Abstract
A major complication of recanalization therapy after an acute arterial occlusion in brain is hemorrhagic transformation (HT). Although it is known that prolonged ischemia is important in the development of HT, the role of reperfusion in ischemia reperfusion induced HT is less well studied. To address the effect of reperfusion on HT, we assessed the incidence and severity of hemorrhage in rats after five hours of middle cerebral artery occlusion (MCAO) followed by 19h reperfusion compared to rats with permanent occlusion (PMCAO) at the same 24h time point. The incidence and amount of hemorrhage, neurological function, and mortality rates were measured. MCAO (5h) with 19h reperfusion was associated with a significantly higher incidence of cortical hemorrhage compared to PMCAO (81.8% vs 18.2%, p < 0.05). Hemorrhage scores were higher in the 5-hour MCAO/reperfusion group compared to PMCAO rats (17.6 ± 11.5 vs 2.4 ± 5.3 in cortex, 20.4 ± 4.6 vs 9.7 ± 4.5 in striatum, p<0.01). Neurological function was worse in the ischemia-reperfusion group compared to PMCAO (p < 0.05) and mortality rates were insignificantly higher in the five-hour MCAO/reperfusion group vs PMCAO group (54.5 % vs18.1%; p < 0.08). The results suggest that reperfusion after prolonged ischemia is associated with increased hemorrhagic transformation and neurological deterioration as compared to permanent ischemia. Whether pharmacological treatments prior to reperfusion attenuate post-ischemic HT requires further study.
Keywords: Animal model, Cerebral ischemia, Hemorrhage, Rat
Introduction
Recombinant tissue plasminogen activator (rt-PA) is the only FDA-approved therapy for acute ischemic stroke. Intravenous rt-PA administration accelerates early recanalization and increases the proportion of patients with minimal or no disability at 3 months by 30 to 35% (1995). However, the relative rate of complete recanalization of major arterial occlusions with intravenous t-PA is low (Wolpert, et al., 1993) (Mori, et al., 1992). Symptomatic intracranial hemorrhage (sICH) after intravenous rt-PA is associated with severe morbidity and mortality, and this risk may limit its use beyond three hours (Hacke, et al., 2004) (Khatri, et al., 2007). Recently, mechanical recanalization was developed for stroke patients. This treatment, which uses mechanical removal of the thrombus, promoted recanalization and produced favorable outcome in the patients within 8 hours of the onset of stroke symptoms. However, symptomatic intracranial hemorrhage was still a primary complication (6.7%–9.9%) (Smith, et al., 2005) (Smith, 2006).
HT after mechanical recanalization is likely to be affected by two factors: ischemia and reperfusion injury. In animal models, it has been shown that prolonged ischemia is important in the development of HT. Specifically, in the rat suture MCAO model, no animals developed HT following temporary MCAO of short duration (1 hour), and the probability of HT increased from 25% to 50% and 75% after 1.5, 2.5 and 3.5 hours of ischemia (Fagan, et al., 2003). No patients had HT among a small cohort of cardioembolic stroke patients with middle cerebral artery occlusions and spontaneous recanalization within six hours, as measured by transcranial Doppler (TCD). Among patients with ischemia for greater than six hours, the HT rate was 60%. All three patients with parenchymal hematoma (PH) type 2 showed recanalization between 6 and 12 hours (Molina, et al., 2001). Previous studies showed that reperfusion promoted reactive oxygen species (ROS) formation, inflammation, blood brain barrier injury, neuronal apoptosis and brain infarction (Peters, et al., 1998) (Clark, et al., 1994)(Morita-Fujimura, et al., 2001)(Yang and Betz, 1994) (Aronowski, et al., 1997). Reperfusion injury may play an important role in the development of HT.
In the present study, we tested whether reperfusion, after prolonged cerebral ischemia, worsens HT compared to no reperfusion in permanent ischemia. A rat suture middle cerebral artery occlusion model (MCAO) was used to produce mechanical reperfusion and to precisely control reperfusion time. The incidence and the amount of hemorrhage were assessed along with neurological function and mortality at 24 hours after either 5 hour MCAO with 19 hours reperfusion or 24 hours after permanent MCAO.
Methods and Materials
Rat Focal Cerebral Ischemia Model
Animal protocols were approved by the University of Cincinnati Animal Care Committee and conform to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing approximately 290–320 grams had unrestricted access to food and water and were housed with a 12-hour light-dark cycle.
Focal ischemia was produced as described in our previous studies (Lu, et al., 2002). Briefly, rats were anaesthetized with 3% isoflurane and maintained with 1.5% isoflurane in a mixture of 70% N2 and 28.5% O2. Rectal temperature was monitored and maintained at 37 ± 0.5°C with a feedback-controlled heating blanket. Blood pressure, blood gases (pO2, pCO2, and pH) and blood glucose concentration were monitored by tail artery catheter and maintained in the normal range. The left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were isolated via a ventral midline incision. A 3/0 monofilament nylon suture was used to occlude the MCA. The suture was inserted into the ECA and advanced into the ICA until the tip occluded the junction of the MCA and anterior cerebral artery. Cerebral blood flow (CBF) was measured using Laser Doppler (5 mm lateral and 2 mm posterior to bregma) (Nito, et al., 2004). The wound was closed and the suture was kept in place for 5 hours. Rats were re-anesthetized, and the filament was pulled back to achieve reperfusion. After the operation, rats were transferred to a temperature-controlled incubator at 37°C for 30 minutes until animals completely woke up, and then to cages with the Delta Phase Isothermal Pad (Braintree scientific, Inc) to prevent hypothermia. Two groups of animals were studied (n = 11). (1) For the permanent ischemia group, the nylon suture was secured in place - in order to permanently occlude the MCA - and rats were sacrificed at 24 h after the onset of ischemia. (2) For the ischemia-reperfusion group, reperfusion was performed at 5 h after MCAO, and rats were sacrificed at 19 h after reperfusion (24 h after the onset of ischemia). Total 3 animals were excluded from experiment due to unsuccessful MCAO.
Hemorrhage rates and volumes
A previously reported and verified visual method of estimating the cerebral hemorrhage was used (Lapchak, et al., 2000) (Lapchak, et al., 2002). At 24 hours after ischemia, rats were sacrificed. The brain was sliced into six 2-mm coronal sections (12 faces). The sections were fixed in 4% paraformaldehyde for 15 minutes. Both sides of the section were scanned. The rate of hemorrhage was first calculated as rats with hemorrhage/total rats in striatum or cortex in each group. Following that, the surface numbers of sections showing hemorrhage in striatum or cortex were counted for each rat in each group. Finally, the hemorrhage area on each hemorrhage section face was measured with an MCID imaging system and hemorrhage scores were calculated. The hemorrhage scores were defined as hemorrhage area seen on any section in the cortex or basal ganglia of each rat (0: no hemorrhage; 1: < 0.8 mm2; 2: 0.8–3.1 mm2; 3: 3.1–7.1 mm2; 4: > 7.1 mm2). (Choudhri, et al., 1997). The hemorrhage surface number and hemorrhage scores represent the hemorrhage volume. The types of hemorrhage were also noted. Hemorrhagic transformation (HT) can be classified as either hemorrhagic infarction (HI) in which blood is interspersed with intact brain elements, or as parenchymal hemorrhage or haematoma (PH) in which a pool of blood destroys brain parenchyma (Petty and Wettstein, 2001). Four major types of HT were assessed: HI1 (small petechiae), HI2 (more confluent petechiae), PH1 (<30% of the infarcted area with some mild space-occupying effect) and PH2 (>30% of the infarcted area with significant space-occupying effect) (Tambasco, et al., 2002).
Determination of Infarct Volume, Brain Swelling and Neurological deficits
These were performed as described in our previous study (Lu, et al., 2002). After the slices were scanned, they were frozen at −80 °C. Coronal sections (20 μm) were cut in a cryostat (−20°C), collected at 1 mm intervals, and stained with Cresyl violet. The standard indirect method was used for measuring the areas of infarction on each section and the volumes of infarction using an MCID digital image analysis system (Imaging Research, Inc, St. Catherines, Ontario, Canada). Brain edema (swelling) was calculated as follows: (volume of infarcted hemisphere − volume of contralateral normal hemisphere)/volume of contralateral hemisphere × 100%. Neurological examinations were also performed. The neurological findings were scored on a 5-point scale: no neurological deficit = 0; forelimb flexion = 1; circling to right = 2; loss of walking = 3; animal death = 4. Average scores were calculated for each group.
Statistical analysis
The rates of hemorrhage and mortality were expressed as percentages. Statistical comparisons for numbers in each group were conducted using a Chi-Square Test. Quantitative data were expressed as mean + SEM. Statistical comparisons were conducted using ANOVA for group comparisons. Differences with p < 0.05 were considered statistically significant.
Results
Physiological data
Table 1 summarized the physiological data including the mean arterial pressure (MABP), pH, PO2, PCO2, glucose and body temperature. There were no significant differences in above parameters between permanent ischemia group and ischemia-reperfusion group at measured time points, although both groups exhibited similar increase in body temperature at 5 h 10 min after MCAO.
Table 1.
Physiological parameters
| Parameter | Group | Before MCAO | 10 min after MCAO | 5 h 10 min after MCAO (10 min after reperfusion) |
|---|---|---|---|---|
| MABP (mmHg) | Per Isch | 110.0 ± 6.3 | 110.6 ± 5.8 | 108.6 ± 7.8 |
| Isch-reper | 113.5 ± 11.7 | 120.6 ± 14.2 | 111.9 ± 21.6 | |
| pH | Per Isch | 7.37 ± 0.04 | 7.38 ± 0.02 | 7.40 ± 0.03 |
| Isch-reper | 7.38 ± 0.02 | 7.38 ± 0.03 | 7.39 ± 0.03 | |
| PO2 (mmHg) | Per Isch | 129.2 ± 14.7 | 127.5 ± 12.6 | 132.5 ± 13.4 |
| Isch-reper | 130.8 ± 13.5 | 129.8 ± 7.8 | 127.8 ± 12.2 | |
| PCO2 (mmHg) | Per Isch | 45.6 ± 2.9 | 46.6 ± 4.0 | 44.5 ± 1.2 |
| Isch-reper | 45.1 ± 4.4 | 44.7 ± 3.9 | 45.5 ± 4.9 | |
| Glucose (mg/dL) | Per Isch | 109.4 ± 32.8 | 108.6 ± 30.4 | 105.0 ± 27.7 |
| Isch-reper | 100.3 ± 37.8 | 98.3 ± 35.1 | 105.5 ± 34.7 | |
| Temperature (°C) | Per Isch | 36.6 ± 0.3 | 36.7 ± 0.3 | 37.8 ± 0.7 |
| Isch-reper | 36.8 ± 0.3 | 36.9 ± 0.4 | 37.9 ± 0.3 |
Perm Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group.
Hemorrhage rate and types
The total hemorrhage rate in the cortex was 81.8% in the ischemia-reperfusion group, which was significantly higher compared to 18.2% in permanent ischemia group (Tables 2) (p<0.01). There were no significant differences between the two groups in the hemorrhage rate of the basal ganglia (Table 3). The hemorrhage types were HI1 and HI2 (Fig. 1). Some of the animals had more than one type of hemorrhage present. Permanent MCAO resulted in hemorrhage mainly in the basal ganglia. Reperfusion after 5 hours of ischemia resulted in hemorrhages in both the basal ganglia and cortex, though there were no statistically significant differences between the groups in the distribution of hemorrhage types.
Table 2.
Rate of cortex hemorrhage
| Groups | Rats with hemorrhage | Rats with no hemorrhage | Number of rats | Hemorrhage rate (%) | Type of Hemorrhage |
|||
|---|---|---|---|---|---|---|---|---|
| HI1 | HI2 | PH1 | PH2 | |||||
| Per Isch | 2 | 9 | 11 | 18.2 | 2 | 2 | 0 | 0 |
| Isch-reper | 9 | 2 | 11 | 81.8** | 4 | 8 | 0 | 0 |
Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group.
p < 0.01 compared with Permanent ischemia group.
Table 3.
Rate of hemorrhage in the basal ganglia
| Groups | Rats with hemorrhage | Rats with no hemorrhage | Number of rats | Hemorrhage rate (%) | Type of Hemorrhage |
|||
|---|---|---|---|---|---|---|---|---|
| HI1 | HI2 | PH1 | PH2 | |||||
| Per Isch | 10 | 1 | 11 | 90.9 | 6 | 10 | 0 | 0 |
| Isch-reper | 11 | 0 | 11 | 100 | 4 | 11 | 0 | 0 |
Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group.
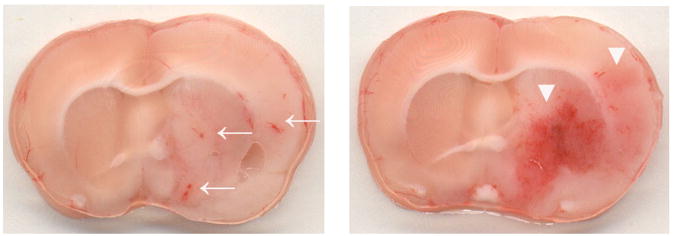
Fig. 1.
Representative brain sections showing the types of hemorrhage that occurred in the ischemia-reperfusion group. Left, HI1 (small petechiae, arrow). Right, HI2 (more confluent petechiae, arrowhead).
Hemorrhage Volume
Fig. 2 shows hemorrhage surface number of sections in the cortex and the basal ganglia. In the permanent ischemia group, there were 4.0 ± 0.0 surfaces of sections with hemorrhage in the cortex (Fig. 2a) and 3.2 ± 1.03 surfaces of sections with hemorrhage in the basal ganglia (Fig. 2b). In the ischemia-reperfusion group 6.1 ± 1.9 surfaces in the cortex (Fig. 2a) and 5.5 ± 1.1 in the basal ganglia (Fig. 2b) showed hemorrhage. The ischemia reperfusion group had statistically greater surface numbers with hemorrhage compared to permanent ischemia in both the cortex and basal ganglia (p ≤ 0.01).
Fig. 2.
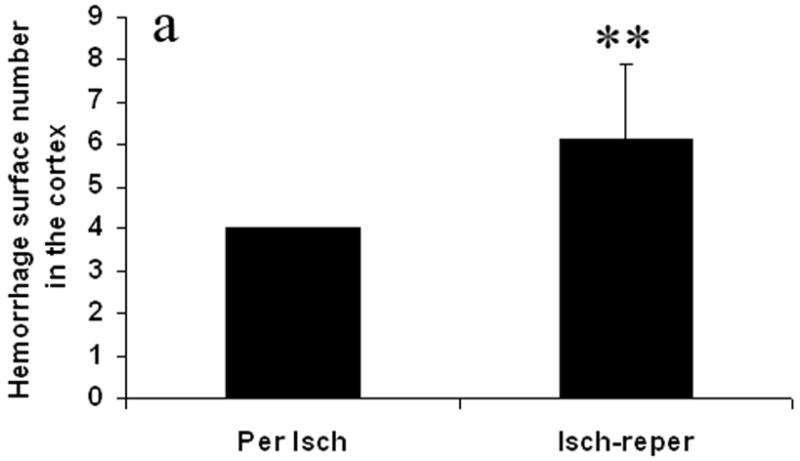

Effect of the reperfusion on hemorrhage surface number in the cortex (Fig. 2a) and the basal ganglia (Fig. 2b). The number of surfaces that show macroscopically visible hemorrhage was counted. The reperfusion after prolonged ischemia increased hemorrhage surface number. Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group. **p < 0.01 compared with permanent ischemia group.
Fig. 3 shows hemorrhage scores. The hemorrhage scores were higher in the ischemia-reperfusion group compared with the permanent ischemia group in the cortex and the basal ganglia. They were 17.6 ± 11.5 vs 2.4 ± 5.3 in the cortex (p < 0.01) (Fig. 3a), and 20.4 ± 4.6 vs 9.7 ± 4.5 in the basal ganglia (p < 0.01) (Fig. 3b).
Fig. 3.
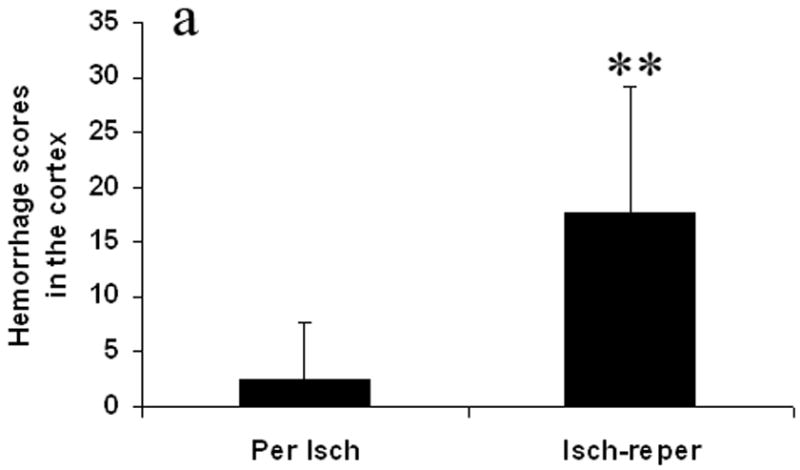
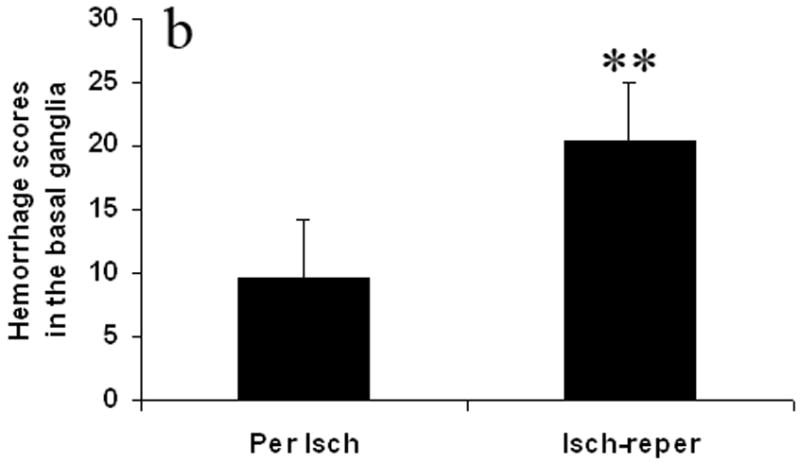
Effect of the reperfusion on hemorrhage score in the cortex (Fig. 3a) and the basal ganglia (Fig. 3b). The hemorrhage scores were calculated as hemorrhage area seen on any of sections in the cortex or basal ganglia. Reperfusion after prolonged ischemia increased hemorrhage score. Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group. **p < 0.01 compared with permanent ischemia group.
Infarct volume and brain swelling
Fig. 4 and Table 4 illustrates the areas and volume of infarction. There was no statistical difference between the ischemia-reperfusion group and the permanent ischemia group in the infarction areas and volume of cortex, basal ganglia and the combination of cortex and basal.
Fig. 4.
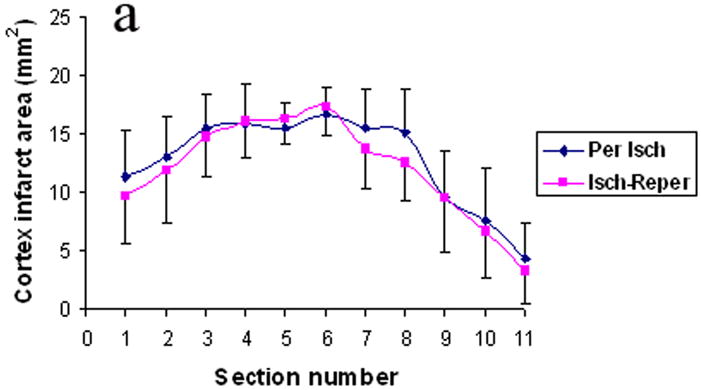
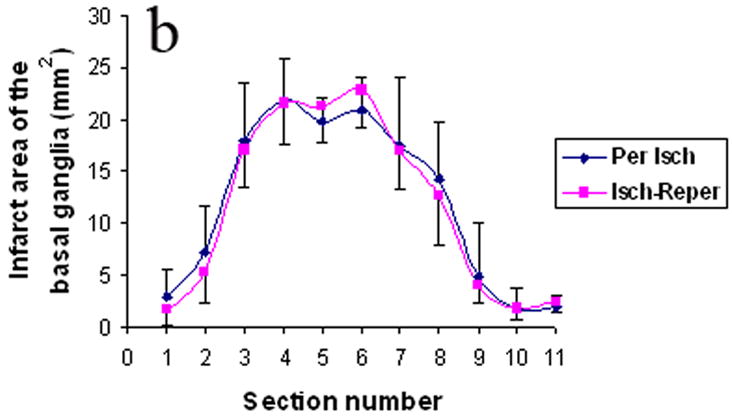
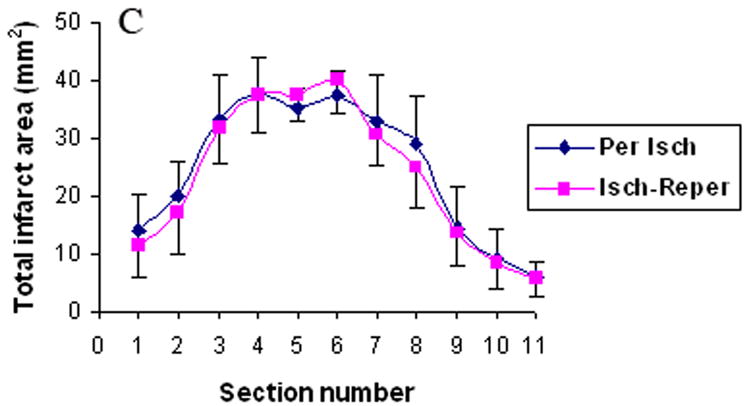
The infarct areas in cortex (Fig. 4a), basal ganglia (Fig. 4b), and the combination of cortex and basal (Fig. 4c). Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group.
Table 4.
Infarct volume
| Groups | Cortex (mm3) | Basal ganglia (mm3) | Total (mm3) |
|---|---|---|---|
| Per Isch | 140.0 ± 28.6 | 130.4 ± 26.3 | 270.4 ± 46.3 |
| Isch-reper | 131.5 ± 24.7 | 127.2 ± 17.8 | 258.7 ± 41.7 |
Per Isch: Permanent ischemia group.
Isch-reper: Ischemia-reperfusion group.
Table 5 illustrates brain swelling. Both the permanent ischemia group and the ischemia-reperfusion group showed brain swelling in cortex and the basal ganglia. There was no statistical difference between the two groups.
Table 5.
Brain swelling
| Groups | Cortex (%) | Basal ganglia (%) | Total (%) |
|---|---|---|---|
| Per Isch | 40.6 ± 8.7 | 24.3 ± 4.7 | 31.2 ± 5.9 |
| Isch-reper | 43.7 ± 9.7 | 28.4 ± 7.3 | 35.1 ± 7.8 |
Per Isch: Permanent ischemia group.
Isch-reper: Ischemia-reperfusion group.
Behavior and mortality
The neurological scores were higher (indicating a greater deficit) in the ischemia-reperfusion group compared with the permanent ischemia group (Fig. 5, p < 0.05). Permanent ischemia caused 18.2% mortality. Ischemia-reperfusion increased mortality to 54.5% (p < 0.08, marginal significance). The animals in the ischemia-reperfusion group died mainly between 3 and 6 hours after onset of reperfusion (8 to 11 hours after ischemia, Fig. 6). The animals that died in ischemia-reperfusion group had evidence of HT in both cortex and basal ganglia.
Fig. 5.
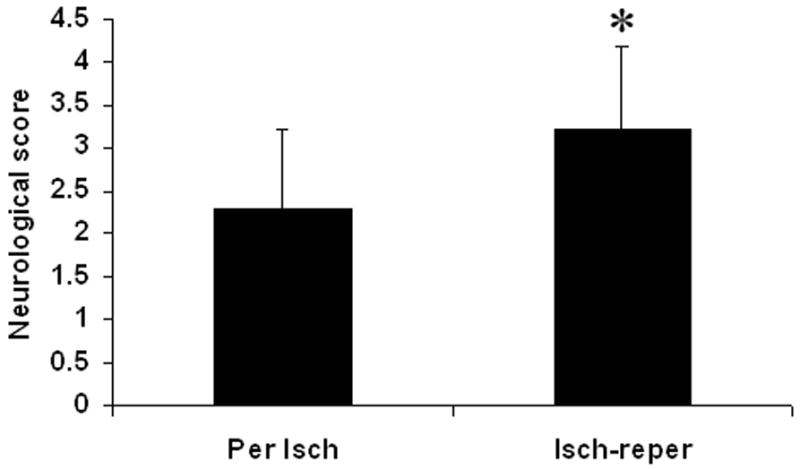
Effect of the reperfusion on neurological score. Increasing neurological scores represent decreased behavioral performance. Reperfusion after prolonged ischemia increased neurological score. Per Isch: Permanent ischemia group. Isch-reper: Ischemia-reperfusion group. *p < 0.05 compared with permanent ischemia group.
Fig. 6.
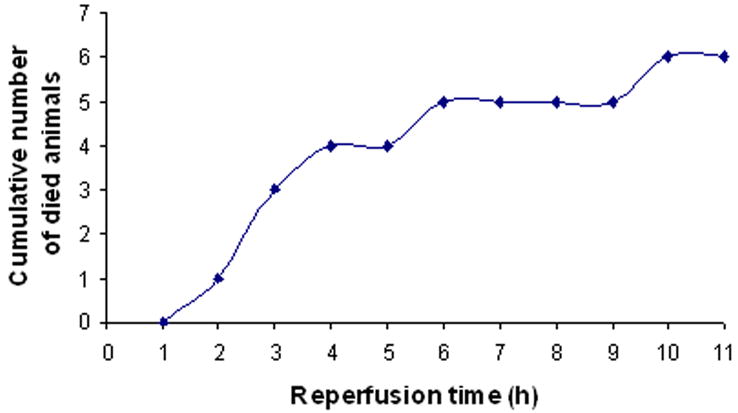
Time course of animal death with reperfusion after 5 hours of ischemia. The animals died mainly between 3 and 6 hours after reperfusion.
Discussion
Even with mechanical removal of clots and recanalization, symptomatic intracranial hemorrhage occurs in 6.7%–9.9% of patients (Smith et al., 2005; Smith, 2006). We performed prolonged ischemia (5 h MCAO) followed by perfusion to produce a HT model in rats that mimics the mechanical removal of clot in humans. This model resulted in more severe intracerebral hemorrhage in the cortex and the basal ganglia compared with permanent ischemia. Because this model is similar to mechanical recanalization, it could be used to test the effects of neuroprotective drugs on HT that results from mechanical reperfusion. In addition, this model would allow for the comparison of mechanisms of tPA and mechanical induced reperfusion injury and whether the same therapy can be used for both.
The current study provides direct evidence that reperfusion after prolonged cerebral ischemia, in the absence of tPA, is associated with more severe HT compared to permanent ischemia. The rate of hemorrhage in the cortex and the hemorrhage volume were both greater following reperfusion compared to permanent MCAO. Previous studies have shown that reperfusion exacerbates blood-brain barrier injury after ischemia in rats (Yang and Betz, 1994) (Aronowski, et al., 1997). Neutrophil infiltration occurred sooner and to a greater extent in reperfused tissues than in permanently occluded tissues (Clark, et al., 1994). Reperfusion also produced a burst in ROS formation in rats (Peters, et al., 1998). Increased reactive oxygen species production during reperfusion contributes to the induction of caspase-8, and exacerbates apoptosis in mice (Morita-Fujimura, et al., 2001). These reports support the concept that reperfusion could worsen HT. Although reperfusion after 5 hours of ischemia in our study produced similar infarction volumes and brain edema compared to permanent ischemia at the end of experiment, our previous study showed that the activation of MMP-2, -9 and neurovascular injury were accelerated by reperfusion, which could contribute to hemorrhage exacerbation (Lu, et al., 2008).
Both permanent and transient MCAO resulted in a high incidence of hemorrhage in the basal ganglia. This may be caused by more rapid and severe ischemic injury in the basal ganglia. Previous studies showed that focal ischemia produced a more severe injury in rat striatum (Lin and Ginsberg, 2000). In a non-human primate MCAO model, HT is most often seen in the corpus striatum where ischemic injury is also greatest (del Zoppo, et al., 1998).
This study also shows that reperfusion worsened neurological function and tended to increase animal mortality. It seems likely that the poorer neurological function in the reperfusion group related to the higher incidence of hemorrhagic transformation in cortex and greater hemorrhagic volume in the reperfusion group compared to the permanent occlusion group. Since 6 animals of 11 died in the reperfusion group, this could have some influence in statistic of infarction volume and brain edema. Future study is needed to clarify whether the differences in neurological function between two groups were due to differences in infarct volume or to edema volumes although they were similar in current study.
In summary, we have shown that reperfusion is important for HT occurrence after prolonged ischemia. Therefore, administration of a hemorrhage protection agent just prior to a reperfusion could be effective on preventing post-ischemic HT, which should be studied in the future.
Acknowledgments
This study was supported by NIH grants NS54652, NS42774, NS43252, NS28167 (Sharp FR), NS44283 (Broderick J and Clark J), NS50569 (Clark J), NS49428 (Pyne-Geithman G), NS30652 (Wagner K) and NS57367 (Lu A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 4.Clark RK, Lee EV, White RF, Jonak ZL, Feuerstein GZ, Barone FC. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Res Bull. 1994;35:387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagan SC, Nagaraja TN, Fenstermacher JD, Zheng J, Johnson M, Knight RA. Hemorrhagic transformation is related to the duration of occlusion and treatment with tissue plasminogen activator in a nonembolic stroke model. Neurol Res. 2003;25:377–382. doi: 10.1179/016164103101201526. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 8.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 9.Lapchak PA, Araujo DM, Song D, Zivin JA. The nonpeptide glycoprotein IIb/IIIa platelet receptor antagonist SM-20302 reduces tissue plasminogen activator-induced intracerebral hemorrhage after thromboembolic stroke. Stroke. 2002;33:147–152. doi: 10.1161/hs0102.100530. [DOI] [PubMed] [Google Scholar]
- 10.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Ginsberg MD. Quantitative assessment of the normal cerebral microvasculature by endothelial barrier antigen (EBA) immunohistochemistry: application to focal cerebral ischemia. Brain Res. 2000;865:237–244. doi: 10.1016/s0006-8993(00)02228-9. [DOI] [PubMed] [Google Scholar]
- 12.Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Ran R, Khatri P, Tomsick T, Sharp FR. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp Neurol. 2008;210:549–559. doi: 10.1016/j.expneurol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, Alvarez-Sabin J. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 15.Mori E, Yoneda Y, Tabuchi M, Yoshida T, Ohkawa S, Ohsumi Y, Kitano K, Tsutsumi A, Yamadori A. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 16.Morita-Fujimura Y, Fujimura M, Yoshimoto T, Chan PH. Superoxide during reperfusion contributes to caspase-8 expression and apoptosis after transient focal stroke. Stroke. 2001;32:2356–2361. doi: 10.1161/hs1001.097241. [DOI] [PubMed] [Google Scholar]
- 17.Nito C, Kamiya T, Ueda M, Arii T, Katayama Y. Mild hypothermia enhances the neuroprotective effects of FK506 and expands its therapeutic window following transient focal ischemia in rats. Brain Res. 2004;1008:179–185. doi: 10.1016/j.brainres.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, Dirnagl U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Petty MA, Wettstein JG. Elements of cerebral microvascular ischaemia. Brain Res Brain Res Rev. 2001;36:23–34. doi: 10.1016/s0165-0173(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 20.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 22.Tambasco N, Corea F, Luccioli R, Ciorba E, Parnetti L, Gallai V. Brain CT scan in acute ischemic stroke: early signs and functional outcome. Clin Exp Hypertens. 2002;24:687–696. doi: 10.1081/ceh-120015345. [DOI] [PubMed] [Google Scholar]
- 23.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. 1664–1655. doi: 10.1161/01.str.25.8.1658. [DOI] [PubMed] [Google Scholar]