Abstract
Sphingosine 1-phosphate (S1P), an abundant lipid mediator in plasma, regulates vascular and immune cells by activating S1P receptors. In this report, we investigated the mechanisms by which high plasma S1P levels are maintained in mice. We found that plasma S1P turns over rapidly with a half-life of ≈15 minutes, suggesting the existence of a high-capacity biosynthetic source(s). Transplantation of bone marrow from wild-type to Sphk1−/−Sphk2+/− mice restored plasma S1P levels, suggesting that hematopoietic cells are capable of secreting S1P into plasma. However, plasma S1P levels were not appreciably altered in mice that were thrombocytopenic, anemic, or leukopenic. Surprisingly, reconstitution of Sphk1−/−Sphk2+/− bone marrow cells into wild-type hosts failed to reduce plasma S1P, suggesting the existence of an additional, nonhematopoietic source for plasma S1P. Adenoviral expression of Sphk1 in the liver of Sphk1−/− mice restored plasma S1P levels. In vitro, vascular endothelial cells, but not hepatocytes, secreted S1P in a constitutive manner. Interestingly, laminar shear stress downregulated the expression of S1P lyase (Sgpl) and S1P phosphatase-1 (Sgpp1) while concomitantly stimulating S1P release from endothelial cells in vitro. Modulation of expression of endothelial S1P lyase with small interfering RNA and adenoviral expression altered S1P secretion, suggesting an important role played by this enzyme. These data suggest that the vascular endothelium, in addition to the hematopoietic system, is a major contributor of plasma S1P.
Keywords: sphingosine 1-phosphate (S1P), sphingosine kinase (Sphk), S1P lyase (Sgpl), plasma S1P gradient, Shear stress
The bioactive lipid sphingosine 1-phosphate (S1P) is a potent regulator of numerous biological responses, the most well characterized being cardiovascular and immune effects. S1P binds to and activates a widely expressed family of G protein–coupled receptors, termed S1PRs. Intracellular signaling of these receptors are thought to mediate most of the effects of S1P.1–4
S1P is abundant (0.1 to 1.2 μmol/L) in plasma, where it is mainly bound to albumin and high-density lipoprotein (HDL).5,6 Thus, S1P receptors on blood-borne cells are likely to be constitutively activated. In contrast, S1P levels in tissues are considerably lower (0.5 to 75 pmol/mg wet weight), although tissues with high blood content, such as spleen, are exceptions. This concentration difference of S1P between plasma and tissues has been termed the vascular S1P gradient, which was shown to be functionally important in lymphocyte egress from the lymphoid tissues and the thymus.7,8
The regulation of S1P production and release is not well understood. Secretion of S1P is observed in a variety of cells including platelets,9–11 erythrocytes,9,12,13 mononuclear cells, neutrophils,9 mast cells,14,15 and endothelial cells.16 The concentration of S1P in the cell is determined by the activity of biosynthetic enzymes (sphingosine kinase [Sphk]-1 and -2) and the degradative enzymes (S1P lyase [Sgpl] and S1P phosphatase [Sgpp]-1 and -2). Recent studies have shown that Sphk1 activity is an important factor in determining plasma S1P levels.15,17,18 High Sphk1 activity is present in blood, and Sphk1−/− plasma contains significantly attenuated S1P levels.19
The cellular source(s) of plasma S1P was widely assumed to be from the platelets.10,20 However, 2 recent studies have suggested that red blood cells (RBCs) and other hematopoietic cells are major cellular sources of plasma S1P.8,12 However, the existence of additional sources of S1P was postulated.8
In this report, we show that the vascular system, in addition to the hematopoietic system, is important for the maintenance of high plasma S1P levels. Moreover, vascular endothelial cells release S1P in response to laminar shear stress. We propose that laminar shear stress–induced release of S1P from the endothelium may be a homeostatic mechanism to maintain high plasma S1P levels.
Materials and Methods
Animals
C57BL/6 and FVB/N mice (4 to 6 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, Me). Sphk1−/− and Sphk2−/− mice were obtained from Dr Richard L. Proia (National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, Md).19 All animal procedures were carried out in accordance with the University of Connecticut Health Center animal care committee guidelines.
Isolation of Blood and Plasma
Whole blood was collected in acid– citrate– dextrose (20 mmol/L citric acid, 110 mmol/L sodium citrate, and 5 mmol/L dextrose; 0.1 mL/mL blood) and heparin (50 U/mL blood) via cardiac puncture.
S1P Half-Life Measurement In Vivo
A 15 μmol/L stock solution of C17-S1P (Avanti Polar Lipids Inc, Alabaster, Ala) was prepared with 4% mouse serum albumin in sterile PBS. Activation of S1P receptors was assayed by the S1P1-GFP internalization assay as described previously.2 The approximate blood volume of mouse is assumed to be ≈1.65 mL per 22 g mouse body weight. After administering 100 μL of C17-S1P stock solution (theoretical blood concentration; 1.0 μmol/L) into tail veins, mice in groups of 4 at time points 5, 15, 30, 60, and 120 minutes after injection were euthanized to collect blood. The residual C17-S1P in plasma was measured by high-performance liquid chromatography (HPLC). Half-life for the initial phase of disappearance is calculated with the following formula21:
S1P Analysis by HPLC
The S1P levels in 20 to 100 μL of plasma were analyzed by HPLC with fluorescent detection system (Shimadzu HPLC system; Shimadzu, Kyoto, Japan) as previously described.22
Whole Body Irradiation and Bone Marrow Transplant Experiments
Mice were subjected to whole body irradiation from a 137Cs radiation source (Gammacell-40, MDS Nordion, Kanata, Ontario, Canada) in 2 fractions 4 hours apart at total doses of 10 Gy.23 For bone marrow transplant experiments, recipient mice (WT or Sphk1−/−Sphk2+/−) were reconstituted with 10×106 bone marrow cells (WT or Sphk1−/− Sphk2+/−) via the lateral tail vein immediately after total body irradiation. Engraftments were confirmed by PCR of bone marrow. Hematologic indices for blood samples collected were analyzed either by standard microcapillary techniques or by using a MaxM Counter (Beckman Coulter Co, Hialeah, Fla) and software designed for murine blood studies.
Platelet Depletion in Mice
A purified rat monoclonal antibody against mouse platelet glycoprotein GPIba24 (CD42b) (Emfret Analytics, Würzburg, Germany) was used to induce thrombocytopenia. Blood was collected after 24 hours, and S1P levels in plasma were measured as described above. Platelet number in diluted whole blood was gated and analyzed on a FACSCaliber (BD Biosciences, San Diego, Calif).
Phenylhydrazine-Induced Anemia
Anemia in mice was induced by an intraperitoneal injection of phenylhydrazine (PHZ) (60 mg/kg) for 2 consecutive days.25 Blood was collected at 48 hours after final injection. RBC counts and hematocrit levels were determined.
Adenoviral Transduction of Sphk1
Recombinant Sphk1 and GFP virus were grown in HEK293 cells, purified by CsCl gradient, dialyzed, and titered as described.16 For gene transduction studies, 2×1010 Sphk1 or GFP Adenovirus particles in 100 μL of PBS were injected intravenously into the tail vein of 8- to 12-week male mice. Mice were euthanized 72 hours postinjection, and tissues were analyzed for histology and Sphk activity.
Immunohistochemistry
Immunohistochemistry using the Sphk1 antibody was performed as previously described.26
Sphk Activity Assay
Sphk assay for blood and tissue homogenates were performed as previously described.19
Cell Culture
HUVECs (Clonetics Corp, San Diego, Calif) were cultured up to passage 9 as previously described.27 Mouse primary hepatocytes were isolated as previously described28 and cultured in Hepatozyme-SFM 1X (Invitrogen) supplemented with 10% FBS, antibiotics, and antimycotics. In addition, mouse embryonic endothelial cells (MEECs), EOMA (cell line derived from a mixed hemangioendothelioma arising in an adult mouse), HuH7, and HepG2 cells were cultured in DMEM supplemented with 10% FBS, antibiotics, and antimycotics.
Measurement of S1P in Cultured Cells and Conditioned Media
HUVECs, MEECs, EOMA, primary murine hepatocytes, Huh7, and HepG2 cells were grown to confluence in 60-mm dishes and incubated with 2 mL of DMEM containing 20 mmol/L Hepes-KOH, pH 7.4, 10 mmol/L sodium glycerophosphate, 5 mmol/L sodium fluoride, and 1 mmol/L semicarbazide and 0.1 or 0.5% fatty acid–free BSA, as a carrier for S1P. Release of S1P to the extracellular medium was determined by supplementing conditioned media with 5 or 10 pmol of C17-S1P, followed by affinity isolation with immobilized metal-affinity beads and HPLC analysis as previously described.22
Laminar Shear Stress
MEECs were cultured to confluence on fibronectin-coated glass slides (75×25 mm) in 4-well Nunc dishes. MEECs were subjected to fluid shear stress at 8 dyne/cm2 force in a parallel flow chamber as described earlier.29 The flow medium was maintained at 37°C.
Mitogen-Activated Protein Kinase Phosphorylation
MEECs were serum-starved for 2 to 3 hours in DMEM, and cells were harvested in 20 mmol/L Hepes-KOH, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1.0% NP40, 1 mmol/L phenyl methyl sulfonyl fluoride, and Calbiochem 1× protease inhibitor cocktail. Forty to 50 micrograms of total cellular protein were immunoblotted with phospho–mitogen-activated protein kinase (MAPK) antibody (Cell Signaling Technology).
Immunoblot Analysis for S1P Lyase
MEECs subjected to laminar shear stress for 2 hours and proteins were extracted as described above. Twenty to 50 micrograms of total homogenate was immunoblotted with affinity purified S1P lyase polyclonal antiserum (provided by Dr Richard L. Proia).
Quantitative RT-PCR
Quantitative RT-PCR analysis using SYBR Green I DNA binding dye technology was carried out as described.30
Downregulation of S1P Lyase by Small Interfering RNA
Double-stranded small interfering (si)RNA for murine S1P lyase (sense, 5′-AATTCAGCTTGAGGAGGTCGGCCTGTCTC-3′; anti-sense, 5′-AACCGACCTCCTCAAGCTGAACCTGTCTC -3′) and corresponding scrambled oligonucleotides (sense, 5′-AATGTAGA-TGGGGTACGCGTCCCTGTCTC-3′; antisense, 5′-AAGACGC-GTACCCCATCTACACCTGTCTC-3′) were synthesized using a Silencer siRNA construction kit (Ambion, Austin, Tex). siRNA (200 nmol/L) was transfected to MEECs using Oligofectamine. Subsequently, MEECs were labeled with 0.35 μCi of [3H]-sphingosine (1 μmol/L), and the formation and release of [3H]-S1P were carried out as described.31
Statistical Analysis
All results are expressed as means±SD. Data were analyzed using Student’s t test or 1-way ANOVA with Tukey’s multiple comparison test.
Results
Rapid Turnover of Plasma S1P
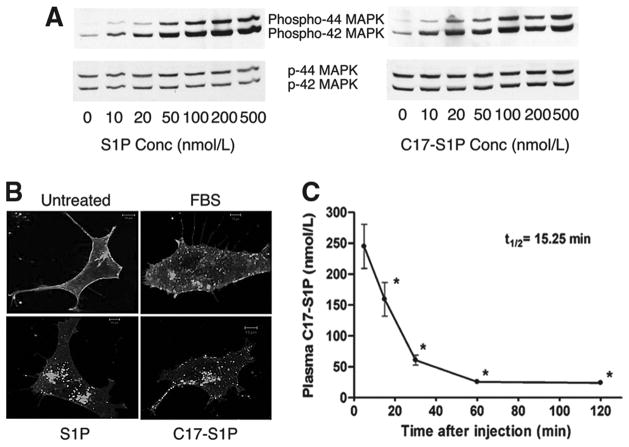
To determine the turnover rate of S1P in plasma, we used C17-S1P, which is structurally and functionally similar to the endogenous C18-S1P but can be readily distinguished by HPLC analysis. Both C17- and C18-S1P stimulated phosphorylation of MAPK in a concentration- and time-dependent manner (Figure 1A and data not shown). In addition, using the GFP-tagged S1P1 receptor internalization assay, both preparations induced receptor internalization to an equivalent degree. Furthermore, BSA-complexed S1P behaved similarly to FBS, which contained ≈ 100 nmol/L S1P (Figure 1B).
Figure 1.
High turnover of S1P in plasma. A and B, Functional similarity between C17- and C18-S1P. A, MEECs were stimulated at indicated concentrations of C17- and C18-S1P for 5 minutes, and phosphorylation of MAPK was analyzed by immunoblot analysis. B, Serum-starved HEK293 cells expressing S1P1R-GFP were treated with FBS or 100 nmol/L C17- or C18-S1P for 30 minutes, fixed, and imaged by a confocal microscope. C, Rapid disappearance of S1P in plasma. C17-S1P (1.5 nmol) complexed with 4% mouse serum albumin was injected intravenously, C17-S1P in plasma was quantified, and the half-life of C17-S1P was calculated (n=3). *Differs from 5 minutes (P<0.01).
We measured the half-life of plasma S1P by injecting mouse serum albumin conjugated C17-S1P intravenously and measuring plasma C17-S1P by HPLC. As shown in Figure 1C, C17-S1P rapidly disappeared from plasma, followed by a much slower phase of decay. The half-life of S1P in plasma was ≈ 15 minutes, suggesting rapid clearance by degradative enzymes such as S1P phosphatases and/or S1P lyase. The rapid turnover of plasma S1P implies the presence of a high-capacity cellular source(s) involved in the maintenance of high plasma S1P levels.
Reciprocal Transplant of Wild-Type and Sphk1−/−Sphk2+/− Bone Marrow Cells Suggests Multiple Cellular Sources of S1P
To define the cellular sources of S1P, we conducted reciprocal bone marrow transplants between wild-type and Sphk1−/−Sphk2+/−mice [Sphk(3N)], which have 471.5±59.1 nmol/L and 155±4.4 nmol/L plasma S1P, respectively (Figure 2A). We reasoned that the difference in plasma S1P levels between the wild-type and Sphk(3N) mice would allow us to delineate the contributions of the hematopoietic system in the bone marrow transplants. S1P levels were restored when wild-type bone marrow was transplanted to Sphk1−/−Sphk2+/− mice (Figure 2B), suggesting that hematopoietic cells are capable of generating plasma S1P. Surprisingly, wild-type mice transplanted with Sphk1−/−Sphk2+/− bone marrow did not show decreased plasma S1P levels (Figure 2B). This suggests that sources from nonhematopoietic cells can also be called on in the absence of diminished secretion from hematopoietic cells.
Figure 2.
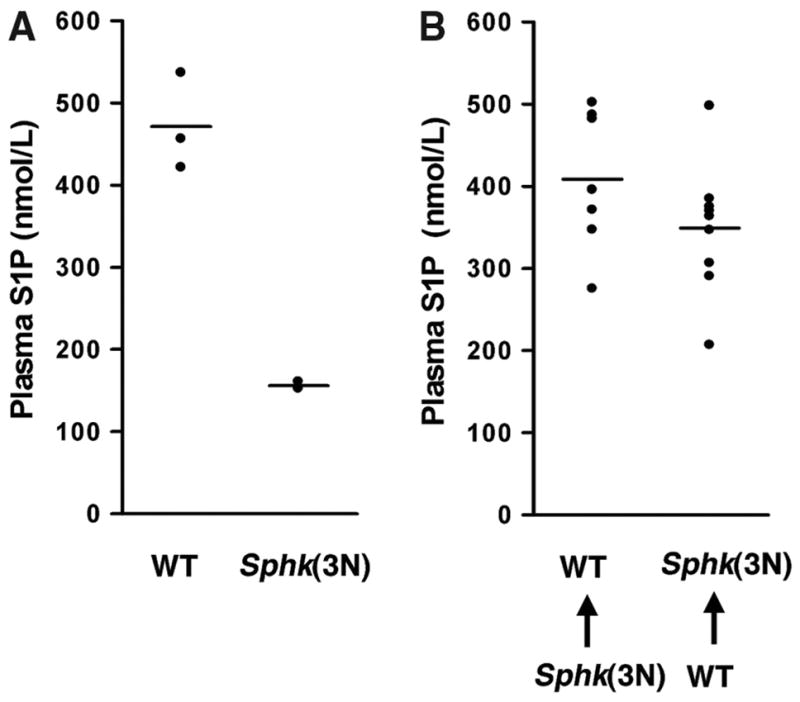
Hematopoietic and nonhematopoietic sources of plasma S1P. A, Plasma S1P levels in wild-type and Sphk1−/− Sphk2+/− Sphk(3N) mice. B, Reciprocal bone marrow transplants in wild-type and Sphk(3N) mice. Wild-type mice receiving Sphk(3N) bone marrow maintained normal plasma S1P levels (left), whereas wild-type marrow restored plasma S1P levels when transplanted into Sphk(3N) mice.
Platelet Depletion Does Not Alter Plasma S1P
We tested the widely held notion that platelets are a major source of plasma S1P.9,10,20 For this, we used the anti-GPIba antibody to induce thrombocytopenia in mice.24 Intraperitoneal injection of anti-GPIb reduced circulating platelet numbers by 93% within 24 hours (Figure 3A). However, plasma S1P levels in platelet-depleted mice were not altered (Figure 3B), suggesting that platelets are not a major source of S1P under physiological conditions. However, thrombocytopenic serum contained ≈ 15% less S1P than the normal counterparts (control serum, 830±39 nmol/L; thrombocytopenic serum, 711±67 nmol/L; N=4), suggesting that platelets may secrete S1P during activation and thrombosis. Thus, under physiological conditions, plasma S1P is unlikely to be derived from platelets.
Figure 3.
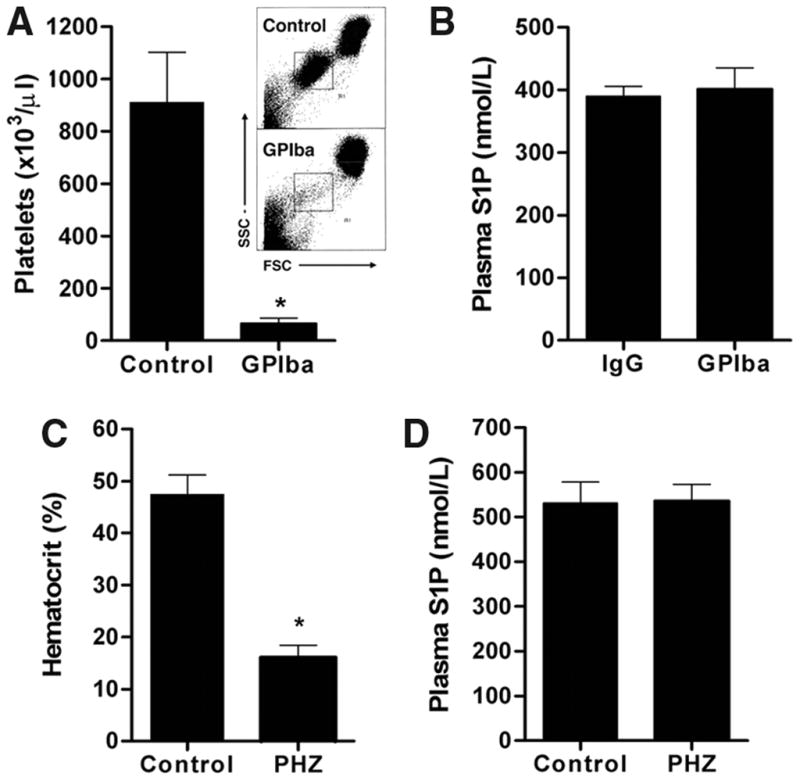
Thrombocytopenia and anemia do not affect plasma S1P. A and B, Lack of effect of platelet depletion on plasma S1P levels. A, Injection of anti-GPIba antibody (2 μg/g) greatly reduced platelet number, as determined by fluorescence-activated cell-sorting analysis (inset), when compared with control (IgG 2 μg/g) (n=5 per group; **P<0.01). B, Plasma S1P levels in control and anti-GPIba antibody–injected mice. C and D, Lack of effect of PHZ-induced anemia on plasma S1P levels. C, Hematocrit values 48 hours after PHZ treatment (n=4 per group; **P<0.01). D, Plasma S1P levels in control and PHZ-treated mice.
Anemic Mice Have Normal Levels of Plasma S1P
The role of RBC in maintaining plasma S1P was tested using PHZ, which denatures hemoglobin and causes severe hemolytic anemia.25 Mice were injected with PHZ (1.2 mg PHZ per 20 g body weight) for 2 consecutive days, and 2 days later, RBC counts and S1P measurements were made. PHZ treatment reduced hematocrit to 34% of control (Figure 3C). Sphk activity in whole blood was concomitantly reduced in PHZ-induced anemia (control versus anemic mice: 45.4±4.4 versus 24.6±3.5 nmol/min · g−1; n=4 to 5), consistent with the notion that Sphk activity is enriched in RBCs. Remarkably, plasma S1P levels of anemic mice were similar to control animals (Figure 3D). Given that RBC contain Sphk activity, and release S1P in vitro12,13 and in vivo,8 these findings suggest that even a small fraction of RBCs can potentially contribute to plasma S1P levels. Alternatively, other cellular sources may supply S1P in the plasma of anemic mice.
Bone Marrow Suppression Does Not Alter Plasma S1P
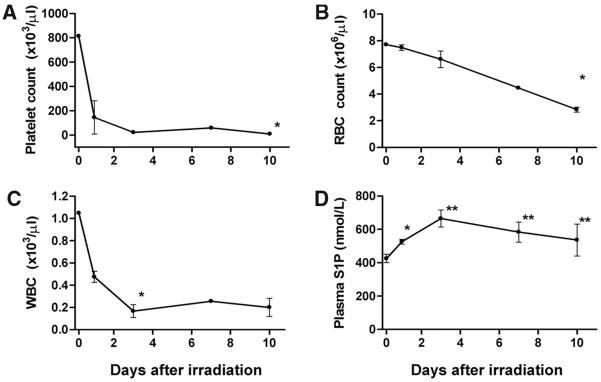
To determine whether generalized suppression of hematopoietic cells affects plasma S1P, C57BL/6 mice were subjected to whole body gamma irradiation, which rapidly induces apoptosis of hematopoietic progenitor cells, leading to pancytopenia.23 Within a day, platelet and white blood cell counts in irradiated mice rapidly declined >80% and >95%, respectively (Figure 4A and 4C). RBC numbers gradually decreased with a slower kinetics, and ≈70% reduction was achieved by day 10 (Figure 4B). Interestingly, plasma S1P levels were transiently increased at 3 days but returned to normal levels by 10 days following irradiation (Figure 4D). The brief increase in S1P coincided with the stimulation of whole blood Sphk activity (Figure I in the online data supplement, available at http://circres.ahajournals.org). The lack of reduction of plasma S1P is surprising despite near total decrease in leukocytes and platelets and significant suppression of RBCs. This suggests that either a small fraction of remaining hematopoietic cells can maintain high plasma S1P or that a nonhematopoietic source exists that is called on to contribute to plasma S1P.
Figure 4.
Total bone marrow suppression does not reduce plasma S1P. Mice were subjected to total body irradiation and platelets (A), red blood cells (B), and white blood cells (C) were quantified. D, Plasma S1P levels were quantified by HPLC as described (n=3; *P<0.05, **P<0.01 vs day 0).
Adenoviral-Mediated Transduction of Sphk1 Alters Plasma S1P Levels via a Nonhematopoietic Source
To further explore the nonhematopoietic sources of plasma S1P, we used Sphk1−/− mice, which contain only ≈60% of plasma S1P of the wild-type counterparts. We transduced Sphk1−/− mice with adenovirus expressing Sphk1 (AdSphk1) or GFP (AdGFP) and measured plasma S1P. Interestingly, transduction of AdSphk1 resulted in the restoration of plasma S1P levels (Figure 5A and 5B). As expected, recombinant Sphk1 was expressed mainly in the liver32 (supplemental Figure II). Immunohistochemical analysis of liver sections, using an anti-Sphk1 antibody, showed Sphk1 in endothelial cells and hepatocytes (Figure 5C through 5F). These results indicate that expression of Sphk1 in the liver resulted in the release of S1P into the circulation, implying that S1P synthesis by a nonhematopoietic source (ie, the liver) can contribute in a significant manner to plasma S1P.
Figure 5.
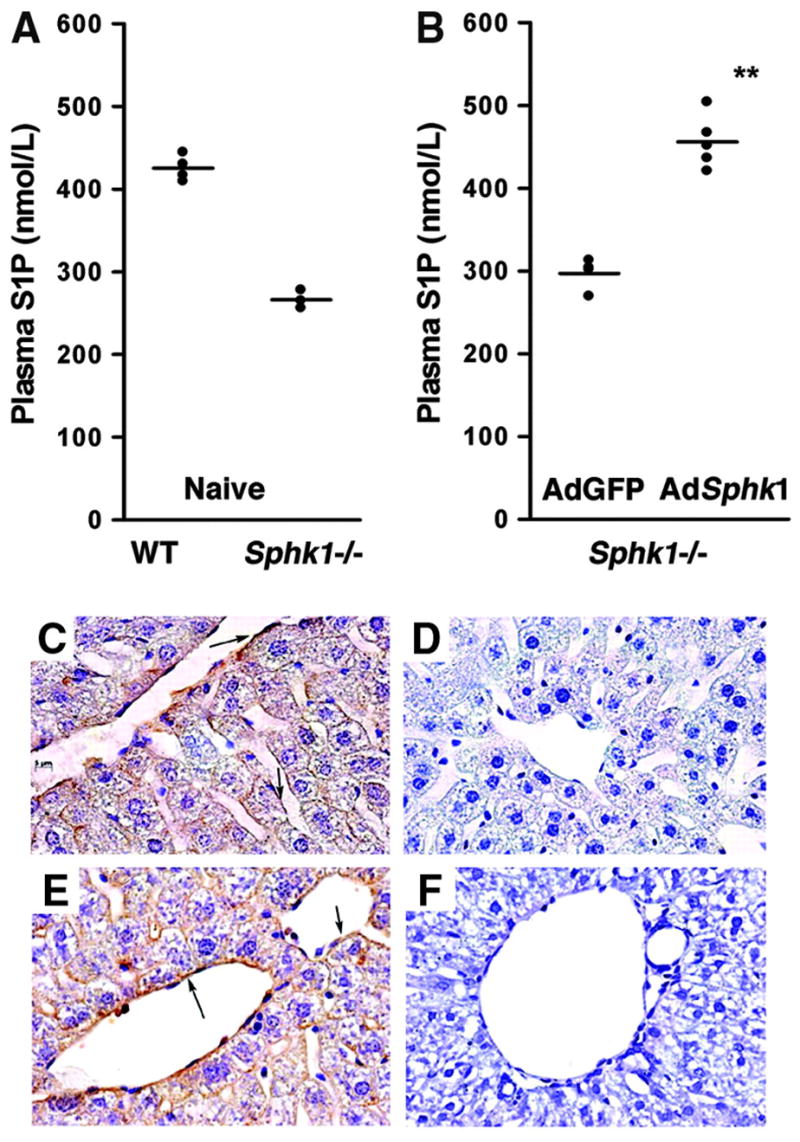
Expression of Sphk1 in liver restores plasma S1P in Sphk1−/− mice. A, Plasma S1P levels in wild-type and Sphk1−/− mice. B, Adenoviral transduction of Sphk1 into Sphk1−/− mice restored plasma S1P levels. **P<0.01. C through F, Immunohistochemistry of liver sections stained with an Sphk1 antibody (brown) and counterstained with hematoxylin: wild-type (C), Sphk1−/− (D), Sphk1−/−transduced with Sphk1 adenovirus (E), and Sphk1−/− transduced with GFP adenovirus (F). Note the strong expression of Sphk1 in sinusoidal endothelial cells of liver (E arrows).
Secretion of S1P by Vascular Endothelial Cells In Vitro
Because Sphk1 is primarily expressed in the endothelial cells and hepatocytes in vivo, we compared the ability of primary hepatocytes, hepatoma cell lines (HuH7 and HepG2), and endothelial cells (HUVECs, MEECs, and EOMA) to synthesize and release S1P. HUVECs, MEECs, and EOMA cells efficiently secreted S1P into the media. In sharp contrast, human hepatoma cell lines (HuH7 cells and HepG2) synthesized low levels of S1P and did not release S1P into the extracellular medium (Figure 6A). Moreover, mouse primary hepatocytes synthesized and released negligible levels of S1P (Figure 6A). In addition, treatment of MEECs with the sphingosine kinase inhibitor SKI reduced both intracellular and extracellular S1P by 29% and 85%, respectively, suggesting that S1P accumulation in the conditioned media is Sphk-dependent (supplemental Figure III). S1P secretion was time-dependent, and a concomitant reduction in intracellular levels was seen (Figure 6B). These data suggest that the vascular endothelium possesses the machinery to produce and secrete S1P.
Figure 6.
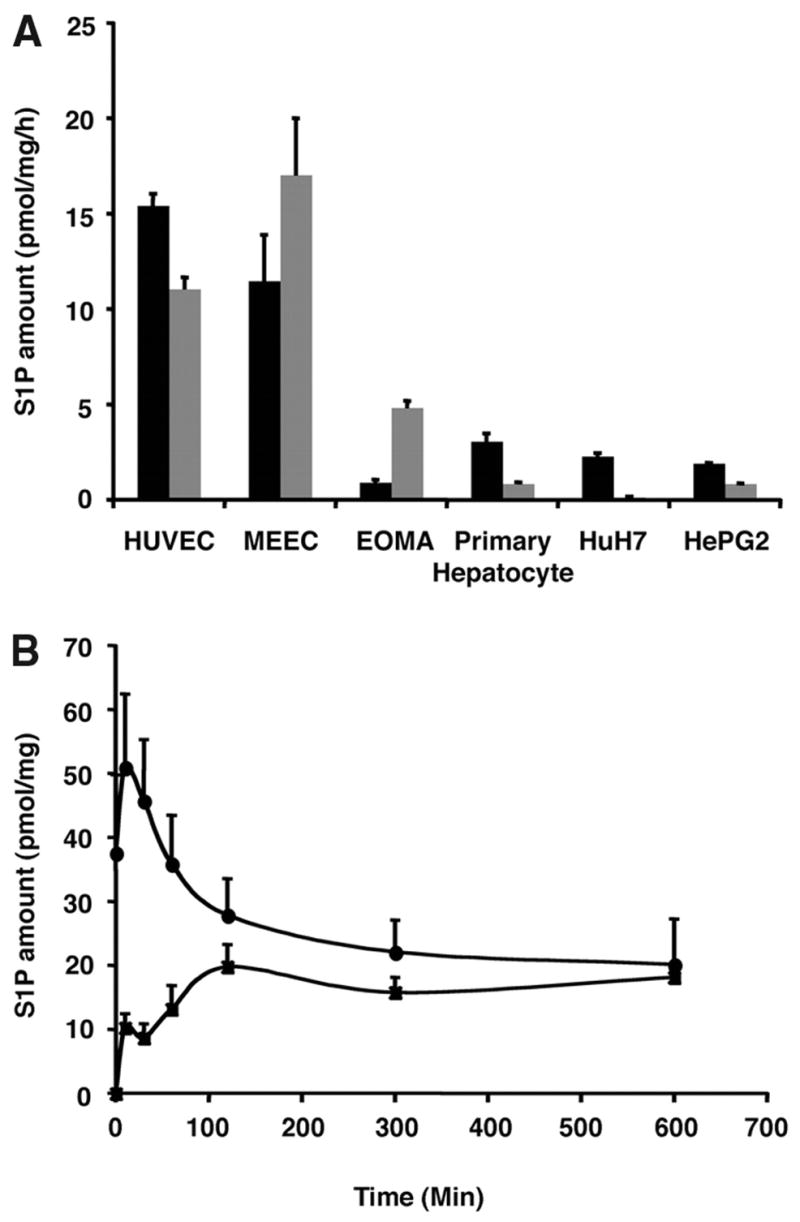
S1P release by endothelial cells in vitro. A, S1P formation in intracellular (black) and extracellular compartments (gray). Note that endothelial cells avidly secrete S1P. B, Formation of endogenous S1P in the intracellular compartment (circles) and time-dependent accumulation of S1P in the extracellular compartment (squares) of HUVECs on medium change.
Shear Stress Induces S1P Release by Endothelial Cells
Vascular endothelium in vivo is subject to laminar shear stress.33 In fact, laminar shear stress induces the secretion of endothelial-derived vasoprotective molecules such as prostacyclin and NO.34 It has also been reported that sphingomyelin hydrolysis is activated on shear stress.35 To examine the effect of shear stress on S1P synthesis and release by endothelial cells, we subjected MEECs to laminar shear stress. The p42/p44 extracellular signal-regulated kinase was rapidly phosphory-lated when the endothelial cells were subjected to laminar shear stress (supplemental Figure IV). Concomitantly, S1P synthesis and secretion were stimulated ≈3-fold (Figure 7A) in a time-dependent manner (Figure 7B).
Figure 7.
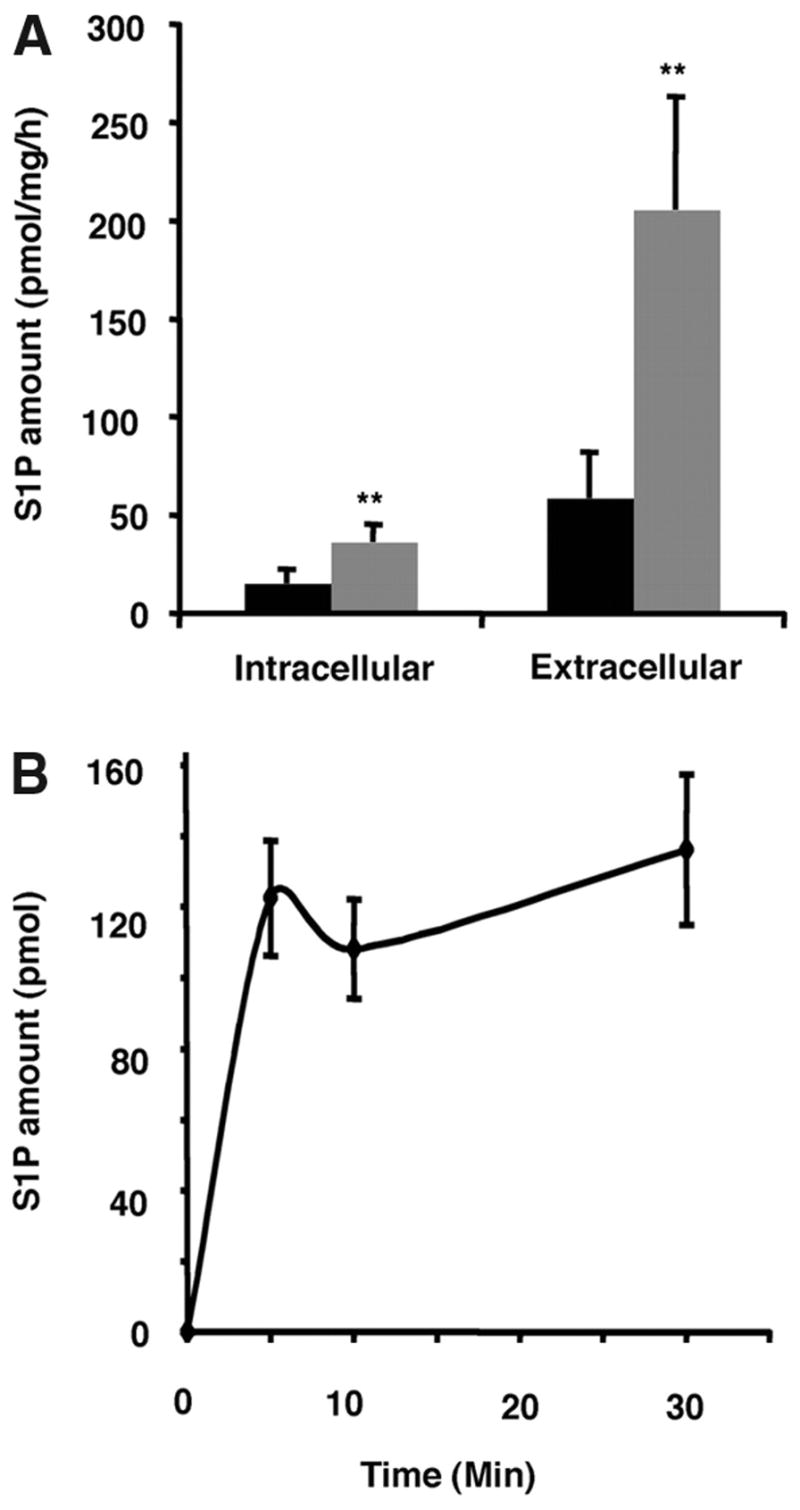
Shear stress induces S1P synthesis and release from MEECs. A, Formation of S1P in the intracellular and extracellular compartments under static (black) and laminar shear-treated (gray) conditions. B, Time-dependent accumulation of S1P in the media of MEECs subjected to laminar shear stress.
Quantitative RT-PCR analysis of MEECs subjected to laminar shear stress showed that S1P lyase (Sgpl) and S1P phosphatase 1 (Sgpp1) transcripts were downregulated by ≈90% and ≈98%, respectively, whereas transcripts for other S1P metabolic enzyme levels were not altered (Figure 8A and supplemental Figure V). In addition, S1P lyase polypeptide was also downregulated (Figure 8B). These data suggest that laminar shear stress suppresses the activity of S1P degradative machinery and thereby stimulates S1P release from vascular endothelial cells in vitro.
Figure 8.
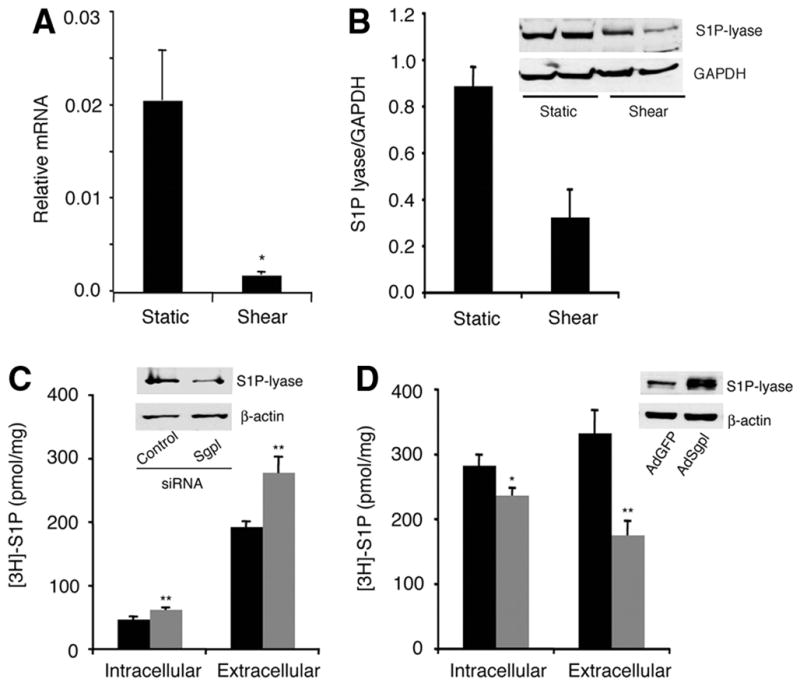
Laminar shear stress downregulates S1P lyase expression. A, Quantitative RT-PCR analysis of S1P lyase (Sgpl) transcript in static and shear stress–treated MEECs. Sgpl transcript was normalized to Gapdh mRNA. B, Immunoblot analysis of S1P lyase. MEECs were subjected to laminar shear stress as described in Materials and Methods. Immunoblot analysis on total homogenate was performed with affinity-purified anti-S1P lyase antibody. GAPDH was used as a loading control. Densitometric analysis of immunoblot is shown. C, Downregulation of S1P lyase by double-stranded siRNA in MEECs stimulated intracellular synthesis of [3H]-S1P and its release to extracellular medium (black: scrambled siRNA; gray: S1P lyase si). Down-regulation of S1P lyase by siRNA and corresponding control (scrambled siRNA) is also shown by immunoblot analysis (n=3). D, Transduction of human adeno-Sgpl in HUVECs decreased the formation of [3H]-S1P in intracellular and extracellular compartments (black: AdGFP; gray: AdSgpl). Stimulated expression of S1P lyase is shown by immunoblot analysis (n=3).
To further confirm that changes in S1P lyase levels can regulate secretion of S1P, we treated endothelial cells with S1P lyase siRNA, which reduced the expression of S1P lyase by ≈50% within 48 hours. This increased intracellular S1P levels and subsequent release into the extracellular compartment (Figure 8C). In contrast, overexpression of S1P lyase by adenoviral transduction decreased the accumulation of S1P in both intracellular and extracellular compartments (Figure 8D). These data suggest that intracellular level of the S1P lyase enzyme is an important determinant of S1P secretion by the endothelial cells.
Discussion
In this report, we address the issue of S1P secretion in vivo. Mammalian plasma is a rich source of S1P; indeed, plasma-derived S1P is thought to be important in immune cell migration and vascular function. Therefore, we explored the mechanisms involved in the accumulation of S1P in plasma. Previous studies have shown that S1P can be formed inside and outside of cells. Intracellular formation of S1P is ubiquitous and is well established. However, our previous analysis of endothelial cells indicated that Sphk is secreted and is capable of forming S1P in the extracellular environment in minor amounts.16,19 However, the bulk of Sphk activity is cell-associated, implying that S1P is formed in the cytosolic phase of the plasma membrane and is exported to the external leaflet of the plasma membrane so that it can be extracted by plasma chaperones such as HDL and albumin.
A major finding of this work is that plasma S1P has a short half-life. Intravenously injected C17-S1P that was complexed with albumin decayed with a half-life of ≈ 15 minutes (Figure 1). In contrast, incubation of C17-S1P with whole blood at 37°C did not result in appreciable degradation (data not shown), suggesting that metabolism in the blood vessel wall is required. It is likely that S1P is dephosphorylated rapidly either by S1P-specific phosphatases or lysolipid phosphate phosphatase enzymes.36,37 Dephosphorylation of S1P is needed for intracellular uptake, and a recent report from Natarajan and colleagues indicated that endothelial cells dephosphorylate S1P to allow intracellular uptake of sphingosine.38 Alternatively, S1P lyase could degrade intracellular S1P. Rapid metabolism of S1P in vivo suggests that a high-capacity biosynthetic source is needed for the maintenance of high plasma S1P levels.
It is generally assumed that plasma S1P is derived from platelets. However, 2 recent reports challenged this notion and suggested RBCs may be a major source of plasma S1P.8,12 By conducting bone marrow transplants from inducible Sphk1 and Sphk2 double allele knockout mice,8 the authors suggested that hematopoietic cells are needed to maintain plasma S1P. In addition, the authors performed RBC transfusion experiments and showed that wild-type RBCs could supply plasma S1P in Sphk1 and Sphk2 double allele knockout mice. They also suggested the existence of an additional source that is not sensitive to bone marrow transplantation.8
We used the Sphk triple allele knockout mice [Sphk1−/− Sphk2+/−; Sphk(3N)], which has dramatically suppressed plasma S1P levels, to examine whether S1P in plasma is derived from hematopoietic cells (Figure 2A). When Sphk(3N) mice were irradiated and the hematopoietic cells were reconstituted with wild-type bone marrow, plasma S1P levels increased to wild-type levels, suggesting that wild-type hematopoietic cells are capable of secreting S1P (Figure 2B). Surprisingly, in the reciprocal bone marrow transplant, when wild-type mice were transplanted with Sphk(3N) bone marrow, plasma S1P levels were not reduced and remained similar to wild-type levels (Figure 2B). This suggests that hematopoietic cells are not the only cellular source that is capable of producing S1P to maintain high plasma levels. Our hematopoietic cell depletion experiments support this notion. Thus, mice that are severely anemic (>50% reduction in hematocrit), thrombocytopenic (>90% suppression of platelets), and irradiated (leukopenic, thrombocytopenic, and anemic) possessed wild-type S1P levels in plasma (Figures 3 and 4). These data suggest that plasma S1P is derived from multiple cellular sources of both hematopoietic and nonhematopoietic origin.
We next attempted to define the nonhematopoietic source of plasma S1P. Injection of Ad-Sphk1 intravenously into mice resulted in predominant expression in the liver (Figure 5). When Ad-Sphk1 was injected intravenously into Sphk1−/− mice, plasma S1P levels were restored to wild-type levels. Analysis of transgene expression showed positivity in endothelial cells and hepatocytes. However, only endothelial cells secreted S1P in vitro. These experiments strongly suggest that the endothelium is the major nonhematopoietic source of plasma S1P.
Experiments with cultured endothelial cells indicate robust expression of Sphk1, Sphk2, Sgpp1, Sgpp2, and Sgpl transcripts, suggesting that S1P metabolism is high in this cell type. In static cell culture conditions, S1P accumulation is constitutive and time-dependent accumulation in the extracellular medium can be seen when BSA is used to trap the secreted S1P (Figure 6). However, laminar shear stress strongly increased the intracellular accumulation and extracellular secretion of S1P (Figure 7).
Quantitatively, MEECs secreted ≈120 pmol/106 cells per 30 minutes in vitro. Assuming similar values in vivo, we speculate that total theoretical output of S1P from a human being is (1012 endothelial cells) estimated to be ≈120 μmol/30 minutes. In a human being with ≈6 L of blood, 3 μmol of S1P would need to be replenished every 15 minutes (1 half-life) to maintain a constant plasma S1P level of 1 μmol/L. Therefore, from theoretical considerations, the endothelium does possess sufficient capacity to produce and maintain plasma S1P levels.
The endothelial cell is a multitalented cell and produces mediators such as NO and prostacyclin.34 Laminar shear stress is a major physiological stimulus for the endothelium and is needed for normal functionality of the vascular system. Thus, secretion of S1P by endothelial cell may be critical for the maintenance of plasma S1P gradient and thereby contribute to vascular homeostasis.
In conclusion, we show here that plasma S1P turns over rapidly. Secondly, an extrahematopoietic source is involved in the maintenance of μmol/L plasma S1P levels. We identify this source as the vascular endothelium. We further demonstrate that laminar shear stress induces the secretion of S1P by the endothelial cells by downregulation of S1P degradation in endothelial cells.
Supplementary Material
Acknowledgments
We thank Drs Carol Pilbeam and Donald Peterson (University of Connecticut Health Center) for helpful comments and for providing facilities to carry out laminar flow studies and Dr Richard Proia (National Institute of Diabetes and Digestive and Kidney Diseases, NIH) for the gift of the S1P lyase antibody. T.H. dedicates this work in the memory of Richard D. Berlin.
Sources of Funding
This work is supported by NIH grants HL70694 and HL-67330 (to T.H.) and DK-38825 (to H.L.B.). Y.L. was supported in part by a Korea Research Foundation grant (Ministry of Education & Human Resources Development/Chungbuk BIT Research-Oriented University Consortium).
Footnotes
Disclosures
None.
References
- 1.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 2.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids–receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 5.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Tomura H, Kuwabara A, Kimura T, Miura S, Noda K, Okajima F, Saku K. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis. 2005;178:199–205. doi: 10.1016/j.atherosclerosis.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 8.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Yatomi Y, Miura Y, Satoh K, Ozaki Y. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 10.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 14.Jolly PS, Rosenfeldt HM, Milstien S, Spiegel S. The roles of sphingosine-1-phosphate in asthma. Mol Immunol. 2002;38:1239–1245. doi: 10.1016/s0161-5890(02)00070-6. [DOI] [PubMed] [Google Scholar]
- 15.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 17.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 19.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh N, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 21.Birkett DJ. Pharmacokinetics Made Easy. Revised. Sydney, Australia: McGraw Hill Australia; 2002. [Google Scholar]
- 22.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303:167–175. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieswandt B, Bergmeier W, Rackebrandt K, Gessner JE, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- 25.Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Carrai V, Calzolari A, Centurione L, Lorenzini R, Carta C, Alfani E, Sanchez M, Migliaccio G, Migliaccio AR. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1(low) mice) Blood. 2001;97:3040–3050. doi: 10.1182/blood.v97.10.3040. [DOI] [PubMed] [Google Scholar]
- 26.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hla T, Maciag T. Cyclooxygenase gene expression is down-regulated by heparin-binding (acidic fibroblast) growth factor-1 in human endothelial cells. J Biol Chem. 1991;266:24059–24063. [PubMed] [Google Scholar]
- 28.Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadhwa S, Godwin SL, Peterson DR, Epstein MA, Raisz LG, Pilbeam CC. Fluid flow induction of cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an extracellular signal-regulated kinase signaling pathway. J Bone Miner Res. 2002;17:266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- 30.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 34.Aird WC. Phenotypic heterogeneity of the endothelium: I. structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 35.Czarny M, Schnitzer JE. Neutral sphingomyelinase inhibitor scyphostatin prevents and ceramide mimics mechanotransduction in vascular endothelium. Am J Physiol Heart Circ Physiol. 2004;287:H1344–H1352. doi: 10.1152/ajpheart.00222.2004. [DOI] [PubMed] [Google Scholar]
- 36.Brindley DN, English D, Pilquil C, Buri K, Ling ZC. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim Biophys Acta. 2002;1582:33–44. doi: 10.1016/s1388-1981(02)00135-x. [DOI] [PubMed] [Google Scholar]
- 37.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, Pyne S. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.