Abstract
Newly synthesized proteins are usually exported through the endoplasmic reticulum (ER) and Golgi due to the presence in their primary sequence of a hydrophobic signal peptide that is recognized by the ER translocation system. However, some secreted proteins lack a signal peptide and are exported independently of ER-Golgi. Fibroblast growth factor (FGF)1 is included in this group of polypeptides, as well as S100A13 that is a small calcium-binding protein critical for FGF1 export. Classically secreted proteins are transported into ER in their unfolded states. To determine the role of protein tertiary structure in FGF1 export through the cell membrane, we produced the chimeras of FGF1 and S100A13 with dihydrofolate reductase (DHFR). The specific DHFR inhibitor, aminopterin, prevents its unfolding. We found that aminopterin did not inhibit the release of FGF1:DHFR and S100A13:DHFR. Thus, FGF1 and S100A13 can be exported in folded conformation.
Keywords: FGF1, S100A13, nonclassical release, protein folding, dihydrofolate reductase, aminopterin
INTRODUCTION
Most secreted proteins have in their primary structure a short (7–25 aminoacids) N-terminal hydrophobic signal peptide (SP) that allows the ribosome-protein complex to be recognized by a signal recognition particle (SRP) in the cytosol [1]. Through the GTP-dependent interaction between the SRP and SRP receptor at the endoplasmic reticulum (ER) membrane, the ribosome docks onto the translocon channel, and the nascent protein is released into the ER compartment [1]. Nascent proteins co-translationally enter ER in unfolded conformation, and subsequent folding and covalent modifications occur in the ER and Golgi before the protein is released through the exocytotic fusion of a Golgi-derived secretory vesicle with the cell membrane. Mitochondrial proteins synthesized in the cytosol post-translationally translocate through mitochondrial membranes also in unfolded conformation, using TOM and TIM translocases [2].
The ER-Golgi-dependent secretion pathway is usually referred to as classical or conventional. Secreted proteins that lack a SP generally cannot enter the ER, and their release is not attenuated by Brefeldin A that interferes with ER-Golgi organization. Their secretion pathways are defined as nonclassical or unconventional [3]. Unconventionally secreted proteins include the fibroblast growth factor (FGF)1 [4] and FGF2 [5–7], interleukin (IL)1α [8] and IL1β [9, 10], macrophage migration inhibitory factor [11], morphogen epimorphin [12], HSP70 [13, 14] and HSP90 [15], S100 proteins [16, 17], sphingosine kinase 1, [18, 19], thioredoxin [20, 21], chromatin-associated proteins HMBG1 [22] and Engrailed 2 [23, 24], secretory transglutaminase [25], annexins I [26] and II [27, 28], galectins [29–31], and some secreted viral [32–34] and protozoan [35] polypeptides. While some of these proteins exhibit constitutive release, others are secreted upon specific stimulation [36]. Secretion of different SP-less proteins varies in its sensitivity to chemical inhibitors [3, 36], suggesting that unconventional secretion is not a uniform process.
FGF1 release is induced by cellular stress conditions such as heat shock [4], hypoxia [37], serum starvation [38], and oxidized LDL treatment [39]. FGF1 is released from the cells as a copper-dependent multiprotein complex that includes a small calcium-binding protein S100A13 [40, 41]. Unlike FGF1, this accessory protein can be constitutively released from the cells. However, when coexpressed with FGF1, its export becomes stress-dependent [36].
The use of a dominant negative mutant of S100A13 demonstrated the importance of this protein for FGF1 export [41]. Particularly interesting, S100A13 participates also in the stress-dependent nonclassical export of IL1α [42] and specifically associates with FGF1 at stress in glial cells [43]. Our confocal microscopy study demonstrated that under stress, FGF1 and S100A13 colocalize at the plasma membrane [44]. How these proteins are translocated through the cell membrane, and what is their conformational status during this process remain to be determined.
Studies based on the production of dihydrofolate reductase (DHFR)-containing chimeras are conducted to clarify the role of unfolding in protein translocation across the biological membranes [45]. This method is based on the use of a specific DHFR inhibitor aminopterin (AP) that stabilizes the tertiary structure of the enzyme or its chimeras, and prevents their transport across biological membranes if this transport requires protein unfolding [45]. Using this method, Nickel et al. demonstrated that FGF2 does not require total unfolding for its unconventional export [7]. However, FGF1 and FGF2 are released through distinct pathways [3, 46]. Indeed, unlike FGF1, FGF2 is exported constitutively, and its export is sensitive to the inhibitors of Na+/K+ ATPase [6]. Therefore, we used the DHFR chimera approach to determine whether protein unfolding is a prerequisite of the release of FGF1 and the critical component of FGF1 release complex, S100A13. We found that complete unfolding is not required for the unconventional export of FGF1 and S100A13.
MATERIALS AND METHODS
Production of FGF1:DHFR and 6Myc:S100A13:DHFR chimeras
The original murine DHFR construct was generously gifted by Walter Nickel, University of Heidelberg, Germany. The DHFR cDNA was excised by digestion with BsrGI and PvuII. A BsrGI restriction site was introduced by PCR mutagenesis (Quick Mutagenesis Kit, Stratagene) at the C-termini of FGF1:HA and 6Myc:S100A13 genes in their respective expression plasmids. FGF1α [47] form was used as in all the previous FGF1 export experiments of our laboratory [36, 48]. After mutagenesis, FGF1:HA TOPO construct (generous gift of Andrew Baird, Human BioMolecular Research Institute, San Diego, CA) and 6Myc:S100A13 pcDNA 3.1 Hygro [41] were digested with BsrGI and Pvu II, and the DHFR fragment was ligated in frame with FGF1:HA and 6Myc:S100A13. The DHFR chimeras constructs were sequenced using the DNA Sequencing Kit (Applied Biosystems) according to manufacturer’s instruction.
DHFR chimeras expression in NIH 3T3 cells and their stabilization by aminopterin
NIH 3T3 cells were grown on 10 cm Petri dishes in DMEM containing 10% Bovine Calf Serum (Hyclone) and 1% antimycotic antibiotic solution (GIBCO) to 60% confluency, and then transfected by using the JetPei transfectant reagent (QBiogene) with 1 μg DNA per dish of FGF1 (the above mentioned FGF1:HA construct was used), FGF1:DHFR, 6Myc:S100A13 or 6Myc:S100A13:DHFR. Twenty-four hours after transfection, the cells were collected and lysed in 100 mM Hepes-KOH pH 7.4, 2 mM CaCl2, 0.2% Triton X100. The cell lysates (CL) were resolved by SDS-PAGE, blotted with rabbit anti-FGF1 antibody [4] for FGF1 and FGF1:DHFR or rabbit anti-Myc antibodies (Covance) for 6Myc-S100A13 and 6Myc:S100A13:DHFR, and developed by using the ECL kit (Roche). In trypsin protection experiments, CL were treated with 200 μg/ml trypsin in the presence or absence of 50 μM AP. The CL were resolved by SDS-PAGE and blotted with rabbit anti-DHFR antibody (Sigma).
Study of DHFR chimeras secretion
NIH 3T3 cells were transiently transfected with FGF1:DHFR or with 6Myc:S100A13:DHFR, as described above. Twenty four hours later, each 10 cm dish was split into four 15 cm dishes. Two days later, the cells were incubated for 4 hours in complete culture medium with either 1mM AP or with DMSO (solvent control). Then, heat shock was performed in DMEM containing 5U/ml heparin and either 1 mM AP or DMSO. Conditioned media from cultures incubated for 2 hours at 37°C and 42°C were filtered and treated with 0.1% dithiotreitol at 37°C for 2 hours. FGF1:DHFR was purified from the conditioned media by using heparin chromatography as described [4], whereas 6Myc:S100A13:DHFR was purified by immunoprecipitation [41] with monoclonal anti-Myc antibody (Covance). CL were prepared as controls of protein expression. The CL and conditioned media were analyzed by SDS-PAGE and immunoblotted as described above.
Immunofluorescence control of aminopterin efficiency (prevention of DHFR transmembrane translocation)
NIH 3T3 cells grown on fibronectin-coated glass coverslips were transfected with a reporter construct coding for the chimera comprised of the mitochondrial transport sequence (MTS), GFP and DHFR (MTS:GFP:DHFR) that was generously gifted by Walter Nickel, University of Heidelberg, Germany. Eight hours from transfection, the cells were incubated for four hours in complete culture medium with either 1 mM AP or DMSO. Then, the cells were fixed in 10% formalin for 10 min. Confocal microscopy (LTCS-SP microscope, Leica) was used to observe MTS:GFP:DHFR intracellular distribution in the presence or absence of AP.
RESULTS AND DISCUSSION
The exact mechanism of transmembrane translocation of FGF1 is unknown. To understand this mechanism, it is important to clarify the role of tertiary conformation of FGF1 release pathway components in this process. Indeed, translocation of proteins into the ER and mitochondria is based upon their unfolded conformation [1, 2]. To elucidate whether the nonclassical export of FGF1 requires unfolding of FGF1 and S100A13, we applied the method of DHFR tagging of the released proteins [45]. FGF1 and S100A13, a member of both the FGF1 and IL1α release complexes, were C-terminally tagged with DHFR (Figure 1A), which is an enzyme locked in folded conformation by its specific inhibitor AP [45]. FGF1:DHFR and 6Myc:S100A13:DHFR chimeras were transfected to NIH 3T3 cells and their expression was verified by using, respectively anti-FGF1, and anti-Myc antibodies (Figure 1B). The AP-induced conformational change of the DHFR moiety of chimeras was assessed based on its sensitivity to trypsin-induced proteolysis. Lysates of cells transfected with chimeras were treated with trypsin in the presence and absence of AP, resolved by SDS-PAGE, and immunoblotted using anti-DHFR antibodies. Trypsin treatment resulted in the proteolysis of the chimeras, whereas some free DHFR moiety was still detectable (Figure 1C). AP strongly increased the amount of the trypsin-resistant DHFR moiety.
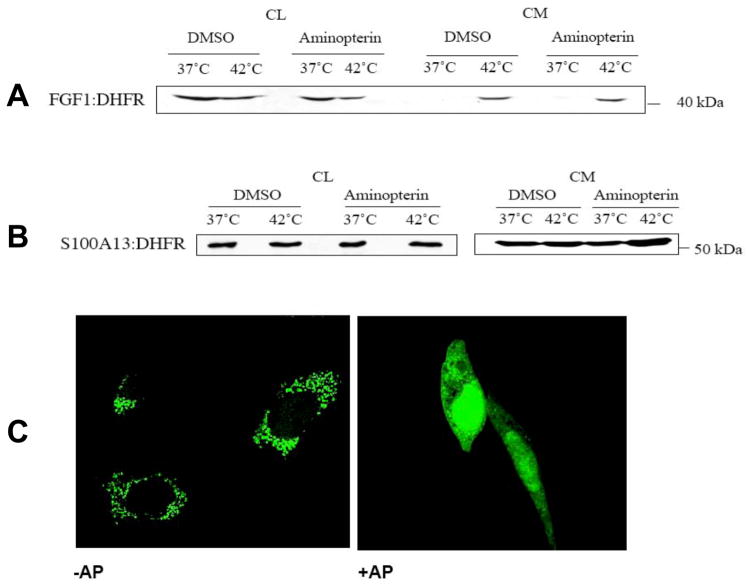
Figure 1. FGF1:DHFR and S100A13:DHFR chimeras.
(A) Structures of FGF1:DHFR and 6Myc:S100:DHFR chimeras. Dotted line (residues 1–21 of FGF1) indicates the residues present in the FGF1β form and absent from the FGF1α form used in our experiments. (B) Expression of FGF1 (FGF1:HA construct was used), FGF1:DHFR, 6Myc:S100A13 and 6Myc:S100A13:DHFR in NIH 3T3 cells, 24 hours after transfection. Cell lysates (CL) were resolved by SDS-PAGE and blotted with anti-FGF1 or anti-Myc antibodies. (C). CL prepared from NIH 3T3 cultures transiently transfected with FGF1:DHFR and 6Myc:S100A13:DHFR were treated with 200 μg/ml trypsin in the presence or absence of 50 μM AP. Untreated CL were used as controls. CL were resolved by SDS-PAGE and immunoblotted with anti-DHFR antibodies.
The immunoblotting of the conditioned medium with anti-DHFR antibodies demonstrated that 1 mM AP treatment did not attenuate the release of FGF1:DHFR from NIH 3T3 cells following heat shock (Figure 2A). AP also did not interfere with the spontaneous export of S100A13:DHFR chimera (Figure 2B). Conversely, 1 mM AP applied to NIH3T3 cells blocked the mitochondrial localization of the GFP:DHFR chimera tagged to the MTS (Figure 2C). Thus, protein folding does not interfere with the nonclassical release of S100A13 and FGF1.
Figure 2. AP does not prevent the secretion of FGF1:DHFR and 6Myc:S100A13:DHFR.
NIH 3T3 cells were transiently transfected with FGF1:DHFR (A) or 6Myc:S100A13:DHFR (B). Transfected cells were pre-incubated for 4 hours with 1 mM AP or with DMSO (solvent control). Then, cells were incubated for 2 hours at 37°C or 42°C, and CL and conditioned media (CM) were collected. FGF1:DHFR and 6Myc:S100A13:DHFR were isolated from CM, resolved by SDS-PAGE and immunoblotted with anti-DHFR antibodies. (C). AP in the concentration of 1 mM abolishes mitochondrial translocation of MTS:GFP:DHFR reporter. NIH 3T3 cells were transfected with MTS:GFP:DHFR. AP was added to the cells 8 h after transfection. Cells were incubated for additional 4 h with or without AP and then fixed. Confocal microscopy (objective X63) demonstrated that incubation with AP results in diffuse cytosolic and nuclear distribution of MTS:GFP:DHFR instead of punctate mitochondrial.
The results of DHFR chimera and experiments indicate that FGF1 and S100A13 export does not require total protein unfolding. How membrane translocation of unfolded proteins proceed without impairing cell viability remains to be determined. Other nonclassically released proteins, such as IL1β and HMBG1 [10, 22] overcome this problem by translocation into lysosome-like vesicles, which further fuse with the cell membrane and release their contents into the extracellular compartment. However, confocal microscopy demonstrated diffuse but not punctate (vesicular) distribution of FGF1 and S100A13 in the cytoplasm [44]. Moreover, recombinant FGF2 translocates through the isolated plasma membrane [7], and we recently obtained similar results with FGF1(unpublished data). Based on the specific interaction of members of the FGF1 release complex with acidic pL [49], we hypothesized [36] that these proteins could be co-exported with acidic phospholipids during their stress-induced translocation from the inner to the outer leaflet of the cell membrane. Recently, Hirai et al. [12] demonstrated that the nonclassical export of epimorphin is associated with the transmembrane translocation of phosphatidylserine. Our results permit further consideration of the problem of nonclassical release of FGF1 and S100A13 from the angle of tertiary protein structure. Significantly, several nonclassically released proteins such as FGF1, FGF2, IL1α and IL1β [3] present a β–barrel in their structure that is also found in many integral membrane proteins [50]. Several members of S100 protein family are nonclassically released, and they all exhibit two characteristic EF-hand domains in their structure [51]. The results of the present study prompt us to further explore the role of specific features of the three-dimensional organization of FGF1 and S100A13 (β-barrel and EF-hands, respectively) in the nonclassical export of these proteins and their interaction with the phospholipid bilayers.
Acknowledgments
The authors thank Walter Nickel from the University of Heidelberg Germany, for generously donating DHFR and MTS:GFP:DHFR constructs. We are also grateful to Andrew Baird, Human BioMolecular Research Institute, San Diego, CA, for the generous gift of the FGF1:HA construct. Irene Graziani was supported in part by a fellowship from the Department of Gerontology, University of Florence, Italy. This work was performed as a partial fulfilment of the PhD dissertations of Andrew Doyle and Sarah Sterling from the University of Maine, Orono. The work was supported in part by NIH grants RR15555 (Project 4), HL35627 and HL32348 to IP.
Abbreviations
- AP
Aminopterin
- DHFR
Dihydrofolate Reductase
- ER
Endoplasmic Reticulum
- FGF
Fibroblast Growth Factor
- IL1
Interleukin 1
- MTS
Mitochondrial Transport Sequence
- SP
Signal Peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blobel G. Protein targeting (Nobel lecture) Chembiochem. 2000;1:86–102. doi: 10.1002/1439-7633(20000818)1:2<86::AID-CBIC86>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Baker MJ, Frazier AE, Gulbis JM, Ryan MT. Mitochondrial protein-import machinery: correlating structure with function. Trends Cell Biol. 2007;17:456–464. doi: 10.1016/j.tcb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 4.Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A. 1992;89:10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol. 1992;151:81–93. doi: 10.1002/jcp.1041510113. [DOI] [PubMed] [Google Scholar]
- 6.Florkiewicz RZ, Anchin J, Baird A. The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na+, K+-ATPase. J Biol Chem. 1998;273:544–551. doi: 10.1074/jbc.273.1.544. [DOI] [PubMed] [Google Scholar]
- 7.Backhaus R, Zehe C, Wegehingel S, Kehlenbach A, Schwappach B, Nickel W. Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding. J Cell Sci. 2004;117:1727–1736. doi: 10.1242/jcs.01027. [DOI] [PubMed] [Google Scholar]
- 8.Tarantini F, Micucci I, Bellum S, Landriscina M, Garfinkel S, Prudovsky I, Maciag T. The precursor but not the mature form of IL1alpha blocks the release of FGF1 in response to heat shock. J Biol Chem. 2001;276:5147–5151. doi: 10.1074/jbc.C000714200. [DOI] [PubMed] [Google Scholar]
- 9.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 12.Hirai Y, Nelson CM, Yamazaki K, Takebe K, Przybylo J, Madden B, Radisky DC. Non-classical export of epimorphin and its adhesion to {alpha}v-integrin in regulation of epithelial morphogenesis. J Cell Sci. 2007;120:2032–2043. doi: 10.1242/jcs.006247. [DOI] [PubMed] [Google Scholar]
- 13.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 14.Mambula SS, Calderwood SK. Heat induced release of Hsp70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. Int J Hyperthermia. 2006;22:575–585. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 16.Davey GE, Murmann P, Heizmann CW. Intracellular Ca2+ and Zn2+ levels regulate the alternative cell density-dependent secretion of S100B in human glioblastoma cells. J Biol Chem. 2001;276:30819–30826. doi: 10.1074/jbc.M103541200. [DOI] [PubMed] [Google Scholar]
- 17.Flatmark K, Maelandsmo GM, Mikalsen SO, Nustad K, Varaas T, Rasmussen H, Meling GI, Fodstad O, Paus E. Immunofluorometric assay for the metastasis-related protein S100A4: release of S100A4 from normal blood cells prohibits the use of S100A4 as a tumor marker in plasma and serum. Tumour Biol. 2004;25:31–40. doi: 10.1159/000077721. [DOI] [PubMed] [Google Scholar]
- 18.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 19.Soldi R, Mandinova A, Venkataraman K, Hla T, Vadas M, Pitson S, Duarte M, Graziani I, Kolev V, Kacer D, Kirov A, Maciag T, Prudovsky I. Sphingosine kinase 1 is a critical component of the copper-dependent FGF1 export pathway. Exp Cell Res. 2007;313:3308–3318. doi: 10.1016/j.yexcr.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 21.Tanudji M, Hevi S, Chuck SL. The nonclassic secretion of thioredoxin is not sensitive to redox state. Am J Physiol Cell Physiol. 2003;284:C1272–1279. doi: 10.1152/ajpcell.00521.2002. [DOI] [PubMed] [Google Scholar]
- 22.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joliot A, Trembleau A, Raposo G, Calvet S, Volovitch M, Prochiantz A. Association of Engrailed homeoproteins with vesicles presenting caveolae-like properties. Development. 1997;124:1865–1875. doi: 10.1242/dev.124.10.1865. [DOI] [PubMed] [Google Scholar]
- 24.Maizel A, Bensaude O, Prochiantz A, Joliot A. A short region of its homeodomain is necessary for engrailed nuclear export and secretion. Development. 1999;126:3183–3190. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- 25.Aumuller G, Wilhelm B, Seitz J. Apocrine secretion--fact or artifact? Anat Anz. 1999;181:437–446. doi: 10.1016/S0940-9602(99)80020-X. [DOI] [PubMed] [Google Scholar]
- 26.Chapman LP, Epton MJ, Buckingham JC, Morris JF, Christian HC. Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology. 2003;144:1062–1073. doi: 10.1210/en.2002-220650. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Hajjar KA. Annexin II: a plasminogen-plasminogen activator co-receptor. Front Biosci. 2002;7:d341–348. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- 28.Peterson EA, Sutherland MR, Nesheim ME, Pryzdial EL. Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J Cell Sci. 2003;116:2399–2408. doi: 10.1242/jcs.00434. [DOI] [PubMed] [Google Scholar]
- 29.Sango K, Tokashiki A, Ajiki K, Horie M, Kawano H, Watabe K, Horie H, Kadoya T. Synthesis, localization and externalization of galectin-1 in mature dorsal root ganglion neurons and Schwann cells. Eur J Neurosci. 2004;19:55–64. doi: 10.1046/j.1460-9568.2003.03102.x. [DOI] [PubMed] [Google Scholar]
- 30.Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110(Pt 10):1169–1178. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- 31.Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquere S, Nishi N, Hirashima M, Middeldorp J, Busson P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. Aids. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 34.Lecellier CH, Vermeulen W, Bachelerie F, Giron ML, Saib A. Intra- and intercellular trafficking of the foamy virus auxiliary bet protein. J Virol. 2002;76:3388–3394. doi: 10.1128/JVI.76.7.3388-3394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- 36.Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, Kirov A, Small D, Kathir KM, Rajalingam D, Kumar TK. Secretion without Golgi. J Cell Biochem. 2008;103:1327–1343. doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouta Carreira C, Landriscina M, Bellum S, Prudovsky I, Maciag T. The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors. 2001;18:277–285. doi: 10.3109/08977190109029116. [DOI] [PubMed] [Google Scholar]
- 38.Shin JT, Opalenik SR, Wehby JN, Mahesh VK, Jackson A, Tarantini F, Maciag T, Thompson JA. Serum-starvation induces the extracellular appearance of FGF-1. Biochim Biophys Acta. 1996;1312:27–38. doi: 10.1016/0167-4889(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 39.Ananyeva NM, Tijurmin AV, Berliner JA, Chisolm GM, Liau G, Winkles JA, Haudenschild CC. Oxidized LDL mediates the release of fibroblast growth factor-1. Arterioscler Thromb Vasc Biol. 1997;17:445–453. doi: 10.1161/01.atv.17.3.445. [DOI] [PubMed] [Google Scholar]
- 40.Landriscina M, Bagala C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem. 2001;276:25549–25557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- 41.Landriscina M, Soldi R, Bagala C, Micucci I, Bellum S, Tarantini F, Prudovsky I, Maciag T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J Biol Chem. 2001;276:22544–22552. doi: 10.1074/jbc.M100546200. [DOI] [PubMed] [Google Scholar]
- 42.Mandinova A, Soldi R, Graziani I, Bagala C, Bellum S, Landriscina M, Tarantini F, Prudovsky I, Maciag T. S100A13 mediates the copper-dependent stress-induced release of IL-1{alpha} from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116:2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- 43.Matsunaga H, Ueda H. Voltage-Dependent N-Type Ca2+ Channel Activity Regulates the Interaction Between FGF-1 and S100A13 for Stress-Induced Non-Vesicular Release. Cell Mol Neurobiol. 2006 doi: 10.1007/s10571-006-9016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prudovsky I, Bagala C, Tarantini F, Mandinova A, Soldi R, Bellum S, Maciag T. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J Cell Biol. 2002;158:201–208. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.America T, Hageman J, Guera A, Rook F, Archer K, Keegstra K, Weisbeek P. Methotrexate does not block import of a DHFR fusion protein into chloroplasts. Plant Mol Biol. 1994;24:283–294. doi: 10.1007/BF00020168. [DOI] [PubMed] [Google Scholar]
- 46.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 47.Forough R, Engleka K, Thompson JA, Jackson A, Imamura T, Maciag T. Differential expression in Escherichia coli of the alpha and beta forms of heparin-binding acidic fibroblast growth factor-1: potential role of RNA secondary structure. Biochim Biophys Acta. 1991;1090:293–298. doi: 10.1016/0167-4781(91)90192-o. [DOI] [PubMed] [Google Scholar]
- 48.Shi J, Friedman S, Maciag T. A carboxyl-terminal domain in fibroblast growth factor (FGF)-2 inhibits FGF-1 release in response to heat shock in vitro. J Biol Chem. 1997;272:1142–1147. doi: 10.1074/jbc.272.2.1142. [DOI] [PubMed] [Google Scholar]
- 49.Graziani I, Bagala C, Duarte M, Soldi R, Kolev V, Tarantini F, Kumar TK, Doyle A, Neivandt D, Yu C, Maciag T, Prudovsky I. Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutik S, Stojanovski D, Becker L, Becker T, Meinecke M, Kruger V, Prinz C, Meisinger C, Guiard B, Wagner R, Pfanner N, Wiedemann N. Dissecting membrane insertion of mitochondrial beta-barrel proteins. Cell. 2008;132:1011–1024. doi: 10.1016/j.cell.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 51.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]