Abstract
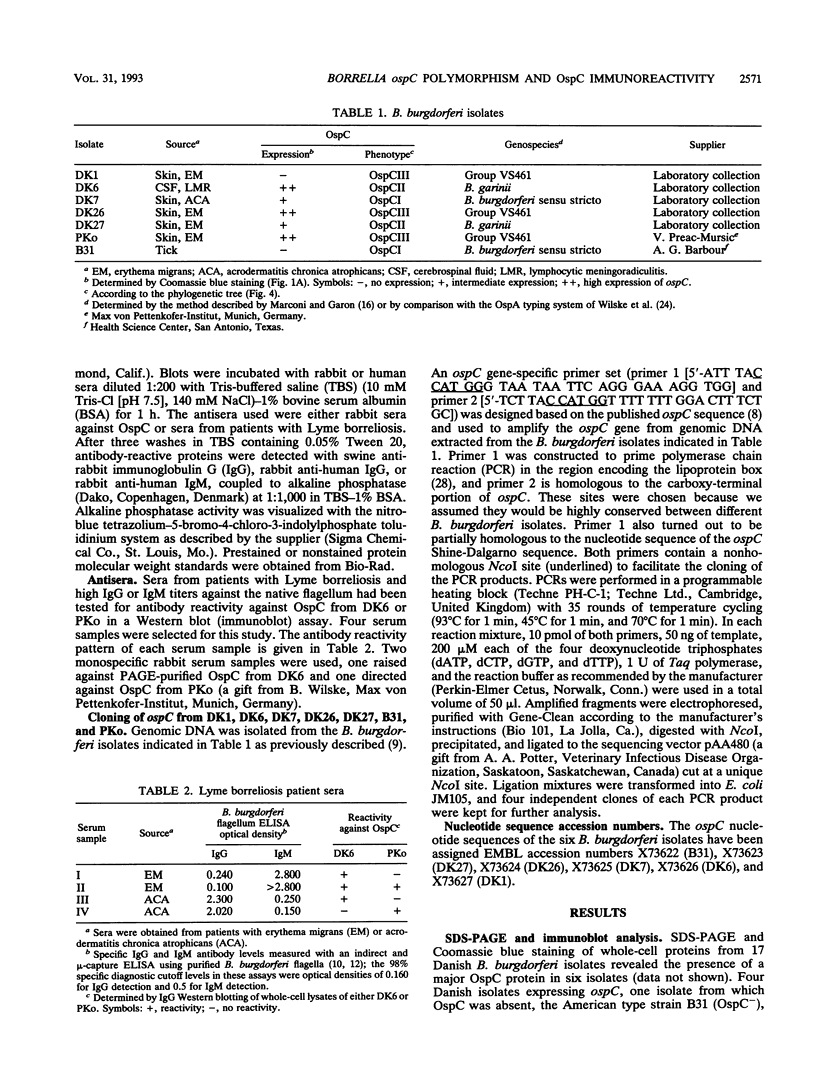
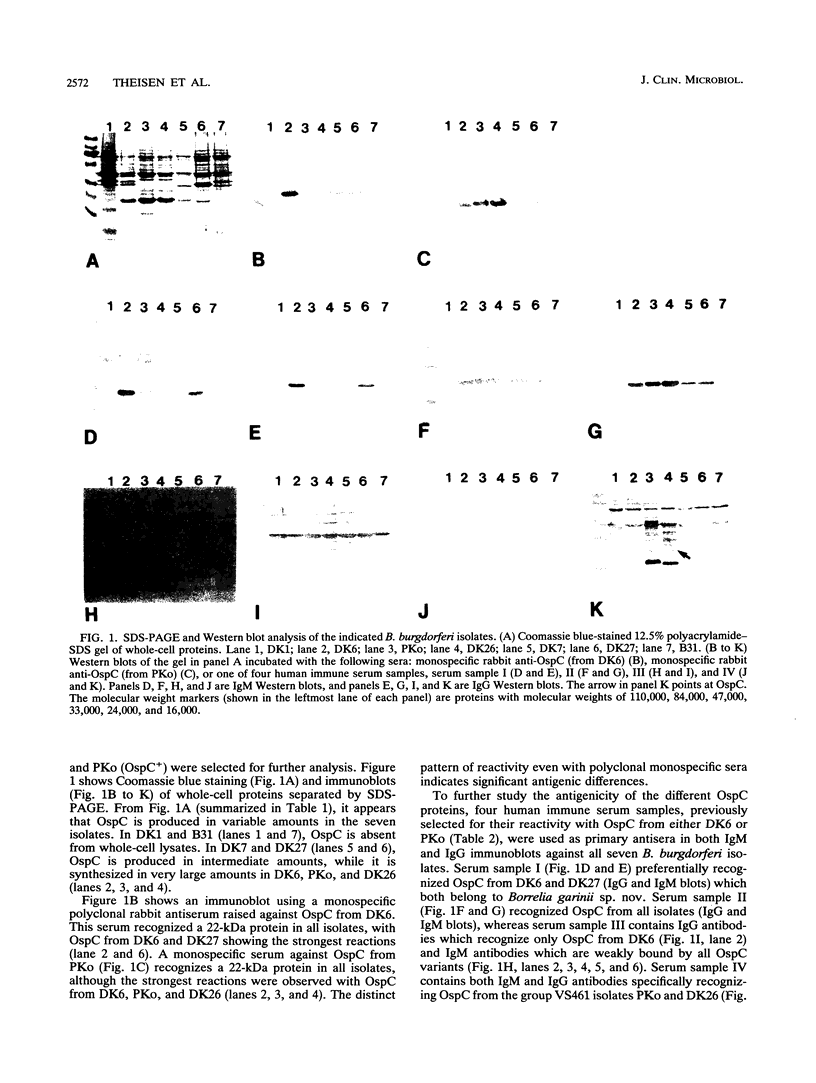
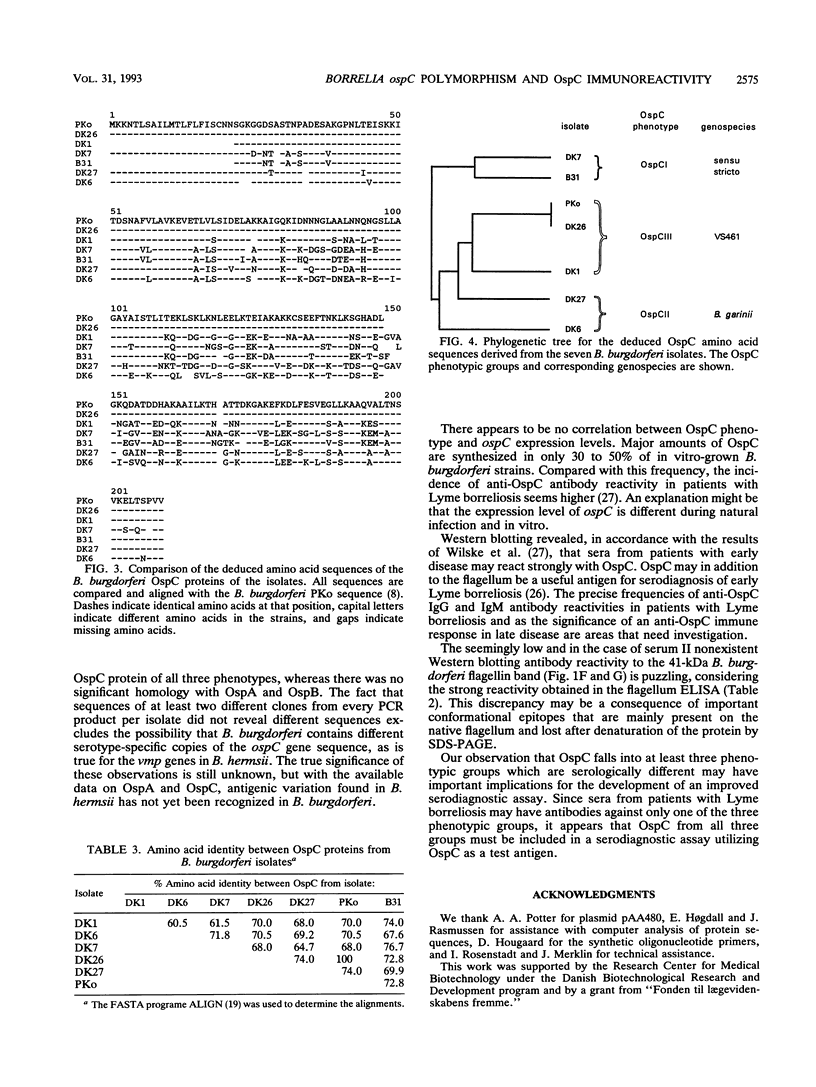
The nucleotide sequences of the ospC gene from five Danish human Borrelia burgdorferi isolates representing all three B. burgdorferi genospecies (B. burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461) and from the American type strain B31 were determined and compared with the published ospC sequence from the German B. burgdorferi isolate PKo (R. Fuchs, S. Jauris, F. Lottspeich, V. Preac-Mursic, B. Wilske, and E. Soutschek, Mol. Microbiol. 6:503-509, 1992). The ospC gene was present in all isolates, regardless of the presence or absence of its product, OspC. The deduced amino acid sequences of OspC from the seven isolates were aligned and revealed pairwise sequence identities ranging from 60.5 to 100%. Differences were scattered throughout the amino acid sequences. A phylogenetic tree was constructed and revealed three distinct phenotypic groups OspCI to OspCIII corresponding to the three delineated genospecies. Immunoblot analysis revealed that the seven OspC proteins tested have both common and specific epitopes. There is significant epitope diversity, since even polyclonal antisera showed serotype-restricted specificity. Therefore, a serodiagnostic assay for Lyme borreliosis utilizing OspC as a test antigen should include all three OspC phenotypes in order to obtain a species-wide sensitivity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbrink E., Hovmark A. Early and late cutaneous manifestations in Ixodes-borne borreliosis (erythema migrans borreliosis, Lyme borreliosis). Ann N Y Acad Sci. 1988;539:4–15. doi: 10.1111/j.1749-6632.1988.tb31833.x. [DOI] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Molecular biology of antigenic variation in Lyme borreliosis and relapsing fever: a comparative analysis. Scand J Infect Dis Suppl. 1991;77:88–93. [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Jauris S., Lottspeich F., Preac-Mursic V., Wilske B., Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992 Feb;6(4):503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Lebech A. M. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985-1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain. 1992 Apr;115(Pt 2):399–423. doi: 10.1093/brain/115.2.399. [DOI] [PubMed] [Google Scholar]
- Hansen K., Pii K., Lebech A. M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991 Jan;29(1):166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauris-Heipke S., Fuchs R., Motz M., Preac-Mursic V., Schwab E., Soutschek E., Will G., Wilske B. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol. 1993 Mar;182(1):37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Stiernstedt G., Granström M., Asbrink E., Wretlind B. Comparison of flagellum and sonicate antigens for serological diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):169–177. doi: 10.1007/BF01963833. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992 Nov;30(11):2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken R. N. Polymerase chain reaction primers and probes derived from flagellin gene sequences for specific detection of the agents of Lyme disease and North American relapsing fever. J Clin Microbiol. 1992 Jan;30(1):99–114. doi: 10.1128/jcm.30.1.99-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C. Clinical definitions and differential diagnosis of Lyme arthritis. Scand J Infect Dis Suppl. 1991;77:51–54. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Göbel U. B., Graf B., Jauris S., Soutschek E., Schwab E., Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993 Feb;31(2):340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Jauris S., Hofmann A., Pradel I., Soutschek E., Schwab E., Will G., Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- van der Linde M. R., Crijns H. J., de Koning J., Hoogkamp-Korstanje J. A., de Graaf J. J., Piers D. A., van der Galiën A., Lie K. I. Range of atrioventricular conduction disturbances in Lyme borreliosis: a report of four cases and review of other published reports. Br Heart J. 1990 Mar;63(3):162–168. doi: 10.1136/hrt.63.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]



