Abstract
Objectives
Microglial activation and thrombin formation contribute to brain injury after intracerebral hemorrhage. Tumor necrosis factor-alpha and interleukin-1beta are two major pro-inflammatory cytokines. The present study investigated if thrombin stimulates tumor necrosis factor-alpha and interleukin-1beta secretion in vitro and if microglial inhibition reduces intracerebral hemorrhage -induced brain injury in vivo.
Methods
There were two parts in this study. In the first part, cultured rat microglial cells were treated with vehicle, thrombin (10 U/ml), or thrombin plus minocycline (1 or 10 μM), an inhibitor of microglia activation. Levels of tumor necrosis factor-alpha and interleukin-1beta in culture medium were measured by Enzyme-Linked ImmunoSorbent Assay at 24 hours after thrombin treatment. In the second part, rats had an intracerebral injection of 100-μl autologous whole blood. Rats received minocycline or vehicle treatment. Brain edema was measured at day 3 and brain atrophy was determined at day 28 after intracerebral hemorrhage.
Results
Thrombin receptors were expressed in cultured microglia cells, and tumor necrosis factor-alpha and interleukin-1beta levels in the culture medium were increased after thrombin treatment. Minocycline reduced thrombin-induced upregulation of tumor necrosis factor-alpha and interleukin-1beta. In vivo, minocycline reduced perihematomal brain edema, neurological deficits and brain atrophy.
Discussion
Thrombin stimulates microglia to release the pro-inflammatory cytokines, tumor necrosis factor-alpha and interleukin-1beta, and microglial inhibition with minocycline reduces brain injury after intracerebral hemorrhage suggesting a critical role of microglia activation in intracerebral hemorrhage -related brain injury.
Keywords: brain edema, cerebral hemorrhage, interleukin-1β, microglia, minocycline, tumor necrosis factor-α
Introduction
Microglia are cells within the brain that are activated in response to injury. Depending upon specific conditions, they can have neurotrophic or neurotoxic actions 1. In normal brain, microglia are in a quiescent state, but in the event of injury they become highly phagocytic and they are involved in clearing debris from the damaged site 1. Activated microglia are found associated with ischemic and hemorrhagic brain injury, including intracerebral hemorrhage and there is evidence that microglia contribute to intracerebral hemorrhage-induced brain damage 2-4.
Thrombin is a serine protease and an essential component in the coagulation cascade. It is produced immediately in the brain after intracerebral hemorrhage. Thrombin, at high concentrations, kills neurons and astrocytes in vitro 5-7 and we have demonstrated that thrombin is responsible for early brain damage following intracerebral hemorrhage in vivo 8-11. Thrombin can activate microglia and activated microglia release proinflammatory cytokines such as tumor necrosis factor-alpha and interleukin-1beta, which are harmful to the brain 12.
Minocycline, a second generation tetracycline-based molecule, is a potent inhibitor of microglia activation 13. It is a highly lipophilic compound and penetrates the brain-blood barrier easily 14. Minocycline has been reported to provide neuroprotection by inhibiting microglia. An in vitro study showed that minocycline reduced excitotoxicity in primary neuronal culture by preventing excitotoxin-induced microglial proliferation 15. It also inhibits macrophage/microglia activation after intracerebral hemorrhage in the rat 16, 17.
In the present study, we examined whether or not thrombin can stimulate tumor necrosis factor-alpha and interleukin-1beta secretion in cultured microglial cells and whether or not microglial inhibition with minocycline can reduce brain injury in a rat model of intracerebral hemorrhage.
Materials and Methods
Experiments in vitro
Microglia Culture
Cultures of microglia were established based on the differential adherence of cells harvested from neonatal (day 1-3) Sprague-Dawley rat cortex. The methods used were modified from McCarthy and deVellis 18. Mixed cell cultures were initially established in T75 Falcon flasks. Cerebra were dissected and placed in a medium for astrocytes. Meninges and blood vessels were removed. The medium was then removed and cerebra were minced with a blade. Tissues were suspended in modified Hanks' Balanced Solution (GIBCO). After centrifugation, the cell pellet was digested in 0.5% trypsin at 37°C for 20 minutes, then re-suspended in Buffer T. The cells were then re-centrifuged. After centrifugation, the pellet was re-suspended in astrocyte medium, and the cells plated into T75 Falcon flasks coated with poly-L-lysine at the density of 1×106/ml. The cells were cultured at 37°C in an atmosphere of 5% CO2 in air. The medium was changed after 3-4 days. After 7-10 days, the flask was shaken at 200 rpm for 1 hour on a gyratory shaker at 4°C. The supernatant cells were plated on uncoated T25 flasks and incubated for 30 minutes at 37°C. The cells were then washed with Tris-buffered saline and the supernatant discarded. The remaining microglia cells were fed with microglia culture medium.
Experimental Groups
Cultured microglia were treated with human thrombin (10 U/mL; Sigma) or thrombin (10 U/mL) plus minocycline (1 or 10μM; Sigma). Culture medium was collected for interleukin-1beta and tumor necrosis factor-alpha measurements 24 hours after treatment.
Measurements of interleukin-1beta and tumor necrosis factor-alpha
Cells were seeded at a density of 1×105 in 24 well plates. After 24 hours in growth media, the cells were cultured in serum-free media for 18 hours prior to treatment with thrombin at doses of 0 and 10 U/ml. Microglia-conditioned medium from each well was collected at 24 hours and the concentrations of interleukin-1beta and tumor necrosis factor-alpha were quantified by Enzyme-Linked ImmunoSorbent Assay (R & D Systems). The final interleukin-1beta and tumor necrosis factor-alpha concentration from each well were standardized by protein concentration.
Experiments in vivo
Animal Preparation and Intracerebral Injection
The University of Michigan Committee on the Use and Care of Animals approved the protocols for these animal studies that used male Sprague-Dawley rats aged 3-4 months (Charles River Laboratories). Septic precautions were utilized in all surgical procedures and body temperature was maintained at 37.5 °C using a feedback-controlled heating pad. Rats were anesthetized with pentobarbital (50 mg/kg, I.P.) and the right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood from the catheter was used to determine pH, PaO2, PaCO2, hematocrit and glucose. It was also the source for the intracerebral blood infusion. The animals were positioned in a stereotactic frame (Kopf Instruments). Autologous blood was injected into the right caudate through a 26-gauge needle at a rate of 10 μL per minute using a microinfusion pump (Harvard Apparatus Inc.). The coordinates were 0.2 mm anterior to bregma, 5.5 mm ventral, and 4.0 mm lateral to midline. After intracerebral infusion, the needle was removed and the skin incision closed with suture.
Experimental Groups
Male Sprague-Dawley rats received an infusion of 100-μl autologous whole blood into the right basal ganglia. Rats received minocycline injection intraperitoneally at dose of 45 mg/kg immediately and 12 hours after intracerebral hemorrhage, followed by 22.5mg/kg twice a day for 2 days. In the control group, rats received vehicle only. There were two sets of experiments in this study. In the first set of experiments, the effects of minocycline on intracerebral hemorrhage-induced brain edema were examined at day 3. In the second set, behavioral tests were performed at days 1, 3, 7, 14 and 28. Rats were killed at day 28 for brain atrophy measurement (caudate and lateral ventricle size).
Brain Water and Ion Content Measurements
Rats were killed under deep pentobarbital anesthesia at day 3. The brains were removed immediately and a 4-mm thick coronal section was taken 3 mm from the frontal pole. The brain sample was then divided into cortex or basal ganglia (ipsilateral or contralateral). Tissue samples were weighed to obtain the wet weight. The tissue was then dried in a gravity oven at 100°C for more than 24 hours to determine the dry weight. Tissue water content (%) was calculated as ([wet weight-dry weight]/ wet weight)*100. Dehydrated brain samples were digested in 1 mL of 1 N nitric acid for 1 week. Sodium and potassium ion contents in this solution were measured by flame photometry and expressed in micro-equivalents per gram of dehydrated brain tissue (μEq/g dry wt).
Brain atrophy examination
Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and perfused with 4% paraformaldehyde in 0.1 M pH 7.4 phosphate-buffered saline. Removed brains were kept in 4% paraformaldehyde for four to six hours, then immersed in 25% sucrose for three to four days at 4 °C. The brains were embedded and sectioned on a cryostat (18 μm thick). Coronal sections from 1 mm anterior and 1 mm posterior to the blood injection site were used for hematoxylin and eosin staining 19. Caudate and lateral ventricular areas were measured.
Behavioral Tests
All animals underwent behavioral testing before and after surgery and were scored by experimenters who were blind to both neurological and treatment conditions. To assess behavioral deficits both forelimb placing and forelimb use asymmetry tests were used 9.
Statistical Analysis
All data in this study were presented as mean ± standard deviation. Data were analyzed using Student t test or analysis of variance with a Scheffe's post-hoc multiple comparison test. Behavioral data were analyzed by Kruskal-Wallis test. Significance levels were set at p < 0.05.
Results
Experiments in vitro
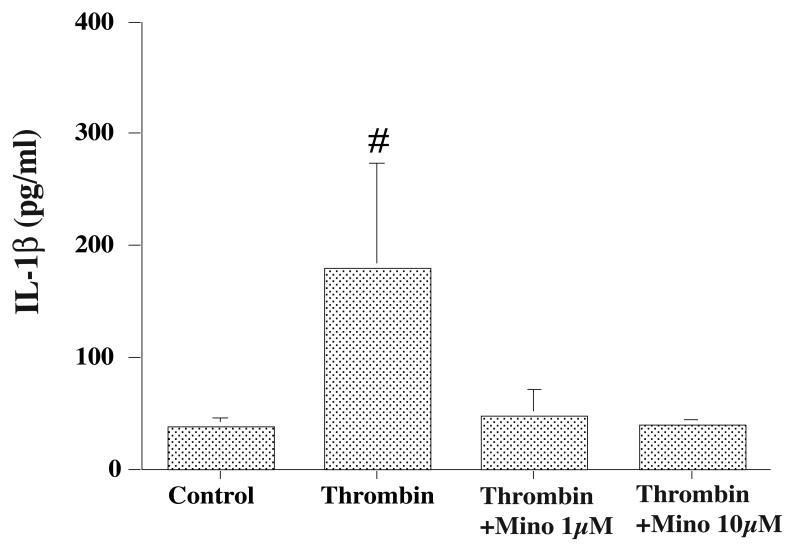
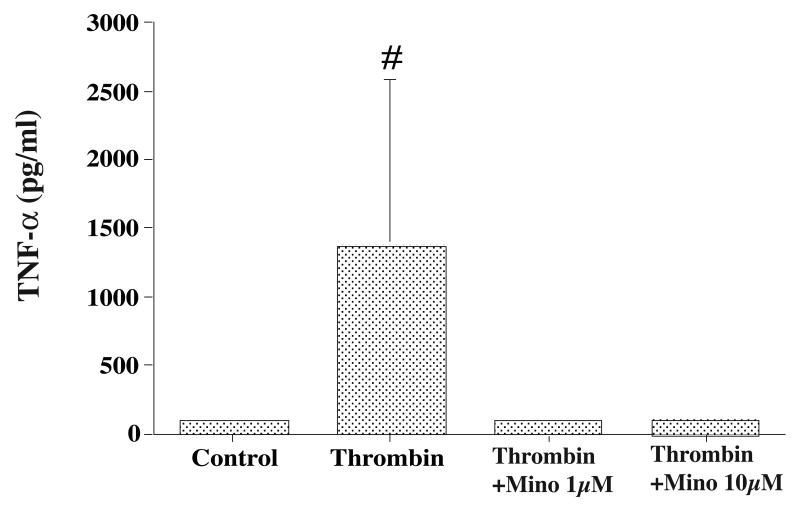
Cultured microglia (Figure 1) released interleukin-1beta and tumor necrosis factor-alpha into the culture medium. Thrombin treatment (10 U/ml) increased interleukin-1beta (180 ± 94 vs. 38 ± 8 pg/ml in the control, p<0.05) and tumor necrosis factor-alpha (1362 ±1224 vs. 101± 21 pg/ml in the control, p<0.01) secretion at 24 hours. Co-treatment of thrombin (10 U/ml) for 24 hours with minocycline (1 and 10 μM) significantly reduced the levels of interleukin-1beta (more than 70% decreases compared with the vehicle-treated group, p<0.01, Figure 2) and tumor necrosis factor-alpha (more than 90% decreases compared with the vehicle treated group, p<0.01, Figure 3) in the culture medium.
Figure 1.
Cultured rat microglia. Scale bar = 20 μm.
Figure 2.
Interleukin-1beta (IL-1β) levels in the microglia culture medium after thrombin treatment. Cultured microglia were treated with either vehicle, thrombin (10 U/ml) or thrombin (10 U/ml) plus minocycline (Mino, 1 or 10 mM). Values are mean ±SD, # p<0.01 vs. the other groups.
Figure 3.
Tumor necrosis factor-alpha (TNF-α) levels in the microglia culture medium after thrombin treatment. Cultured microglia were treated with either vehicle, thrombin (10 U/ml) or thrombin (10 U/ml) plus minocycline (Mino, 1 or 10 mM). Values are mean ±SD, # p<0.01 vs. the other groups.
Experiments in vivo
All physiologic variables were measured immediately before intracerebral injections. Mean arterial blood pressure, pH, arterial oxygen and carbon dioxide tensions (PO2 and PCO2), hematocrit, and blood glucose were controlled within normal range (mean arterial blood pressure, 80-120 mmHg; PO2, 80-120 mmHg; PCO2, 35-45 mmHg; hematocrit, 38-43 %; blood glucose, 80-120 mg/dl).
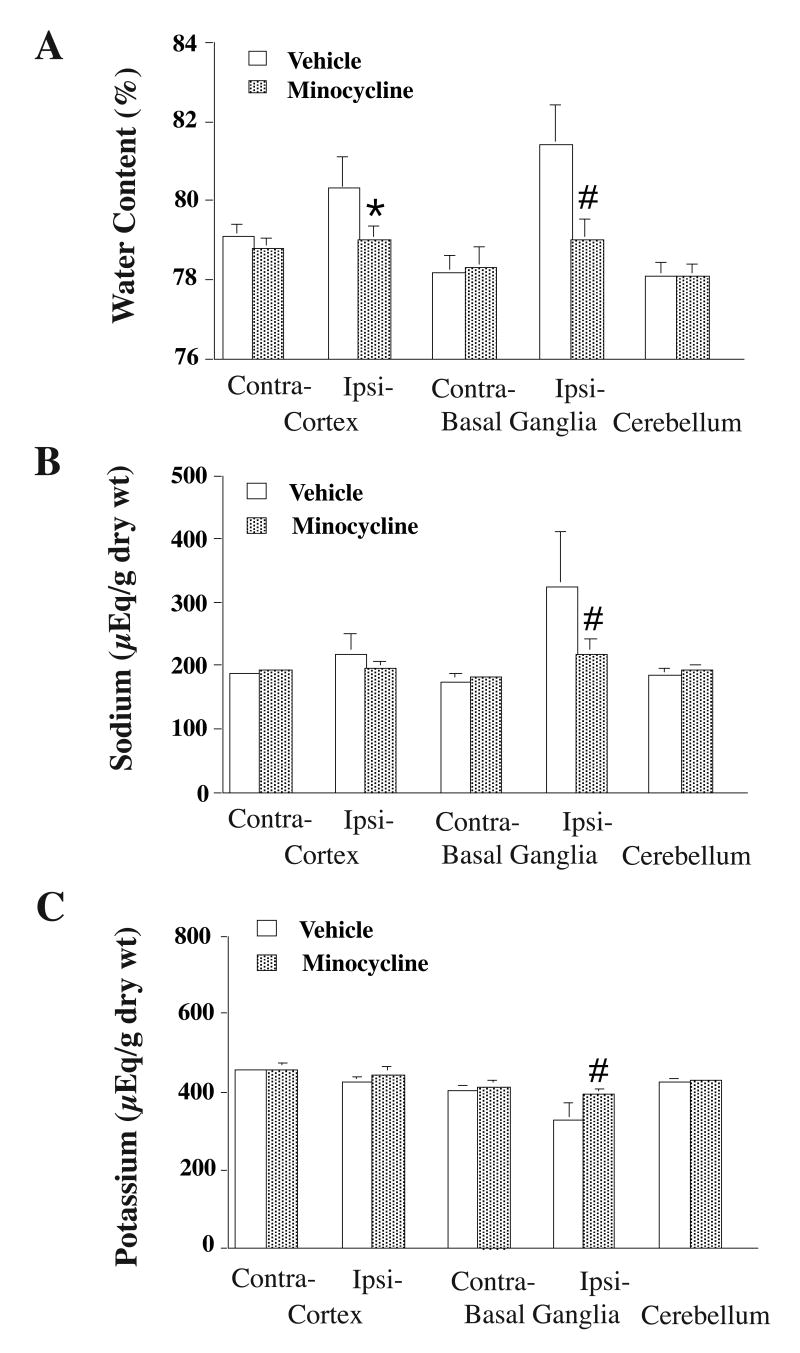
Normal rat brain water content in the basal ganglia is about 78% and intracerebral hemorrhage causes brain edema in the perihematomal zone. Minocycline reduced perihematomal brain edema in the ipsilateral basal ganglia (78.8±0.4 vs. 80.9±1.1% in the vehicle-treated group, p<0.01, Figure 4A). Minocycline also reduced sodium ion accumulation (219 ±22 vs. 324 ± 89 μEq/g dry wt in the vehicle-treated group, p<0.01, Figure 4B) and potassium ion loss (394 ±16 vs. 327 ± 48 μEq/g dry wt in the vehicle-treated group, p<0.01, Figure 4C) in the perihematomal zone.
Figure 4.
Brain water (A), sodium (B) and potassium (C) contents 3 days after intracerebral hemorrhage (ICH). Rats received an injection of autologous blood into the right basal ganglia. The rats were treated with either vehicle or minocycline. Values are mean ± SD. # p < 0.01 vs. vehicle.
Minocycline also improved functional outcome. For forelimb placing, normal rats have a score of 100% and lower scores indicate a deficit. The forelimb placing score was increased by minocycline treatment (day 1: 12±10 vs. 0±0% in the vehicle-treated group, p<0.05; day 14: 98±4 vs. 60±36%, p<0.05). For forelimb use asymmetry, normal rats have a score of 0% and high scores indicate a deficit. Minocycline reduced forelimb using asymmetry score (day 1: 13±24 vs. 47± 16% in the vehicle-treated group, p<0.05; day 3: 13±18 vs. 41±15%, p<0.05).
In addition, minocycline reduced brain tissue loss in the ipsilateral caudate (5±7% vs. 17±5% loss in the vehicle-treated group, p<0.01) and ventricular enlargement (p<0.05, Figure 5).
Figure 5.
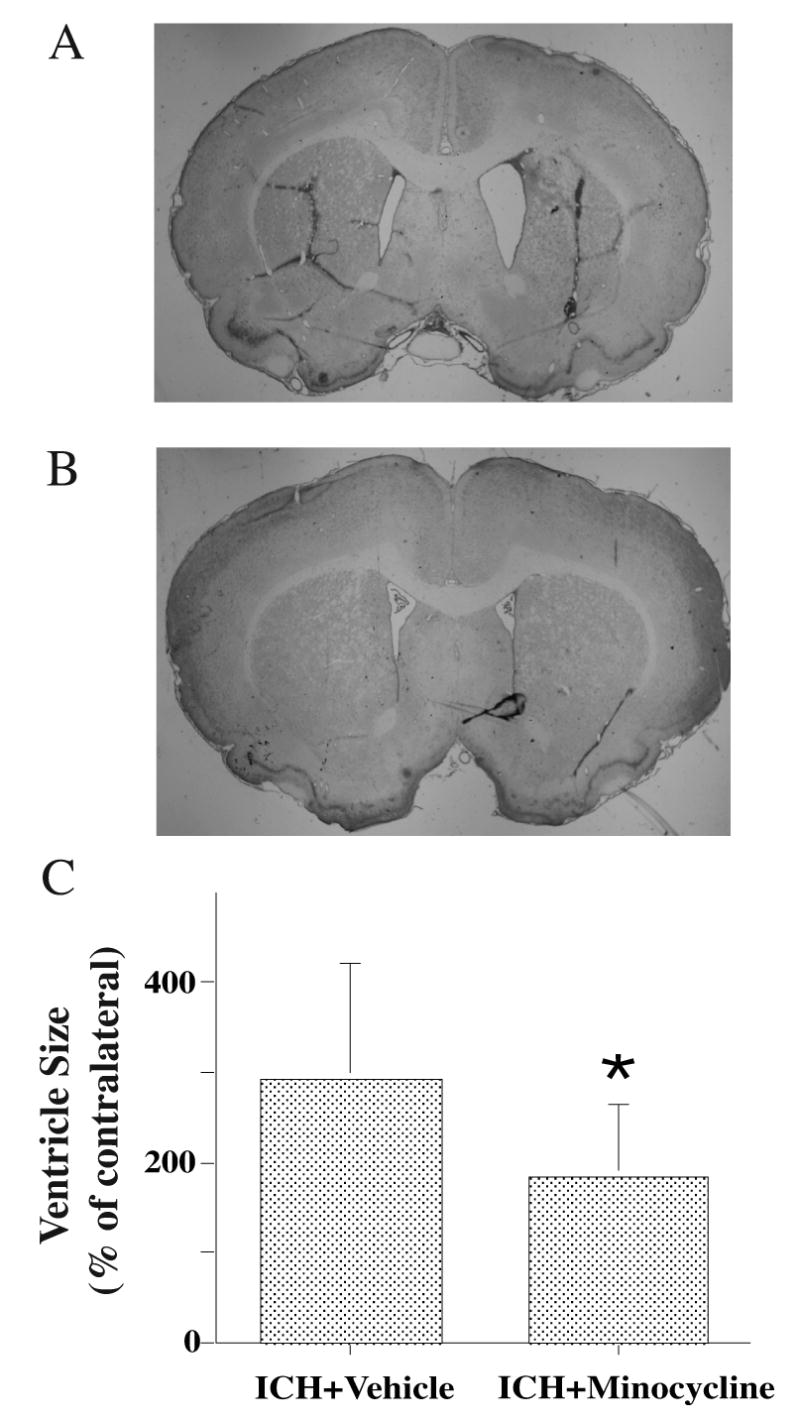
Ventricle sizes 28 days after intracerebral hemorrhage (ICH) in rats treated with vehicle (A) or minocycline (B). For ventricle measurement, the ipsilateral size was expressed a % of contralateral (C). Values are mean ±SD, *p<0.05 vs. vehicle.
Discussion
In the present study, we found that thrombin can stimulate microglia to secrete interleukin-1beta and tumor necrosis factor-alpha, and that microglia inhibition with minocycline reduces intracerebral hemorrhage-induced brain injury. These results suggest an important role of thrombin-activated microglia in brain injury following intracerebral hemorrhage and that this injury may be via pro-inflammatory cytokines.
Thrombin is produced immediately in the brain after an intracerebral hemorrhage. Thrombin is necessary to stop the bleeding and it can also have direct neuroprotective actions at very low concentrations 5, 6, 20. However, at high concentrations, thrombin can also activate potentially harmful pathways. In vivo, thrombin contributes to intracerebral hemorrhage-related injury, including brain edema formation and neuronal death 2, 21. We have shown that thrombin-induced edema is partly from a direct opening of the blood-brain barrier 22. In vitro, thrombin induces apoptosis in cultured neurons and astrocytes 23, potentiates N-methyl-D-aspartic acid receptor function 24 and activates rodent microglia 12 25, 26. The current study focused on the effects of the latter, examining the effects of thrombin on the secretion of pro-inflammatory cytokines by microglia and the results of microglia inhibition on intracerebral hemorrhage-induced brain injury.
Activated microglia secrete many toxic materials such as free radicals 27. Here, we found that rat microglia can secrete interleukin-1beta and tumor necrosis factor-alpha upon exposure to thrombin. Tumor necrosis factor-alpha is a major pro-inflammatory cytokine. Tumor necrosis factor-alpha levels in the brain are increased after intracerebral injection of thrombin and intracerebral hemorrhage 28 and intracerebral hemorrhage-induced brain edema was less in tumor necrosis factor-alpha knockout mice compared with wild type mice 29. It appears that tumor necrosis factor-alpha is involved in intracerebral hemorrhage- and thrombin-induced brain injury. Similarly, interleukin-1 is also a pro-inflammatory cytokine. Although it is important in initiating tissue repair, it can also induce prolonged inflammation and it is associated with certain pathologies in human. Interleukin-1 has multiple potentially harmful effects on the brain, including neurotoxicity, opening the blood–brain barrier, and inducing apoptosis and neutrophil infiltration. Our previous study showed attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist in the brain 30. In all, these results and those of the current study suggest that thrombin-induced production of these pro-inflammatory cytokines by microglia may have an important role in intracerebral hemorrhage-induced brain injury.
Microglia activation contributes to edema formation following intracerebral hemorrhage since minocycline, an inhibitor of microglia activation, results in less perihematomal edema. We have previously found that microglial activation is associated with severe brain swelling after intracerebral hemorrhage4. Many factors, including thrombin and complement factors, contribute to brain edema formation after intracerebral hemorrhage 2, and both thrombin and complement can activate microglia 3, 25. It is unclear, however, whether or not thrombin- or complement-mediated brain edema is through microglia activation.
Minocycline reduces ICH-induced brain tissue loss and improves functional outcomes suggesting that acute microglia activation after intracerebral hemorrhage may be harmful. Microglia secrete many toxic materials such as free radicals and can cause brain damage 27. Our results are supported by a study by Wang et al. who found that microglial inhibition decreases injury volume and improves neurobehavioral outcomes in a mouse intracerebral hemorrhage model 31. However, it should be noted that although microglial activation is a brain injury marker for many central nervous system diseases, microglia can also have neurotrophic actions 1.
In summary, thrombin can stimulate microglia to secrete interleukin-1beta and tumor necrosis factor-alpha. Clarification of the role of microglia activation in intracerebral hemorrhage-induced brain injury should be helpful to develop new therapies for intracerebral hemorrhage.
Acknowledgments
This study was supported by grants NS-017760, NS-039866 and NS-047245 from the National Institutes of Health, 0755717Z from American Heart Association and NSFC30600195 from National Natural Science Foundation of China. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, American Heart Association or National Natural Science Foundation of China.
References
- 1.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Progress in Neurobiology. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang S, Nakamura T, Hua Y, Keep RF, Younger JG, He Y, Hoff JT, Xi G. The role of complement c3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006;26:1490–1495. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- 4.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: Effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. Journal of Neuroscience. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, Xi G. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22:404–410. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: The role of thrombin. J Neurosurg. 1996;84:91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- 9.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 10.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 11.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. The role of blood clot formation on early edema development following experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 12.Moller T, Hanisch UK, Ransom BR. Thrombin-induced activation of cultured rodent microglia. Journal of Neurochemistry. 2000;75:1539–1547. doi: 10.1046/j.1471-4159.2000.0751539.x. [DOI] [PubMed] [Google Scholar]
- 13.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against n-methyl-d-aspartate neurotoxicity by inhibiting microglia. Journal of Immunology. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 14.Klein NC, Cunha BA. Tetracyclines. Medical Clinics of North America. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- 15.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. Journal of Neuroscience. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Annals of Neurology. 2003;53:731–742. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage: Effects of delayed minocycline treatment. Brain Res. 2007;1136:208–218. doi: 10.1016/j.brainres.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: The “Black hole” Model of hemorrhagic damage. Annals of Neurology. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 20.Xi G, Keep RF, Hua Y, Xiang JM, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 21.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? Journal of Neurochemistry. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: Effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 23.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and rhoa activities. Journal of Neuroscience. 1997;17:5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of nmda receptor function by the serine protease thrombin. Journal of Neuroscience. 2000;20:4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- 26.Suo Z, Wu M, Citron BA, Gao C, Festoff BW. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J Bio Chem. 2003;278:31177–31183. doi: 10.1074/jbc.M302137200. [DOI] [PubMed] [Google Scholar]
- 27.Klegeris A, McGeer PL. Interaction of various intracellular signaling mechanisms involved in mononuclear phagocyte toxicity toward neuronal cells. Journal of Leukocyte Biology. 2000;67:127–133. doi: 10.1002/jlb.67.1.127. [DOI] [PubMed] [Google Scholar]
- 28.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. discussion 542-550. [DOI] [PubMed] [Google Scholar]
- 29.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: The role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 30.Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J Neurosurg. 2001;95:680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Annals of Neurology. 2003;54:655–664. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]