Abstract
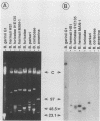
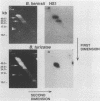
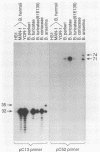
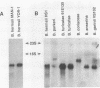
Using oligonucleotide probes which have previously been shown to be specific for the ospC gene found in the Lyme disease spirochete species Borrelia burgdorferi, B. garinii, and group VS461, we detected an ospC homolog in other Borrelia species including B. coriaceae, B. hermsii, B. anserina, B. turicatae, and B. parkeri. In contrast to the Lyme disease spirochetes, which carry the ospC gene on a 26-kb circular plasmid, we mapped the gene in other Borrelia species to linear plasmids which varied in size among the isolates tested. Some isolates carry multiple copies of the gene residing on linear plasmids of different sizes. The analyses conducted here also demonstrate that these Borrelia species contain a linear chromosome. Northern (RNA) blot analyses demonstrated that the gene is transcriptionally expressed in all species examined. High levels of transcriptional expression were observed in some B. hermsii isolates. Transcriptional start site analyses revealed that the length of the untranslated leader sequence was identical to that observed in the Lyme disease spirochete species. By Western blotting (immunoblotting) with antiserum (polyclonal) raised against the OspC protein of B. burgdorferi, we detected an immunoreactive protein of the same molecular weight as the OspC found in Lyme disease spirochete species. The results presented here demonstrate the presence of a protein that is genetically and antigenically related to OspC which is expressed in all species of the genus Borrelia tested.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. The genes encoding major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann N Y Acad Sci. 1988;539:144–153. doi: 10.1111/j.1749-6632.1988.tb31847.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Molecular hybridization of ribonucleic acid with a large excess of deoxyribonucleic acid. Biochem J. 1972 Jan;126(1):171–185. doi: 10.1042/bj1260171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlin P., Peter O., Bretz A. G., Postic D., Baranton G., Piffaretti J. C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992 Apr;60(4):1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiffert H., Ohlenbusch A., Fehling W., Lotter H., Thomssen R. Nucleotide sequence of the ospAB operon of a Borrelia burgdorferi strain expressing OspA but not OspB. Infect Immun. 1992 May;60(5):1864–1868. doi: 10.1128/iai.60.5.1864-1868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Fuchs R., Jauris S., Lottspeich F., Preac-Mursic V., Wilske B., Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992 Feb;6(4):503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Burgdorfer W., Hayes S. F., Barbour A. G. Isolation of a spirochete from the soft tick, Ornithodoros coriaceus: a possible agent of epizootic bovine abortion. Science. 1985 Oct 4;230(4721):85–87. doi: 10.1126/science.3898367. [DOI] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992 Nov;30(11):2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol. 1992 Mar;138(3):533–536. doi: 10.1099/00221287-138-3-533. [DOI] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Phylogenetic analysis of the genus Borrelia: a comparison of North American and European isolates of Borrelia burgdorferi. J Bacteriol. 1992 Jan;174(1):241–244. doi: 10.1128/jb.174.1.241-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Konkel M. E., Garon C. F. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect Immun. 1993 Jun;61(6):2611–2617. doi: 10.1128/iai.61.6.2611-2617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Lubke L., Hauglum W., Garon C. F. Species-specific identification of and distinction between Borrelia burgdorferi genomic groups by using 16S rRNA-directed oligonucleotide probes. J Clin Microbiol. 1992 Mar;30(3):628–632. doi: 10.1128/jcm.30.3.628-632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Samuels D. S., Garon C. F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Carter C. J., Howell J. K., Barbour A. G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992 Nov;60(11):4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E., Weisburg W. G., Tordoff L. A., Fraser G. J., Hespell R. B., Stanton T. B., Zablen L., Mandelco L., Woese C. R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991 Oct;173(19):6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Patsouris E., Jauris S., Will G., Soutschek E., Rainhardt S., Lehnert G., Klockmann U., Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992 Nov-Dec;20(6):342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- Restrepo B. I., Kitten T., Carter C. J., Infante D., Barbour A. G. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol. 1992 Nov;6(22):3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Samuels D. S., Garon C. F. Coumermycin A1 inhibits growth and induces relaxation of supercoiled plasmids in Borrelia burgdorferi, the Lyme disease agent. Antimicrob Agents Chemother. 1993 Jan;37(1):46–50. doi: 10.1128/aac.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Kime K. K., Schrumpf M. E., Coe J. E., Simpson W. J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect Immun. 1989 Nov;57(11):3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Pretzman C., Postic D., Saint Girons I., Baranton G., McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992 Jul;42(3):370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Jauris S., Hofmann A., Pradel I., Soutschek E., Schwab E., Will G., Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]