a recent meta-analysis concluded there were no depression-related differences in frontal lobe levels of NAA, Cho, or mI (Yildiz-Yesiloglu et al., 2006), however this analysis included MRS studies of depression across all ages and was not specifically limited to an older population. The few published studies which do specifically examine older depressed subjects have reported increased frontal lobe concentrations of mI, Cho, and Glx (Kumar et al., 2002; Binesh et al., 2004). Although these metabolite differences could be related to neuronal and glial changes, they may also be related to cerebral hyperintensities (Murata et al., 2001) which are common in depressed elders (Taylor et al., 2005).
In the present study, we performed single-voxel spectroscopy of the medial prefrontal cortex (PFC) and bilateral medial temporal lobe, focused on the amygdala. We examined a cohort of never-depressed older subjects, and a cohort of subjects with a history of depression who had responded to antidepressants and did not currently meet diagnostic criteria for a major depressive episode. We hypothesized that the metabolites like NAA, Cho, Cre, mI and Glu would exhibit different concentration in the elderly depressed population when compared with nondepressed subjects.
2. Methods
2.1. Sample
Subjects were recruited from participants in the NIMH Conte Center for the Neuroscience of Depression in Late Life, located at Duke University Medical Center. Eligibility was limited to age 60 years or older. Depressed subject eligibility included a diagnosis of major depression at entry to the Conte Center based on the NIMH Diagnostic Interview Schedule (DIS) (Robins et al., 1981) and clinical evaluation by a geriatric psychiatrist. At time of enrollment for the current study, subjects did not meet DSM-IV TR diagnostic criteria for a current major depressive episode, although residual depressive symptoms were allowed.
Exclusion criteria included (1) another major psychiatric illness, although coexisting anxiety symptoms considered to be secondary to the depression diagnosis were allowed; (2) lifetime history of alcohol or drug abuse or dependence; (3) primary neurologic illness, including dementia; (4) current use of medications that could cause or contribute to depression (such as systemic corticosteroids); and (5) any contraindication to MRI. As part of screening for dementia, all subjects completed a mini-mental status exam (MMSE) (Folstein et al., 1975), and individuals with a MMSE of 24 or less were excluded.
As part of the Conte Center, subjects were treated for depression following a naturalistic, algorithm-driven protocol (Steffens et al., 2002) where all approved treatments were available, and treatment decisions made based on the algorithm and on the individual’s past treatment history. Although electroconvulsive therapy was an option in this protocol, none of the subjects in the current study had received it. All depressed subjects had depression severity measured using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) at time of imaging, although no a priori MADRS cutoff was used as entry criteria. Use of antidepressant medications and duration of that use at time of imaging was determined by review of the subjects’ electronic medical record.
Nondepressed comparison subjects were also recruited from participants in the Conte Center; these were community-dwelling individuals, initially recruited for the Conte Center from the Aging Center Subject Registry at Duke University. Eligible comparison subjects had a non-focal neurological examination, no neurologic or psychiatric illness including dementia or memory problems, a MMSE score above 24, no evidence of a psychiatric diagnosis based on the DIS, and no contraindication to MRI.
The protocol was reviewed and approved by the Institutional Review Board at Duke University Medical Center. All subjects provided written informed consent before beginning study procedures.
2.2 Proton Magnetic Resonance Spectroscopy
We acquired the data on a 3T Signa EXCITE scanner (GE Medical Systems, Waukesha, WI) using the standard quadrature transmit receive volume coil. The axial T1-weighted slices are used as the localizer to place the spectroscopic voxels. Water suppression was achieved with a CHESS sequence (Frahm et al., 1989) and volume localization used a PRESS sequence (Bottomley, 1987) with numerically optimized Shinnar-Le Roux slice-selective radiofrequency pulses (Pauly et al., 1991). Additionally, volume selective suppression (VSS) pulses were used to reduce lipid contamination (Tran et al., 2000). Parameters for spectral acquisition were: TR = 3000 ms, TE = 30 ms, 128 excitations. With the use of a short echo time (TE = 30 ms) there may be macromolecule contribution to the quantification of metabolite concentration. This was minimized by incorporating macromolecules (MM) in the basis-set.
The volume of interest for all regions was 2 × 2 × 2 cm3 (8mL). The PFC single voxel was placed just above the orbito-prefrontal cortex, about 10 mm above the anterior cerebral artery. Specifically, this voxel included regions of the anterior cingulate cortex and medial prefrontal cortex. Although a single voxel was used, it sampled both hemispheres. Voxels on the left and right amygdala were placed immediately to the left and right side of the optic tract and above the hippocampus. As the amygdala volume ranges between 1.5mL to 2.0mL (Frodl et al., 2004), this voxel also includes surrounding tissue, so we have designated this voxel as examining the medial temporal lobe. See Figure 1 for an example of voxel placement. Linear and higher order shims were adjusted automatically using localized water signal.
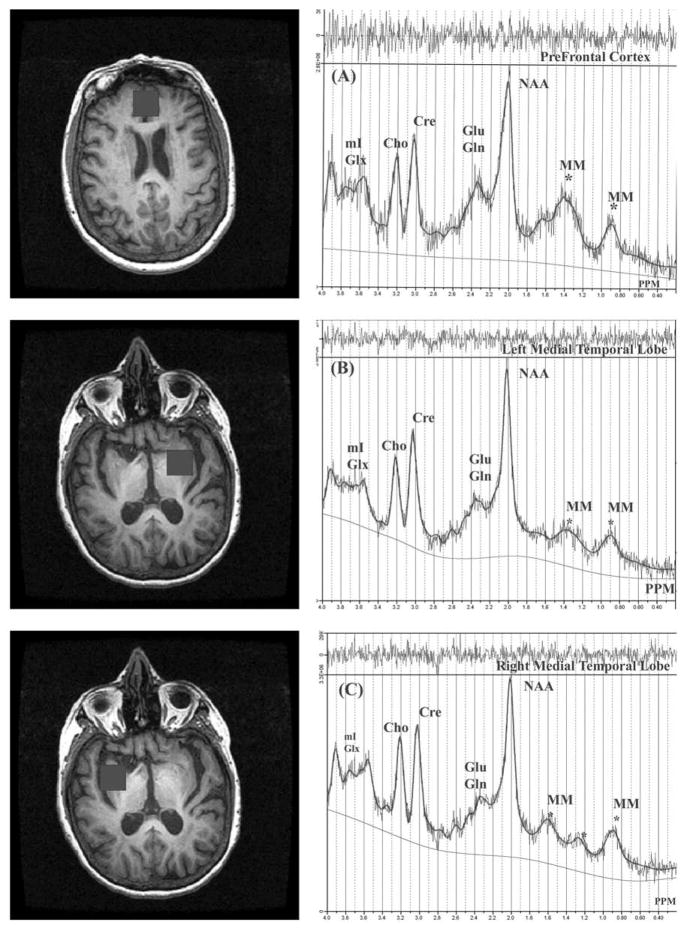
Figure 1.
In Vivo 1H single-voxel (8cc) MRS by region (71 year old female subject) with LCModel fit. The trace above each spectrum is the difference between LCModel fit and the experimental data. All the metabolite peaks are marked.
A) Medial prefrontal cortex; B) left medial temporal lobe; C) right medial temporal lobe. Black boxes on the images are the corresponding regions of interest examined.
The MRS data were transferred to a Linux workstation and metabolites quantified using LCModel (Provencher, 1993). An appropriate basis set with identical parameters are used for each region of interest, including the spectra of Alanine, Aspartate, Choline, Creatine Choline, GABA, Glucose, Glutamate, glutamine, Glycine, Lactate, Myo-Inositol, N-Acetyl Aspartate, N-Acetyl Aspartateglutamate (NAAG), Taurine and macromolecules. The spectral analysis window was between 0.2 and 4.0 ppm. Metabolite concentrations were discarded if the percentage of SD was > 20 for tNAA, Cho, Cre, mI, Glx and >30 for Glu (from LCModel output).
The 3D FSPGR data set was segmented to generate gray matter (GM), white matter (WM) and CSF images using the statistical parametric mapping program (SPM5) [http://www.fil.ion.ucl.ac.uk/spm/]. The segmented images covering 1H single-voxel dimension were summed over each nominal voxel to estimate the proportion of gray matter, white matter and CSF. The absolute metabolite concentrations (Co) were not corrected for T1 and T2 relaxation effects. Co were corrected for the partial volume effect due to CSF, gray matter (GM) and white matter (WM) by determining the fractional content of CSF, GM and WM (FCSF, FGM, FWM) in each voxel and applying the correction: C =Co*((FGM + FWM)/(1 - FCSF)). In LC Model, the visible water concentrations of 43,300 mM and 35,880 mM were used for gray matter and white matter respectively (McLean et al., 2000; Provencher, 2007).
2.3 Statistical Analysis
All analyses were conducted using SAS software, version 8.2 (Cary, NC, USA). Differences in demographic variables between groups were tested using two-tailed pooled t-tests for continuous variables, and the Fisher’s exact test for categorical variables. Differences between groups in MRS metabolite concentrations were assessed using analysis of covariance (ANCOVA), where metabolite concentration was the dependent variable, and age, sex, and diagnosis (depressed or nondepressed) were independent variables. As secondary analyses, we tested for group hemispheric differences between the medial temporal lobe measures. Asymmetry indexes (Nagae-Poetscher et al., 2004) were calculated for medial temporal lobe metabolite concentrations using the formula AI = (left – right) / [(left + right) / 2] and subsequently analyzed as dependent variables in ANCOVA models controlling for age, sex, and diagnosis. We did not correct for multiple comparisons.
3. Results
The sample consisted of 26 older individuals, 14 with a history of depression and 12 with no psychiatric illnesses. There was not a significant difference between groups in age (depressed: 72.1y, SD = 5.3y; nondepressed = 72.7y, SD = 4.6y; t = 0.31, 24 df, P = 0.763), sex representation (depressed: 57.2% female or 8/14; nondepressed: 50.0% female or 6/12; Fisher’s exact test, P = 1.00), or education level (depressed: 15.8y, SD=1.5y; nondepressed: 15.5y, SD=1.5y; 24 df, t = 0.49, P = 0.6306). Neither group exhibited any signs of dementia on screening with MMSE (range = 27–30; depressed = 29.3, SD = 0.9; nondepressed = 29.7, SD = 0.7; t = 1.20, 24 df, P = 0.240).
The depressed group overall had a mean age of onset of major depression at 49.5y (SD = 18.6y, range 12–74y), with current mild residual depressive symptoms by MADRS (mean = 6.3, SD = 5.6, range 0–15). Their medication regimens were relatively stable, being on a consistent medication regimen and dose for an average of 498 days (range 60–808 days, SD = 232.4 days). Three subjects were on SSRI monotherapy, 5 on bupropion, 3 on venlafaxine, and 2 on a combination of bupropion plus a SSRI. One subject had not been taking any antidepressant for approximately 30 days prior to MRS, but previously had been on bupropion for 169 days.
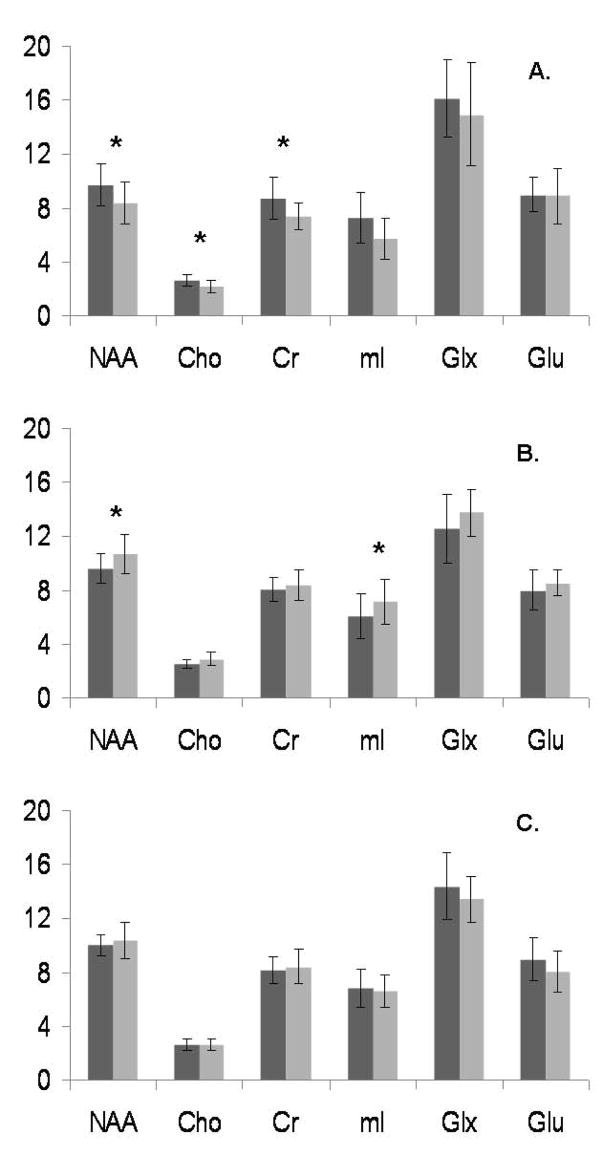
The metabolite concentrations, adjusted for both CSF and ratio of gray to white matter in each voxel, are given in Tables 1 and 2, and displayed in Figure 2. After controlling for age and sex, medial PFC concentrations of NAA, choline, and creatine were significantly decreased in the depressed subjects (Table 1). In the left medial temporal lobe (Table 2), the depressed population exhibited significantly higher concentrations of NAA and myo-inositol. In contrast, in the right medial temporal lobe there were no significant differences between subject groups. Neither the glutamate + glutamine (Glx) composite measure nor glutamate concentration alone were significantly different in any region of interest. There were no significant differences in tissue composition (gray matter, white matter, and CSF) in any of the three regions (Tables 1 and 2). In secondary analyses looking at hemispheric differences in medial temporal lobe metabolite concentrations between groups, there were no significant differences between depressed and nondepressed subjects in the asymmetry indices (data not shown)
Table 1.
Medial prefrontal cortex tissue composition and adjusted metabolite concentrations
| Adjusted Group Differences | |||||
|---|---|---|---|---|---|
| Metabolites | Depressed (N = 14) |
Nondepressed (N = 12) |
F value | Df | P value |
| NAA | 8.42 (1.58) | 9.75 (1.56) | 4.37 | 1 | 0.050 |
| Cho | 2.21 (0.41) | 2.68 (0.45) | 5.81 | 1 | 0.026 |
| Cre | 7.39 (0.98) | 8.78 (1.54) | 5.57 | 1 | 0.029 |
| mI | 5.74 (1.54) | 7.28 (1.85) | 3.66 | 1 | 0.071 |
| Glx | 14.96 (3.85) | 16.10 (2.88) | 0.68 | 1 | 0.460 |
| Glu |
8.90 (2.04) | 9.00 (1.31) | 0.20 | 1 | 0.658 |
| CSF (%) | 28.3 (16.4) | 28.3 (13.7) | 0.03 | 1 | 0.872 |
| Gray Matter (%) | 63.6 (13.8) | 60.4 (11.1) | 0.24 | 1 | 0629 |
| White Matter (%) | 8.0 (8.0) | 11.3 (13.9) | 0.75 | 1 | 0.399 |
Table presents mean concentrations of meatabolites in mM, adjusted for presence of CSF and gray/white matter ratio, presented as mean (SD). Tissue composition displayed as the mean (SD) percent of the voxel filled by that type. Group comparisons performed using ANCOVA, including age and sex as covariates, with the models having 25 degrees of freedom.
Key: NAA = N-Acetyl aspartate; Cho = choline; Cre = creatine; mI = myo-Inositol; Glx = glutamate + glutamine; Glu = glutamate.
Table 2.
Medial temporal lobe tissue composition and adjusted metabolite concentration
| Adjusted Group Differences | |||||
|---|---|---|---|---|---|
| Metabolites | Depressed (N = 14) |
Nondepressed (N = 12) |
F value | Df | P value |
| Left Medial Temporal Lobe | |||||
| NAA | 10.69 (1.43) | 9.61 (1.11) | 5.25 | 1 | 0.035 |
| Cho | 2.88 (0.49) | 2.57 (0.32) | 3.91 | 1 | 0.065 |
| Cre | 8.40 (1.07) | 8.05 (0.87) | 0.91 | 1 | 0.353 |
| mI | 7.16 (1.65) | 6.11 (1.64) | 4.66 | 1 | 0.045 |
| Glx | 13.79 (1.73) | 12.63 (2.54) | 1.69 | 1 | 0.212 |
| Glu |
8.53 (0.96) | 8.01 (1.51) | 0.55 | 1 | 0.469 |
| CSF (%) | 7.4 (19.2) | 3.1 (3.5) | 0.50 | 1 | 0.488 |
| Gray Matter (%) | 67.1 (17.3) | 74.1 (11.1) | 1.12 | 1 | 0.305 |
| White Matter (%) | 25.9 (15.4) | 23.2 (8.6) | 0.24 | 1 | 0.631 |
|
| |||||
| Right Medial Temporal Lobe | |||||
| NAA | 10.37 (1.35) | 10.05 (0.76) | 0.40 | 1 | 0.533 |
| Cho | 2.62 (0.43) | 2.64 (0.41) | 0.06 | 1 | 0.809 |
| Cre | 8.45 (1.26) | 8.18 (1.02) | 0.47 | 1 | 0.502 |
| mI | 6.64 (1.19) | 6.81 (1.44) | 0.19 | 1 | 0.669 |
| Glx | 13.44 (1.73) | 14.41 (2.48) | 1.06 | 1 | 0.315 |
| Glu |
8.06 (1.52) | 8.97 (1.61) | 1.70 | 1 | 0.209 |
| CSF (%) | 2.4 (2.5) | 8.1 (17.8) | 0.97 | 1 | 0.337 |
| Gray Matter (%) | 69.8 (8.8) | 61.1 (13.2) | 3.14 | 1 | 0.092 |
| White Matter (%) | 27.8 (9.3) | 30.8 (14.2) | 0.33 | 1 | 0.573 |
Table presents absolute concentrations of meatabolites in mM, adjusted for presence of CSF and gray/white matter ratio, presented as mean (SD). Tissue composition displayed as the mean (SD) percent of the voxel filled by that type. Group comparisons performed using ANCOVA, including age and sex as covariates; the models had 25 degrees of freedom.
Key: NAA = N-Acetyl aspartate; Cho = choline; Cre = creatine; mI = myo-Inositol; Glx = glutamate + glutamine; Glu = glutamate.
Figure 2.
Absolute metabolite concentrations (in millimolar, or mM) by region.
A) Medial prefrontal cortex; B) left medial temporal lobe; C) right medial temporal lobe. Dark gray bars are control subjects, light gray are depressed subjects. Differences significant at p ≤ 0.05 marked with an asterisk.
4. Discussion
We observed significant reductions in tNAA, Cho and Cre in a predominantly gray matter region of the medial prefrontal cortex in patients with geriatric depression who responded to treatment. These findings demonstrate that older subjects who are generally recovered from depression, albeit with residual depressive symptoms, exhibit reductions in medial PFC NAA (by 14%) and choline (by 18%). This is accompanied by decreased energy expenditure, as seen by a mean 16% reduction in creatine. In contrast, individuals with depression exhibited an 11% increase in NAA and and a 17% increase in myo-insoitol concentrations in the left medial temporal lobe, in a region focused on the amygdala. There were no significant differences between groups in the right medial temporal lobe.
Our results expand upon previous MRS studies in older depressed subjects, although our findings are somewhat discordant with them; this may be due to differences in brain regions examined, MRS techniques used, or the treatment status of our subject population. An early MRS study of the basal ganglia in an elderly population found elevated Cho/Cre concentrations in depressed subjects, which decreased with antidepressant treatment (Charles et al., 1994); similar findings have been reported in younger adult populations (Renshaw et al., 1997; Vythilingam et al., 2003). Other studies have reported elevated levels of choline (Kumar et al., 2002), myo-insositol (Kumar et al., 2002; Binesh et al., 2004), and glutamate/glutamine (Binesh et al., 2004) in the dorsolateral prefrontal cortex of older depressed subjects, but did not find differences in the anterior cingulate cortex (Kumar et al., 2002), which is where we obtained our prefrontal cortex measure. These differences are intriguing as these previous studies in older populations primarily examined acutely depressed subjects. It is possible the current study is identifying trait markers related to risk of depression, while previous studies were examining state markers of depression, although studies in midlife adults found that metabolite differences observed in acute depression were not present on remission (Hasler et al., 2005; Hasler et al., 2007). Testing such a hypothesis requires MRS techniques to be linked to a treatment study.
Regarding our PFC observations, NAA is considered a marker of neuronal viability (Moffett et al., 1991; Simmons et al., 1991; Urenjak et al., 1993), while older depressed populations exhibit reduced density of PFC neurons (Rajkowska et al., 2005). However, more recent work has demonstrated that NAA may be expressed in mature oligodendrocytes (Bhakoo and Pearce, 2000), and so may reflect the formation and maintenance of myelin (Chakraborty et al., 2001), thus it is unclear if this finding represents changes in myelination, neuronal loss, or both. We also found a reduction in choline, which is an essential precursor of acetylcholine and membrane phospholipids, and associated with membrane turnover. As oligodendrocytes are involved in membrane turnover, this finding, in conjunction with the NAA finding, suggests a myelination deficit in the PFC of older depressed, recovered subjects.
Although neurodegenerative processes may contribute to these findings, it is also possible these differences are related to subcortical hyperintensities, which are more severe in older depressed populations (Taylor et al., 2005). This theory is supported by observations that individuals with more severe white matter hyperintensity disease exhibit decreased NAA, choline, or creatine (Auer et al., 2001; Murata et al., 2001). This theory does not preclude our findings being related to local changes in neuronal size or glial density (Miguel-Hidalgo et al., 2000; Cotter et al., 2001; Si et al., 2004), as these microstructural changes may themselves occur as a result of the underlying cerebral injury that the hyperintensities represent. Thus the neuronal or glial changes may be secondary to the same vascular processes that contribute to hyperintensity development, or represent distant effects of more localized hyperintensity ischemia.
The findings in the left medial temporal lobe may have a different explanation. The observed higher concentration of NAA and mI, which are neuronal and glial cell markers, supports previous studies examining the hippocampus of depressed subjects that found increased neuronal and glial cell packing (Stockmeier et al., 2004). However, this finding is in contrast to other studies identifying glial reductions in the amygdala in depressed subjects (Hamidi et al., 2004). In general, the left rather than right amygdala appears more responsive to emotional stimuli, particularly those stimuli with a negative valence (Chen et al., 2007). MRS studies of the hippocampal/amygdala region in other psychiatrically ill populations have similarly found metabolic differences specific to the left hemisphere (Li et al., 2006; van Elst et al., 2007).
One may hypothesize that this finding in the left medial temporal lobe is secondary to reduced frontal lobe inhibition of the amygdala. Reduced inhibition by the frontal lobe may contribute to increased left amygdala activity as seen in depression (Drevets, 1999), which may result in neuronal and glial changes and our current findings. This theory may be supported by our previous finding of depression-related deficits in the left hemisphere’s uncinate fasciculus, a fiber tract which connects the amygdala with the prefrontal cortex (Taylor et al., 2007a). This interpretation of the data should be tempered by the recognition that NAA and mI signals do not derive solely from cell bodies, and increased cell density would also be expected to affect Glx and Glu concentrations. More work is needed to better understand hemispheric differences in medial temporal lobe function and how this is related to psychiatric illnesses.
One methodological advantage to the current study is that MRS data were acquired using 3T with a relatively modest single-voxel volume (8mL). In contrast, most of the previous studies used 1.5T scanners with larger voxel volumes (>8mL). Unfortunately even this approach is not optimal when attempting to measure the amygdala, which is between 1.5–2.0mL in size (Frodl et al., 2004).
The present study does have limitations, including how the single-voxel placement includes both gray and white matter, although this was addressed using tissue segmentation, and results adjusted accordingly. Moreover, we did not adjust for multiple comparisons; had we done so, our results would not have reached an adjusted level of statistical significance. Thus these results should be considered as hypothesis-generating, and require replication in larger samples with greater power to detect definitive differences. Finally, there are issues of the sample, where they were not matched on handedness or premorbid intellect. Additionally, although the depressed sample did not meet diagnostic criteria for a current major depressive episode, some had residual symptoms and would not be considered truly remitted. All but one of the depressed subjects were taking antidepressant medication, so it is unclear how this may have influenced our results. Studies examining antidepressant-free subjects are a superior approach, although such a method is more feasible for examining acute depression, rather than subjects who are in remission where antidepressant withdrawal may increase the risk of relapse.
In conclusion, our study supports theories implicating prefrontal neuronal or glial loss as a contributor to late-life depression, a finding that does not necessarily reverse with treatment. Moreover, we identify findings in the left medial temporal lobe which may be related to reduced frontal inhibition of the amygdala. These findings suggest this reduced inhibition may have persistent microstructural effects in the left temporal lobe. Future studies should address the limitations of this study, while also examining the relationship between MRS findings and structural brain measures including measures of white matter hyperintensity severity.
Acknowledgments
We would like to thank Grazyna Rajkowska, PhD, for her careful review of an early version of this manuscript.
Financial Support: This study was supported by NIH grants MH65939, MH60451, NS41328, and NS50329.
Footnotes
Disclosure of Conflicts of Interest: None.
References
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Auer DP, Schirmer T, Heidenreich JO, Herzog J, Pütz B, Dichgans M. Altered white and gray matter metabolism in CADASIL: a proton MR spectroscopy and 1H-MRSI study. Neurology. 2001;56:635–642. doi: 10.1212/wnl.56.5.635. [DOI] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: An MRI-based parcellation of the prefrontal cortex. American Journal of Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Bhakoo KK, Pearce D. In vivo expression of N-acetyl aspartate by oligodendrocytes: Implications for proton magnetic resonance spectroscopy. Journal of Neurochemistry. 2000;74:254–262. doi: 10.1046/j.1471-4159.2000.0740254.x. [DOI] [PubMed] [Google Scholar]
- Binesh N, Kumar A, Hwang S, Mintz J, Thomas MA. Neurochemistry of late-life major depression: a pilot two-dimensional MR spectroscopic study. Journal of Magnetic Resonance Imaging. 2004;20:1039–1045. doi: 10.1002/jmri.20214. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: Evidence for myelin-associated aspartoacylase. Journal of Neurochemistry. 2001;78:736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Payne ME, Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore ET. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301593. Epub Nov 7. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of General Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial application to human brain in vivo. Magnetic Resonance in Medicine. 1989;9:79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, Jager M, Leinsinger G, Bottlender R, Reiser M, Moller HJ. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. Journal of Clinical Psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biological Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, Drevets WC, Charney DS. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biological Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Archives of General Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thomas A, Lavretsky H, Yue K, Huda A, Curran J, Venkatraman T, Estanol L, Mintz J, Mega M, Toga A. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. American Journal of Psychiatry. 2002;159:630–636. doi: 10.1176/appi.ajp.159.4.630. [DOI] [PubMed] [Google Scholar]
- Kusumakar V, MacMaster FP, Gates L, Sparkes SJ, Khan SC. Left medial temporal cytosolic choline in early onset depression. Canadian Journal of Psychiatry. 2001;46:959–964. doi: 10.1177/070674370104601009. [DOI] [PubMed] [Google Scholar]
- Li L, Chen S, Liu J, Zhang J, He Z, Lin X. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in the hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Canadian Journal of Psychiatry. 2006;51:431–437. doi: 10.1177/070674370605100704. [DOI] [PubMed] [Google Scholar]
- McLean MA, Woermann FG, Barker GJ, Duncan JS. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magnetic Resonance in Medicine. 2000;44:401–411. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen A-K, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychological Medicine. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes in within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychological Medicine. 2003a;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance spectroscopy study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003b;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA, Cangro CB, Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport. 1991;2:131–134. doi: 10.1097/00001756-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murata T, Kimura H, Omori M, Kado H, Kosaka H, Iidaka T, Itoh H, Wada Y. MRI white matter hyperintensities, 1H-MR spectroscopy and cognitive function in geriatric depression: a comparison of early- and late-onset cases. International Journal of Geriatric Psychiatry. 2001;16:1129–1135. doi: 10.1002/gps.501. [DOI] [PubMed] [Google Scholar]
- Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horska A. Asymmetry and gender effects in functionally lateralized cortical regions: A proton MRS imaging study. Journal of Magnetic Resonance Imaging. 2004;19:27–33. doi: 10.1002/jmri.10429. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly J, Leroux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm. IEEE Transactions on Medical Imaging. 1991;10:53–65. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- Pfeiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Research. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Provencher S. LCModel & LCMgui User’s Guide. 2007 http://s-provencher.com/pub/LCModel/manual/manual.pdf.
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS & Neurological Disorders Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biological Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Lafer B, Babb SM, Fava M, Stoll AL, Christensen JD, Moore CM, Yurgelun-Todd DA, Bonello CM, Pillay SS, Rothschild AJ, Nierenberg AA, Rosenbaum JF, Cohen BM. Basal ganglia choline levels in depression and response to fluoxetine treatment: an in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry. 1997;15:837–843. doi: 10.1016/S0006-3223(96)00256-9. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991;45:37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacology Bulletin. 2002;36:58–68. [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Gerig G, Krishnan KR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatric Disease and Treatment. 2007a;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Research Neuroimaging. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychological Medicine. 2007b;37:1763–1773. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- Tran TK, Vigneron DB, Sailasuta N, Tropp J, Le Roux P, Kurhanewicz J, Nielson S, Hurd R. Very selective suppression pulses for clinical MRSI studies of brain and prostate cancer. Magnetic Resonance in Medicine. 2000;43:23–33. doi: 10.1002/(sici)1522-2594(200001)43:1<23::aid-mrm4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. Journal of Neuroscience. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi S, Taylor WD. Neuroimaging in late-life depression. International Review of Psychiatry. 2006;18:443–451. doi: 10.1080/09540260600935454. [DOI] [PubMed] [Google Scholar]
- van Elst LT, Ludaescher P, Thiel T, Büchert M, Hesslinger B, Bohus M, Rüsch N, Hennig J, Ebert D, Lieb K. Evidence of disturbed amygdalar energy metabolism in patients with borderline personality disorder. Neuroscience Letters. 2007;417:36–41. doi: 10.1016/j.neulet.2007.02.071. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Charles HC, Tupler LA, Blitchington T, Kelly L, Krishnan KRR. Focal and lateralized subcortical abnormalities in unipolar major depressive disorder: an automated multivoxel proton magnetic resonance spectroscopy study. Biological Psychiatry. 2003;54:744–750. doi: 10.1016/s0006-3223(02)01908-x. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Research Neuroimaging. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]