Abstract
Objective
The purpose of this analysis was to investigate the effect of oral feeding experience on clinical outcomes (time to full oral feedings and length of stay) in preterm infants.
Study Design
This analysis was completed on 92 infants who participated in a longitudinal, non-experimental study. Data were collected daily for maturity, weight and experience at oral feeding. Additional data were collected to assess overall morbidity.
Result
Time to full oral feedings was predicted by experience at oral feeding and morbidity. Length of stay from the start of oral feedings until discharge was predicted by feeding experience and by maturity at the first oral feeding. Weight gain was not affected by feeding experience.
Conclusion
Experience at feeding may result in more rapid transition to full oral feedings regardless of severity of illness and may contribute to shorter length of stay. These positive clinical outcomes related to feeding experience warrant further research attention.
Keywords: infant premature, bottle feeding, length of stay, child development, intensive care, neonatal, research
Introduction
Competence at oral feeding is a criterion for discharge from the neonatal intensive care unit (NICU) for infants who are born preterm.1 It is the inability to feed orally, either in bottle or breast that often delays discharge, thus increasing the length of NICU stay. Increased NICU stay has been correlated with increasing iatrogenic risk of complications.2 More importantly, the process by which preterm infants attain feeding competence may be important to short- and long-term development as feeding skills are neurologically derived.3
There are no definitive criteria to guide decisions about when to initiate and how to progress oral feedings in preterm infants.4 In fact, preterm infants often progress in oral feedings using a trial-and-error approach that may contribute to poor clinical outcomes and altered development because of the randomness of neurological stimulation that such an approach provides.5 In addition, preterm infant feeding is relatively complex. The infant is born before the normally expected maturation of various systems has occurred, particularly the neurological system, which controls the coordination of sucking, swallowing and breathing.6 In addition, the infant’s other systems, especially those responsible for cardiorespiratory control, have not matured sufficiently.7 Thus, the normal, evolutionary expected pattern of feeding is not present. The infant has to learn to feed, something that would occur almost reflexively at term birth.
Thus, preterm infants are challenged to feed orally much sooner than there is any reason to suspect that they can feed. Moreover, they are expected to achieve oral feeding skills under adverse conditions, with multiple caregivers who may not be attentive to signs of readiness.8 One aspect of preterm infant feeding that has received little research attention is the function that feeding experience has on the achievement of oral feeding consumption.9–12
The purpose of this analysis was to investigate the effect of oral feeding experience on preterm infants’ clinical outcomes. For this analysis, feeding experience was defined as the number of oral (nipple) feeding opportunities the infant received once oral feedings were started. A feeding was considered an oral feeding opportunity if the infant was taken out of the incubator, swaddled and offered the feeding by nipple; the feeding was counted as an oral feeding regardless of the amount of fluid ingested. Clinical outcomes included either the time in days to full oral feeding from the first oral feeding and length of stay or the time to discharge in days from the first oral feeding. Day of full oral feeding was further defined as the second day on which 100% of all prescribed feeding volume was taken orally, or in other words, after 48 h of all oral consumption occurred. In addition, as oral feeding has been considered a source of energy expenditure13 that might lead to slower weight gain, weight gain changes were also analyzed. It was hypothesized that the amount of oral feeding experience would predict days to full oral feeding and length of stay after oral feedings were initiated.
Methods
The data reported here were from a sample that included 95 preterm infants who participated in a non-experimental study of feeding readiness.9 There was no intervention. Data were collected over 3 years. Infants were eligible for the study if they were born at less than 32 weeks post-menstrual age (PMA) and had no known gastrointestinal, craniofacial, cardiovascular, neurological or muscular defects. Infants were also excluded if they were not able to feed orally at 32 weeks PMA; this criterion resulted in infants being excluded who were on mechanical ventilation, including continuous positive airway pressure (CPAP), or who were otherwise physiologically unstable at 32 weeks PMA. Characteristics of the sample are seen in Table 1. The analysis was completed on data from 92 of the subjects; data from 3 subjects were excluded because infants had been orally fed before the start of the study protocol.
Table 1.
Sample characteristics
| Characteristic | n | % | ||
|---|---|---|---|---|
| Gender | ||||
| Male | 46 | 50 | ||
| Female | 46 | 50 | ||
| Morbidity | ||||
| 1 (most well) | 16 | 17 | ||
| 2 | 7 | 8 | ||
| 3 | 45 | 49 | ||
| 4 | 12 | 13 | ||
| 5 (most ill) | 12 | 13 | ||
| Mean | s.d. | Range | ||
| Maturity (at birth) | ||||
| PMA | 29.3 | 2.0 | 24 | 32 |
| NMA | 7.13 | 1.59 | 3 | 10 |
| Weight | 1295.6 | 401.2 | 550 | 2390 |
| Maturity (at first oral feeding) | ||||
| DOL | 22.2 | 13.8 | 1 | 61 |
| PMA | 32.6 | 0.56 | 32 | 34.3 |
| Weight | 1571.7 | 401.2 | 1055 | 2596 |
| Average daily experience | 4.5 | 2.2 | 0.18 | 9.0 |
| Days to full oral feedings | 16.2 | 11.0 | 0 | 76 |
| Days to discharge | 21.23 | 12.18 | 5 | 88 |
Abbreviations: DOL, day of life; NMA, Neurobehavioral Maturity Assessment; PMA, post-menstrual age.
Infants were observed at one oral feeding daily or every other day depending on their morbidity rating for 10–14 observations. Oral feedings were initiated when infants were between 32 and 34 weeks PMA. The research setting provides minimal policies about offering oral feedings. Although infants are fed on an every 2- or 3-h schedule depending on infant weight, once an infant is between 32 and 34 weeks PMA, not mechanically ventilated, and without distress, the nurse care providers decide if a feeding will be offered orally or given by gavage. There is no protocol for the number of oral feedings to offer per day or for frequency or progression of the number of oral feedings.
A number of measures were taken for variables thought to affect feeding including morbidity and neurodevelopmental maturity. Morbidity was measured using the Neonatal Medical Index (NMI),14 which assesses how ill infants are during the hospital stay. NMI scores at 32 weeks PMA have been reported to be predictive of PMA at full oral feedings.10 NMI classifications range from 1 to 5, with 1 describing preterm infants without serious medical problems and 5 describing infants with the most serious complications; birth weight but not gestation is factored into the scale. The NMI was scored at 32 weeks PMA. Neurodevelopmental maturity was measured using PMA, day of life (DOL) and the Neurobehavioral Maturity Assessment (NMA).15 The NMA includes three simple measures of maturity, namely scarf sign, popliteal angle and tone. Data collectors were trained in techniques to assess the NMA, and interrater reliability among data collectors was maintained at >95%. PMA and DOL increase by 1 on each consecutive day using birth PMA as the start for the daily PMA measure and using the day of birth as the start for the daily DOL measure. The NMA was measured before each feeding observation. Weight was recorded daily at the same time each day using a scale accurate to 1 g.
Data were collected using standardized paper and pencil forms. Feeding experience was monitored daily and recorded on the infant’s study record in a prospective manner, thus there was no missing data. Measures of morbidity and maturity were collected by research assistants who were trained to reliability by the principal investigator. All data were entered into a secure database. Data were checked for completeness by the principal investigator and the statistician. Analyses were carried out using SAS.
Ethics
The study was reviewed and approved by the Institutional Review Board and parents of participating infants gave informed written consent.
Statistics
Statistical analyses were performed for each dependent variable using SAS (version 9.1.3). Predictor variables were tested for significance at α<0.05; nonsignificant variables were removed from preliminary models to result in final models.
Results
Because of the non-normality of the distribution for both the time to full oral feedings and the length of time to discharge from the start of full oral feedings, these data were log-transformed using standard procedures. The log transformations resulted in normally distributed values for both outcomes.
In a preliminary analysis, the number of days to full oral feedings was analyzed using multiple regression. This preliminary model included all subject characteristics described in Table 1. The results of this preliminary analysis appear in the top portion of Table 2. A number of factors were not significant in this preliminary analysis, including birth weight, weight at the time of the first oral feed and maturity. The remaining three variables were significant in the preliminary model, namely gender, infant morbidity and feeding experience.
Table 2.
Prediction model of days to full oral feeding from first oral feeding
| Preliminary model | |||
|---|---|---|---|
| Source | d.f. | F | P-value |
| Gender | 1 | 6.61 | 0.0121 |
| Morbidity | 4 | 0.94 | 0.4443 |
| Maturity | |||
| PMA | 1 | 0.01 | 0.9037 |
| NMA | 1 | 0.15 | 0.7026 |
| DOL | 1 | 0.03 | 0.8540 |
| Weight | 1 | 0.43 | 0.5156 |
| Experience | 1 | 42.74 | <0.0001 |
| Error | 77 | ||
| R2=49%, model significance (F=6.7, P<0.0001) | |||
| Final model | |||
| Gender | 1 | 8.05 | 0.0057 |
| Morbidity | 2 | 4.45 | 0.0145 |
| Experience | 1 | 50.57 | <0.0001 |
| Error | 88 | ||
| R2=47%, model significance (F=19.6, P<0.0001) | |||
Abbreviations: DOL, day of life; NMA, Neurobehavioral Maturity Assessment; PMA, post-menstrual age.
It was observed that male infants took longer to achieve full oral feedings than female infants. Gender differences in other sample characteristics were then examined. Male infants were significantly heavier at birth (1489 versus 1314 g) and heavier at the first oral feeding (1739.5 versus 1558.3 g). Male infants did not differ from female infants on birth gestation (30 versus 29.5 weeks PMA), maturity at feeding start (32 weeks PMA for both), morbidity (11 most well and 8 most ill versus 12 most well and 15 most ill) or on the amount of feeding experience received (average of 5.3 versus 4.9 oral feedings per day). Morbidity (NMI) was measured at five levels. However, using Tukey’s honestly significant difference (HSD), it was determined that there was a significant difference only in the days to full oral feeds between the most ill and most well infants. Accordingly, NMI value was used to collapse the sample into three groups (1, 2+3+4 and 5) and then entered into the final model. The average daily experience at oral feeding was also entered into the final model.
The final multiple regression for days to full oral feedings is shown in the bottom portion of Table 2. Forty-seven percent (R2) of the variability in days to full oral feeds from the first day of oral feeding was predicted by infant morbidity (NMI, P<0.01), infant gender (P<0.006) and the average daily experience of the infant (P<0.0001) (F=19.6, P<0.0001).
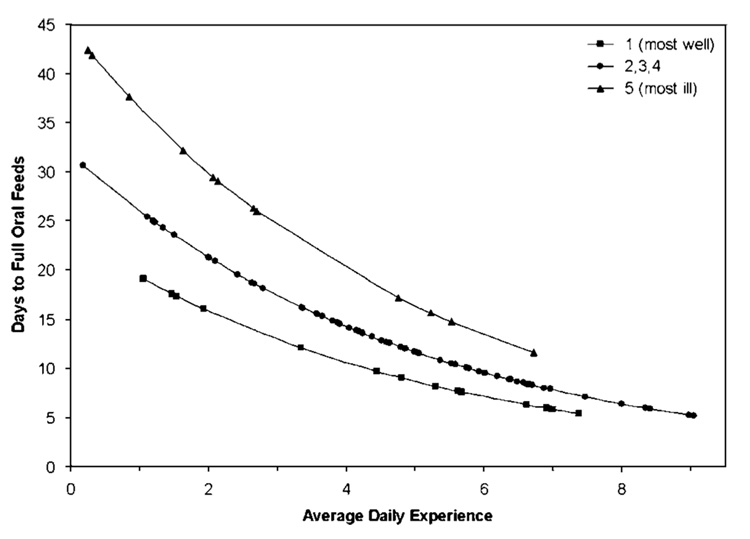
These results are depicted in Figure 1. In the figure, each infant in the study is represented by a point; the legend indicates the morbidity (NMI) rating. As expected, the most ill infants (NMI = five diamonds in the figure) took longer to achieve full oral feedings; these infants are found in the upper curve. The most well infants (NMI = one, square in the figure) achieved full oral feeding in fewer days; these infants are found in the lower curve. In all NMI groups, those with less daily experience at oral feeding took considerably longer time than those with more daily feeding experience. For example, in the most well infants, the infant who had averaged more than six oral feeding opportunities a day achieved full oral feedings after about 8 days. This is in contrast to another infant in the most well group of infants who averaged fewer than two oral feeding opportunities a day. That infant took about 18 days to achieve full oral feedings. This same pattern is seen in all morbidity groups.
Figure 1.
Predicted days to full oral feedings.
Time from the first oral feeding to the time to discharge was modeled in similar manner. In a preliminary analysis, the (log transformed) number of days to discharge was analyzed using multiple regression. This preliminary model included all subject characteristics described in Table 1. The results of this preliminary analysis appear in the top portion of Table 3. A number of factors were not significant in this preliminary analysis, including gender, morbidity, and PMA and NMA at the time of the first oral feed. The remaining three variables were significant in the preliminary model, namely DOL at the first oral feeding, weight at the first oral feeding and feeding experience. When the model was run with these three variables, weight at the first oral feeding was no longer significant. Thus, the final multiple regression model, are shown in the bottom portion of Table 3, includes only DOL at the first oral feeding and feeding experience. Forty-one percent (R2) of the variability in days to discharge from the first day of oral feeding was predicted by DOL at the first oral feeding (P<0.0001) and the average daily experience of the infant (P<0.0001) (F = 29.4, P<0.0001).
Table 3.
Prediction model of days to discharge from first oral feeding
| Preliminary model | |||
|---|---|---|---|
| Source | d.f. | F | p-value |
| Gender | 1 | 1.13 | 0.2917 |
| Morbidity | 4 | 0.41 | 0.8001 |
| Maturity | |||
| PMA | 1 | 0.40 | 0.5273 |
| NMA | 1 | 0.10 | 0.7510 |
| DOL | 1 | 3.59 | 0.0622 |
| Weight | 1 | 5.20 | 0.0255 |
| Experience | 1 | 13.22 | 0.0005 |
| Error | 77 | ||
| R2=47%, model significance (F=5.7, P<0.0001) | |||
| Final model | |||
| DOL | 1 | 23.82 | <0.0001 |
| Experience | 1 | 24.46 | <0.0001 |
| Error | 87 | ||
| R2=41%, model significance (F=29.4, P<0.0001) | |||
Abbreviations: DOL, day of life; NMA, Neurobehavioral Maturity Assessment; PMA, post-menstrual age.
Table 4 shows the predicted days to full oral feeds and days to discharge from the first oral feeding for each NMI morbidity group. Average daily experience is shown here in four groups that summarize the experience at oral feedings that infants received. As the table shows, regardless of the infant’s morbidity (NMI) grouping, infants who received more average daily experience at oral feeding achieved full oral feedings sooner and were discharged to home sooner than infants who received fewer opportunities to feed orally. Within each morbidity group, increased oral feeding experience was associated with a decrease in the length of time to full oral feedings and in the length of time to discharge from the start of oral feedings. Moreover, for length of time to full oral feedings, as feeding experience increased, the range of confidence interval narrowed.
Table 4.
Predicted days to full oral feedings and discharge from first oral feeding
| Days to full oral feedings | Days to discharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NMI | Experience group | n | BW | Mean experience | Estimated days | 95% CI | BW | Estimated days | 95% CI |
| 1 | 2 or less | 4 | 1840 | 1.50 | 17.51 | 12.30, 24.92 | 1840 | 20.4 | 14.37, 26.43 |
| 2.1–4.5 | 2 | 1275 | 3.89 | 10.84 | 8.03, 14.65 | 1275 | 15.6 | 9.56, 21.63 | |
| 4.6–6.5 | 5 | 1639 | 5.34 | 8.12 | 6.02, 10.95 | 1639 | 14.43 | 9.33, 19.53 | |
| More than 6.5 | 6 | 1812 | 6.97 | 5.85 | 4.22, 8.11 | 1812 | 11.50 | 5.99, 17.01 | |
| 2, 3, or 4 | 2 or less | 7 | 1053 | 1.11 | 25.43 | 19.58, 33.02 | 1053 | 28.67 | 18.18, 39.15 |
| 2.1–4.5 | 21 | 1237 | 3.38 | 16.14 | 13.63, 19.11 | 1237 | 24.54 | 19.50, 29.58 | |
| 4.6–6.5 | 22 | 1371 | 5.46 | 10.64 | 9.12, 12.42 | 1371 | 16.44 | 11.90, 20.98 | |
| More than 6.5 | 14 | 1305 | 7.42 | 7.18 | 5.77, 8.93 | 1305 | 15.00 | 8.95, 21.06 | |
| 5 | 2 or less | 4 | 758 | 0.76 | 38.31 | 26.57, 55.23 | 758 | 36.83 | 23.57, 50.10 |
| 2.1–4.5 | 4 | 770 | 2.39 | 27.63 | 19.58, 38.99 | 770 | 36.00 | 22.74, 49.26 | |
| 4.6–6.5 | 3 | 1066 | 5.17 | 15.84 | 10.96, 22.90 | 1066 | 23.50 | 12.00, 34.99 | |
| More than 6.5 | 1 | 950 | 6.72 | 11.62 | 7.70, 17.52 | 950 | 17.67 | 10.09, 36.42 | |
Abbreviations: BW, birth weight; CI, confidence interval; NMI, Neonatal Medical Index.
The effect of feeding experience on weight gain was also examined using a mixed-model analysis of repeated measures. We found that infants in the study gained a minimum of 12.7 g per day and a maximum of 40.1 g per day, with an average daily weight gain of 27.7 g (95% CI = 24.7, 30.75 g). The mean weight gain did not vary by feeding experience.
Discussion
Preterm infants with more oral feeding experience achieved full oral feedings sooner than infants with less experience. In addition, preterm infants who had more oral feeding experience had a shorter length of stay in the NICU once oral feedings were initiated. Moreover, infants with more feeding experience had these improved outcomes without incurring weight loss or slower weight gain.
Although few have studied the effect of feeding experience on feeding outcomes, there are other studies that have achieved similar results. For example, McCain10 has shown that an intervention involving nonnutritive sucking brings infants to an alert state that helps them to feed better, and thus leads to greater feeding experience. This experience in turn has been shown to shorten hospital stay.11 Others have shown that preterm infants can be fed orally earlier and more frequently than previously thought possible, and that these experiences can lead to shortened transition time to full oral feedings and to shortened hospital stays.16
In this current analysis, we found that not only did experience at oral feedings affect the transition time from gavage to full oral feedings but also that feeding experience contributed to this faster transition despite the severity of illness of the infant. For example, the most well infants with the least feeding experience achieved full oral feedings in 17.5 days and were discharged to home 20.4 days after starting oral feedings. Conversely, the most ill infants who received the greatest amount of feeding experience achieved full oral feedings in 13.2 days and were discharged to home 20.5 days after starting oral feedings. Although the numbers in these groups are small, this is a reduction of 6 days in the transition from gavage to full oral feedings.
Experience at oral feedings also predicted days to discharge from the start of oral feeding. However, despite a strong prediction model, there are many more factors that affect discharge besides the consumption of all feedings orally. These additional factors include parental readiness to take the infant home and the stability of the infant’s other health conditions, such as apnea of prematurity. These additional factors are most likely to impinge on the discharge of the most ill infants, making the prediction of their length of stay after the start of oral feedings more difficult.
Preterm birth occurs in 12% of all US births and is a significant financial and social problem in the United States today.17 The financial cost of NICU stays for infants similar to those in this analysis are reported to be as high as $31,000 each, a cost that does not take into account the psychological burden on families associated with having a hospitalized infant.18 Length of NICU stay is one of the predictors of poor developmental outcome in infants who are born preterm,19,20 and one not amenable to quick or easy solutions. However, one potential source of reducing NICU stay is in feeding care. Thus, analyses such as the one presented here may promote a more structured approach to feeding than currently exists in most nurseries. A more structured feeding approach, one that includes more frequent oral feeding opportunities, might facilitate the transition from gavage to full oral feedings. This is not to suggest that infants should be forced to feed orally. However, preterm infants give very subtle cues for readiness, and these may be missed by caregivers unless careful assessment is made. At this time, easy to use, empirically derived tools for clinical assessment of readiness to feed orally at a particular feeding do not exist.21 However, the results of this analysis suggest that increasing opportunities to feed orally on a more regular basis once oral feedings have been initiated and may result in more rapid advancement to full oral feedings. That is, the absence of any adverse behavioral or physiological signs, perhaps preterm infants, may benefit from the predictable offering of oral feedings rather than leaving this up to subjective decision-making.
However, although more rapid discharge is indeed a worthy goal for research and care providers, perhaps a more important benefit may be gained by offering infants more frequent and thus arguably more predictable feeding experiences. That is, there may be evidence to suggest that offering the infant the opportunity to attempt oral feeding at each scheduled feeding may help the infant’s neurological system to develop along more normal pathways, especially in regard to the neurological systems that affect feeding during infancy and childhood.9,22–24
It can be noted that the findings reported here are from a non-experimental study. Thus, they must be viewed carefully. More research is needed on the role of feeding experience on both important feeding outcomes and clinical outcomes such as those reported here.
Acknowledgments
This study is supported by R01 NR005182 and F31 NR007929 from the National Institute of Nursing Research, National Institutes of Health.
References
- 1.American Academy of Pediatrics. Hospital discharge of the high-risk neonate. Pediatrics. 1998;102(2):411–417. [PubMed] [Google Scholar]
- 2.Kirkby SR, Greenspan JS, Kornhauser M, Schneiderman R. Clinical outcomes and cost of the moderately preterm infant. Adv Neonat Care. 2007;7(2):80–87. doi: 10.1097/01.anc.0000267913.58726.f3. [DOI] [PubMed] [Google Scholar]
- 3.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97(1):61–67. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross ES, Browne JV. Developmental progression of feeding skills: an approach to supporting feeding in preterm infants. Sem Neonatol. 2002;7(6):469–475. doi: 10.1053/siny.2002.0152. [DOI] [PubMed] [Google Scholar]
- 5.Dodrill P, McMahon S, Ward E, Weir K, Donovan T, Riddle B. Long-term oral sensitivity and feeding skills of low-risk pre-term infants. Early Hum Dev. 2004;76:23–27. doi: 10.1016/j.earlhumdev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Thoyre SM, Carlson JR. Preterm infants’ behavioral indicators of oxygen decline during bottle feeding. J Adv Nurs. 2003;43(631):641. doi: 10.1046/j.1365-2648.2003.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoyre SM, Brown RL. Factors contributing to preterm infant engagement during bottle-feeding. Nurs Res. 2004;53(5):304–313. doi: 10.1097/00006199-200409000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickler RH, Best AM, Reyna BA, Wetzel PA, Gutcher GR. Prediction of feeding performance in preterm infants. Newborn Infant Nurs Rev. 2005;5(3):116–123. doi: 10.1053/j.nainr.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickler RH, Mauck AG, Geldmaker B. Bottle-feeding histories of preterm infants. J Obstet Gynecol Neonatal Nurs. 1997;26(4):414–420. doi: 10.1111/j.1552-6909.1997.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 10.McCain GC, Gartside P, Greenberg JM, Lott JW. A feeding protocol for healthy preterm infants that shortens time to oral feeding. J Pediatr. 2001;139(3):374–379. doi: 10.1067/mpd.2001.117077. [DOI] [PubMed] [Google Scholar]
- 11.Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. J Pediatr. 2002;141(230):236. doi: 10.1067/mpd.2002.125731. [DOI] [PubMed] [Google Scholar]
- 12.Pridham K, Bhattacharya A, Thoyre S, Steward D, Bamberger J, Wells J, et al. Exploration of the contribution of biobehavioral variables to the energy expenditure of preterm infants. Biol Res Nurs. 2005;6(3):216–229. doi: 10.1177/1099800404272310. [DOI] [PubMed] [Google Scholar]
- 13.Korner AF, Stevenson DK, Kraemer HC, Spiker D, Scott DT, Constantinou J, et al. Prediction of the development of low birth weight preterm infants by a new neonatal medical index. J Dev Behav Pediatr. 1993;14(2):106–111. [PubMed] [Google Scholar]
- 14.Korner AF, Kraemer HC, Reade EP, Forrest T, Dimiceli S, Thom VA. A methodological approach to developing an assessment procedure for testing the neurobehavioral maturity of preterm infants. Child Dev. 1987;58(6):1478–1487. [PubMed] [Google Scholar]
- 15.Pickler RH, Best AM, Reyna BA, Gutcher G, Wetzel PA. Predictors of nutritive sucking in preterm infants. J Perinatol. 2006;26(11):693–699. doi: 10.1038/sj.jp.7211590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson C, Schanler RJ, Lau C. Early introduction of oral feeding in preterm infants. Pediatrics. 2002;110(3):517–522. doi: 10.1542/peds.110.3.517. [DOI] [PubMed] [Google Scholar]
- 17.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990’s. Early Hum Dev. 1999;53(193):218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 18.Cuevas KD, Silver DR, Brooten D, Youngblut JM, Bobo CM. The cost of prematurity: hospital charges at birth and frequency of rehospitalizations and acute care visits over the first year of life: a comparison by gestational age and birth weight. Am J Nurs. 2005;105(7):56–64. doi: 10.1097/00000446-200507000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catelin C, Tordjman S, Morin V, Oger E, Sizun J. Clinical, physiologic, and biologic impact of environmental and behavioral interventions in neonates during a routine nursing procedure. J Pain. 2005;6(12):791–797. doi: 10.1016/j.jpain.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Gressens P, Rogido M, Paindaveine B, Sola A. The impact of neonatal intensive care practices on the developing brain. Pediatrics. 2002;140:646–653. doi: 10.1067/mpd.2002.123214. [DOI] [PubMed] [Google Scholar]
- 21.Howe TH, Lin KC, Su CT, Hsieh CL. A review of psychometric properties of feeding assessment tools used in neonates. J Obstet Gynecol Neonatal Nurs. 2008;37(3):338–349. doi: 10.1111/j.1552-6909.2008.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 23.Pickler RH, Chiaranai C, Reyna BA. Relationship of the first suck burst to feeding outcomes in preterm infants. J Perinat Neonatal Nurs. 2006;20(2):157–162. doi: 10.1097/00005237-200604000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thoyre SM. Feeding outcomes of extremely premature infants after neonatal care. J Obstet Gynecol Neonatal Nurs. 2007;36(4):366–376. doi: 10.1111/j.1552-6909.2007.00158.x. [DOI] [PubMed] [Google Scholar]