Abstract
Sensory information from the urinary bladder is conveyed via lumbar splanchnic (LSN) and sacral pelvic (PN) nerves to the spinal cord. In the present report we compared the mechanosensitive properties of single afferent fibers in these two pathways using an in vitro mouse bladder preparation. Mechanosensitive primary afferents were recorded from the LSN or PN and distinguished based on their response to receptive field stimulation with different mechanical stimuli: probing (160 mg to 2 g), stretch (1–25 g), and stroking of the urothelium (10–1,000 mg). Four different classes of afferent were recorded from the LSN and PN: serosal, muscular, muscular/urothielial, and urothelial. The LSN contained principally serosal and muscular afferents (97% of the total sample), whereas all four afferent classes of afferent were present in the PN (63% of which were muscular afferents). In addition, the respective proportions and receptive field distributions differed between the two pathways. Both low- and high-threshold stretch-sensitive muscular afferents were present in both pathways, and muscular afferents in the PN were shown to sensitize after exposure to an inflammatory soup cocktail. The LSN and PN pathways contain different populations of mechanosensitive afferents capable of detecting a range of mechanical stimuli and individually tuned to detect the type, magnitude, and duration of the stimulus. This knowledge broadens our understanding of the potential roles these two pathways play in conveying mechanical information from the bladder to the spinal cord.
INTRODUCTION
Sensory information from the urinary bladder is conveyed via lumbar splanchnic (LSN) and sacral pelvic (PN) nerves to the spinal cord. Mechanosensitivity of bladder afferents underlies in part the pain and discomfort experienced by patients with interstitial cystitis/painful bladder syndrome and other bladder disorders, yet the afferent pathways and constituent afferent fibers that contribute to bladder sensations are incompletely understood. Peripheral terminals of sensory neurons innervating the bladder are equipped to detect a variety of mechanical stimuli, including distension and circumferential stretch of the organ. The mechanosensitivity of bladder afferents in the mouse (Daly et al. 2007; Rong et al. 2002), rat (Mitsui et al. 2001; Moss et al. 1997; Sengupta and Gebhart 1994; Shea et al. 2000), cat (Bahns et al. 1987; Floyd et al. 1976; Häbler et al. 1990, 1993), and guinea pig (Zagorodnyuk et al. 2006) has been studied previously, but the principal focus of study has been the PN.
It has long been appreciated clinically that the two nerves innervating an internal organ have different functions, but this has not been systematically investigated. Nonhuman animal studies have generally used nerve transection with subsequent study of changes in bladder reflexes (e.g., micturition). Conclusions based on such studies are not all in agreement with respect to nerve function or straightforward (see discussion). More recently, examination of sensory neurons (nerve transection affects both afferent and efferent axons), either afferent fibers or their cell bodies, has provided evidence that the different pathways of sensory innervation of an organ significantly differ with respect to both chemical and mechanical sensitivities (e.g., Brierley et al. 2004; Dang et al. 2005a,b; Sugiura et al. 2005). We recently compared the mechanosensitivity of LSN and PN afferent fibers innervating the mouse colon and noted several important differences (Brierley et al. 2004) between the two pathways, which in conjunction with studies in knockout mice (e.g., Daly et al. 2007; Jones et al. 2005, 2007; Page et al. 2005) could inform development of therapeutic strategies targeting selected molecules and pathways important to visceral disorders.
The availability of knockout mice provides the unique opportunity to examine the role of relevant molecular targets in organ mechanosensitivity and their contributions to visceral hypersensitivity such as characterizes interstitial cystitis/painful bladder syndrome. As a first step to this end, we compared the mechanosensitive properties of single afferent fibers in the two pathways innervating the mouse urinary bladder (i.e., LSN and PN afferents) using an in vitro mouse bladder preparation. We also examined the ability of an inflammatory soup (Handwerker and Reeh 1991; Jones et al. 2005) to sensitize responses of stretch/tension-sensitive fibers to subsequent mechanical stimuli.
METHODS
Male C57BL/6 mice (20–30 g; Taconic Farms, Germantown, NY) were used throughout. All experiments were reviewed and approved by the Institutional Animal Care Use Committee of the University of Iowa.
In vitro preparation and recording of mouse bladder afferents
Mice were killed by CO2 inhalation. The bladder with either the PN or LSN and associated neurovascular bundle was isolated and transferred to ice-cold Krebs solution bubbled with carbogen (95% O2-5% CO2). After carefully removing attached tissue from the neurovascular bundle, the bladder wall was opened from the base to the vertex on the postero-midline of the umbilical fold. After opening the bladder wall, it was pinned flat, urothelium side up, in one chamber of a two-chamber clear acrylic organ bath, the floors of which were lined with Sylgard (Dow Corning, Midland, MI), and superfused with oxygenated Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4(H2O)7, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, and 20 acetate at a temperature of 31°C). The PN or LSN was placed into an immediately adjacent recording chamber filled with light paraffin oil. In all preparations the L-type calcium channel antagonist nifedipine (1 μM, to block spontaneous muscle contractions) and the prostaglandin synthesis inhibitor indomethacin (3 μM, to block synthesis of endogenous prostaglandins) were added to the Krebs solution perfusing the organ chamber. Under a dissection microscope, the nerve sheath was carefully peeled back and the nerve trunk teased apart into 6–10 bundles, each of which could be individually placed onto a platinum recording electrode. A platinum reference electrode was placed in a small pool of Krebs solution on a glass plate near the recording electrode. Unit activity was differentially amplified, filtered, sampled at a rate of 20 kHz using a 1401 interface (Cambridge Electronic Design, Cambridge, UK), and stored on a PC. The amplified signal was also used for on-line audio monitoring. Action potentials were viewed on-line, recorded, and analyzed off-line using the Spike2 wavemark function and discriminated on the basis of distinguishable waveform, amplitude, and duration. If more than two units were present in a nerve filament, the filament was further divided so either one or two clearly discriminable units were present.
Characterization of bladder afferents
The preparation was allowed to rest for 60 min before recording was initiated. Mechanosensitive receptive fields were identified by systematically stroking the urothelial surface with a camel's hair brush of sufficient stiffness to activate all types of mechanosensitive afferent endings. Once identified, receptive fields were tested with three different mechanical stimuli as described previously (Brierley et al. 2004) to enable classification: urothelial stroking with calibrated von Frey nylon monofilaments (10, 200, 500, and 1,000 mg force, each force applied 10 times, once/s), blunt (perpendicular to the tissue) probing with calibrated von Frey nylon monofilaments (160, 400, 1,000, and 2,000 mg, 3-s duration), and stretch (1, 5, 10, 15, 20, and 25 g, each weight applied for 20 s with an interstimulus interval of 1 min). Bladder stretch was achieved using a hook made from a bent dissection pin that was attached to the tissue adjacent to the mechanosensitive receptive field to stretch, either in a transverse or longitudinal direction, whichever in preliminary stretch with a forceps appeared most sensitive to the stimulus. The hook was connected to a cantilever system via thread to which different weights were added.
Chemical activation and sensitization were tested only on PN muscular afferents because stretch/tension was considered the most relevant physiological mechanical stimulus among those tested. Immediately after baseline mechanical testing, a stainless steel cylindrical ring (height, 1 cm; ID, 4 mm) was placed over the receptive field and the Krebs solution removed from within the ring. A mildly acidic (pH 6.0) inflammatory soup (IS) or vehicle,150 μl, was applied directly to the receptive field for 1 min, the IS or vehicle and stainless steel ring were removed, and responses to stretch were tested 5 and 20 min after terminating exposure to the IS or vehicle. pH 6.0 is considered here to be mildly acidic because the mean pH of urine in the bladder measured in 12 mice was 6.1 (range 5.8–6.5). The IS was prepared in aliquots of 20 μl by combining bradykinin, serotonin, and histamine dissolved in distilled water with prostaglandin E2 dissolved in dimethylsulfoxide, frozen, and stored at −20°C. On the day of an experiment, an aliquot was diluted to final concentration (5 μM for all mediators) in acidified (6.0) Krebs solution.
Data analysis
Data are plotted throughout as means ± SE. Two-way ANOVAs or repeated-measures multiway ANOVAs were performed as appropriate using Prism 5 (GraphPad Software, San Diego, CA). Tukey or Bonferroni-corrected post hoc tests were performed as appropriate when F values for main effects were significant. Differences were considered significant when P ≤ 0.05.
Adaptation profiles to probing were calculated as the mean number of spikes per 100-ms bin over the entire 3 s of the probe stimulus. Adaptation profiles to stretch were calculated as the mean number of spikes per 500-ms bin over the entire 20 s of stretch. Slopes were derived from regression analyses performed on stimulus–response functions and adaptation data to compare the gain and rates, respectively, of afferent fiber responses. Extrapolations of threshold values for stimulation based on a linear regression analysis of stimulus–response functions were determined only for muscular fiber responses to stretch. To compare the sensitivity of splanchnic and pelvic afferents to von Frey probing, the percentage of afferent fibers that responded to each probe force was calculated based on the total number of fibers of each type (“percentage responding”) and evaluated by χ2 analysis (Prism 5).
RESULTS
Basic mechanosensory and topographical properties
All fiber types were responsive to perpendicular von Frey probing. However, their receptive field location and response, or lack of response, to mechanical stimuli (i.e., von Frey probing, urothelial stroking, and muscle stretch) distinguished four types of mechanosensitive fibers in the LSN and PN pathways innervating the mouse urinary bladder.
LSN BLADDER AFFERENTS
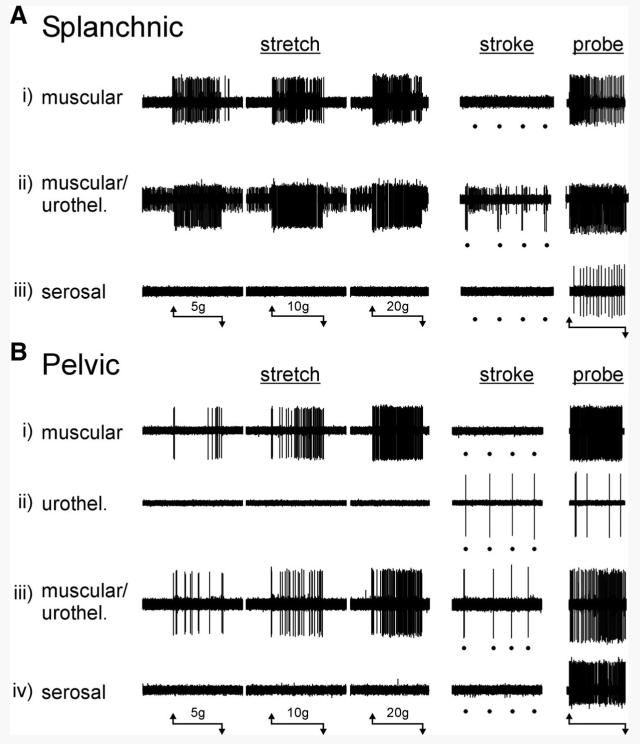
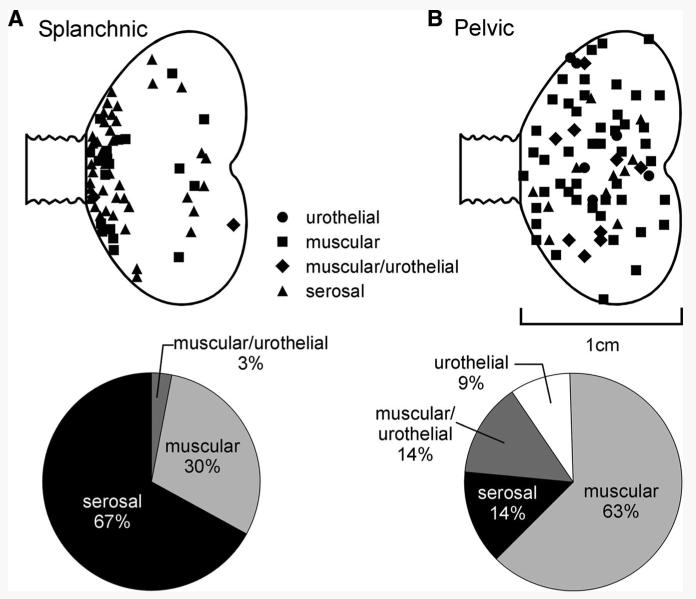
Three types of LSN fibers could be distinguished based on their responses to mechanical stimuli (Fig. 1A): muscular, muscular/urothelial, and serosal. Muscular afferents (20 of 67 LSN fibers studied) had receptive fields in the bladder wall that were optimally activated by probing and stretch, but were not responsive to fine urothelial stroking (Fig. 1Ai). In response to graded intensities of stretch, muscular afferents typically responded with an excitation that typically adapted during the stimulus, particularly at the greater intensities of stretch tested. Muscular afferents also responded to stroking of the urothelium at greater stimulus intensities (500–1,000 mg; data not shown), which we observed to distort the underlying muscle layers. We found no LSN receptive endings sensitive only to stroking of their receptive fields with a 10-mg von Frey filament, but did identify a small proportion of LSN afferent fibers (3% of the sample; 2 of 67 fibers) that responded to both urothelial stroking (10-mg von Frey filament) and graded stretch of the bladder (Fig. 1Aii), which we designated muscular/urothelial. Serosal afferents (45 of 67 fibers studied) had receptive fields located in the bladder wall that were reproducibly activated in a graded fashion only by blunt probing and did not respond either to bladder stretch or fine urothelial stroking with a 10-mg von Frey filament (Fig. 1Aiii). Thus serosal afferents were the most abundant type of mechanosensitive fiber recorded in the LSN; afferent fibers that responded to stretch (muscular and muscular/urothelial) constituted the remainder of the sample (Fig. 2A, bottom). Virtually all LSN afferents possessed a single, small (typically ≤0.5 mm) punctate receptive field from which responses could be most readily evoked; their receptive fields tended to be clustered at the base of the bladder (Fig. 2A, top). Afferents recorded from the LSN were commonly silent at rest. However, some afferents in this pathway (20%) had low rates of spontaneous activity (≤0.5/s), but no formal analysis of spontaneous activity was performed.
FIG. 1.
Types of mouse urinary bladder afferent fibers classified on the basis of responses to mechanical stimuli. A: 3 types of mechanosensitive fibers were identified in the lumbar splanchnic pathway: i) muscular afferents were activated by stretch and von Frey probing, but not by urothelial stroking (10 mg); ii) muscular/urothelial fibers were activated by stretch, urothelial stroking, and von Frey probing; iii) serosal afferents were activated only by von Frey probing and not by either stretch or urothelial stroking. B: 4 types of mechanosensitive fibers were identified in the pelvic pathway: i) muscular afferents were activated by stretch and von Frey probing, but not by urothelial stroking (10 mg); ii) urothelial afferents were activated by urothelial stroking (10 mg) and von Frey probing, but not by stretch; iii) muscular/urothelial afferents were activated by stretch, urothelial stroking, and von Frey probing; iv) serosal afferents were activated only by von Frey probing and not by either stretch or urothelial stroking. The horizontal lines beneath A and B indicate the onset and termination of stretch (20 s) or von Frey probing (1 g, 3 s) stimuli. Stroking of the urothelium is indicated by dots below the respective records.
FIG. 2.
Distribution of receptive fields and proportions of afferent fiber types recorded in the splanchnic (A) and pelvic (B) pathways. Mechanosensitive splanchnic nerve receptive fields tended to cluster at the base of the bladder, whereas mechanosensitive pelvic nerve receptive fields were distributed throughout the bladder. The principal fiber type in the splanchnic pathway responded only to von Frey probing of the bladder (serosal), whereas stretch-responsive muscular and muscular/urothelial fibers characterized the pelvic pathway.
PN BLADDER AFFERENTS
Four types of PN fibers could be distinguished based on their responses to mechanical stimuli (Fig. 1B): muscular, urothelial, muscular/urothelial, and serosal. As with LSN afferents, all PN afferents responded in a graded manner to blunt von Frey probing of their receptive fields and were distinguished by their sensitivity to fine urothelial stroking and bladder stretch. PN muscular afferents (49 of 78 PN fibers studied) responded to stretch but not fine stroking (Fig. 1Bi), urothelial afferents (9% of the sample; 7 of 78 fibers) responded to fine stroking but not stretch (Fig. 1Bii), muscular/urothelial PN fibers (14% of the sample; 11 of 78 fibers) responded to both urothelial stroking (10-mg von Frey filament) and to graded intensities of bladder stretch (Fig. 1Biii), and serosal afferents (also 14% of the sample) responded only to probing of their receptive field (Fig. 1Biv) and not to any other mechanical stimulus. In contrast to LSN afferent fibers, muscular afferents were the most common fiber type recorded in the PN (63% of the sample), and overall three fourths of PN fibers responded to stretch (muscular and muscular/urothelial fibers) (Fig. 2B, bottom). Whereas no fibers with urothelial-only receptive fields were found in the LSN sample, 9% of the PN fiber sample had receptive fields restricted to the urothelium (and overall almost one quarter of the PN sample responded to urothelial stroking). Like LSN afferents, PN afferents typically had single, punctate receptive fields that, in contrast to LSN afferents, were distributed throughout the bladder (Fig. 2B, top). Interestingly, none of the PN fiber receptive fields that responded to urothelial stroking was located in the area of the base of the bladder, where most LSN receptive fields were located (and very few of which responded to urothelial stroking). No PN afferents exhibited spontaneous activity.
Dynamic responses to graded von Frey probing
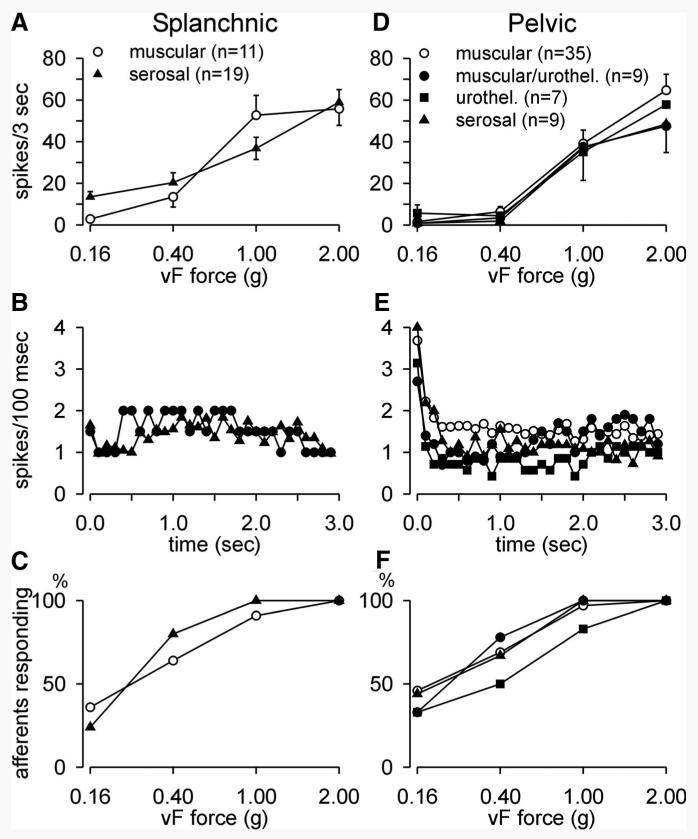
All LSN and PN afferent fibers displayed graded responses to an ascending series of von Frey probing stimuli (0.16–2.0 g, 3 s). Figure 3, A and D illustrates mean stimulus–response functions to von Frey probing of the receptive fields of the principal LSN (serosal and muscular) and all four PN fiber types, respectively. LSN muscular and serosal afferents appear to be more responsive to probing at lower forces (e.g., 0.40 g), but there were no significant differences in response to probing between fiber types within a pathway or between pathways. Unfortunately, we did not examine responses to a less-intense probing force (e.g., 0.07 g), which may have confirmed greater sensitivity in the LSN pathway.
FIG. 3.
Mechanosensitivity, adaptation, and activation characteristics of splanchnic (left column) and pelvic (right column) nerve afferents to von Frey probing (0.16–2.0 g). All fiber types in both pathways displayed graded responses to increasing intensities of von Frey force (A, D). B and E: mean adaptation profiles (error bars omitted for clarity) during application of a 1-g von Frey probe (3 s). All 4 fibers types in the pelvic pathway (E) gave an initial dynamic response to application of the probe, whereas neither muscular nor serosal splanchnic afferents (B) responded in this fashion. Sensitivity to von Frey probing was assessed in C and F by determining the percentage of afferents responding at the 4 intensities of force applied.
The only significant difference between pathways is apparent in the response and adaptation to probing (1 g). Responses of serosal and muscular LSN fibers exhibited neither a dynamic response to probing nor adaptation during the 3-s application of the 1-g stimulus (Fig. 3B), whereas all four mechanosensitive fiber types in the PN pathway gave dynamic responses at the onset of stimulus application before adapting to response magnitudes not different from their LSN counterparts (Fig. 3E). Relative to their splanchnic nerve counterparts (Fig. 3B), pelvic nerve serosal (F = 6.83, P = 0.011, Fig. 3E) and muscle (F = 9.16, P = 0.002, Fig. 3E) afferents gave significantly greater dynamic responses (0.0–0.5 s) to probing (1 g), which adapted quickly within 0.5 s of stimulus application.
Percentages of afferents responding to the range of forces applied are compared in Fig. 3, C and F. Chi-square analysis did not reveal any significant differences either within or between pathways.
RESPONSES TO STRETCH
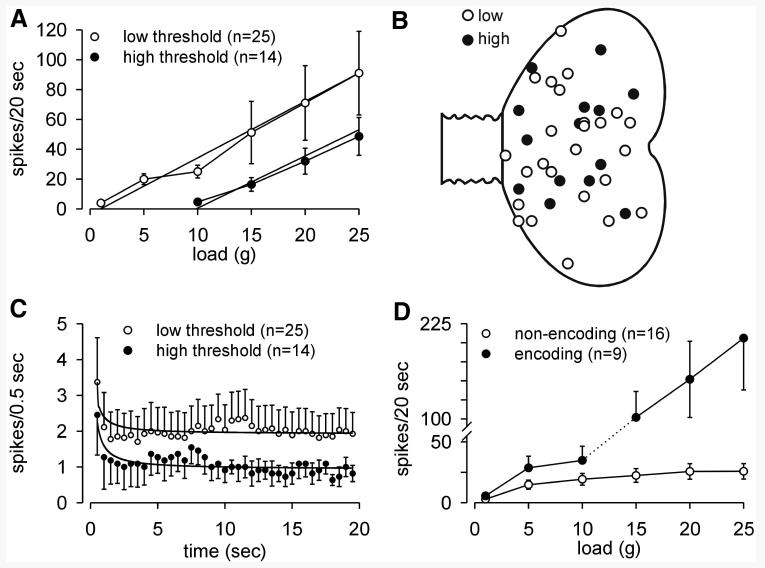
Within the PN group of 39 muscular afferent fibers studied, two principal response types are apparent, although there is no obvious difference in location of receptive fields (Fig. 4, A and B). The larger proportion (64%) of fibers had low thresholds for response to stretch, typically responding at the lowest load tested, 1 g. Interestingly, many of them (see Fig. 4D) encoded stimulus intensity throughout the range of loads applied. A smaller proportion (36%) of muscular fibers had high thresholds for response to stretch, responding first at or >10 g. The estimated (by extrapolation) response thresholds for the low- and high-threshold groups are about 1 and about 10 g, respectively; the slopes of their stimulus–response functions do not differ (mean 3.7 for low- and 3.5 for high-threshold fibers). Despite significant differences in response threshold, both low- and high-threshold PN muscle afferents gave initial dynamic responses at the onset of stretch and adapted similarly to steady rates of discharge during the period of stretch (20 g, 20 s; Fig. 4C). The difference between low- and high-threshold PN fiber response and adaptation within the first 5 s of stimulation was significant (F = 23.36, P < 0.0001). Inspection of Figs. 1 and 5 also reveals that responses to stretch are closely linked to stimulus onset and termination.
FIG. 4.
Characteristics of pelvic nerve muscular fiber responses to stretch. A: low-threshold muscular afferents responded at low stimulus intensity and encoded increasing stimulus intensity throughout the range of loads tested. The estimated response threshold extrapolates to about 1 g. High-threshold muscular afferents did not respond at low intensities of load and first responded at ≥10 g load. The estimated response threshold extrapolates to about 10 g. The difference between low- and high-threshold muscular afferent responses to stretch is significant (F = 7.39, P = 0.007). B: distribution of low- and high-threshold receptive fields. C: both low- and high-threshold muscular afferents gave an initial dynamic response to stretch (20 g) and similarly adapted to relatively stable, but significantly different rates of discharge for the duration of stretch (20 s) (F = 23.36, P < 0.0001). Response magnitudes of low-threshold fibers were on average significantly greater than response magnitudes for high-threshold fibers. D: low-threshold fibers were further divided into encoding and nonencoding types; 16 of the 25 low-threshold fibers encoded stimulus intensity up to about 10 g load and did not further increase response magnitude as load was increased. The remaining 9 low-threshold muscular afferents exhibited load-dependent responses throughout the range of intensities applied.
FIG. 5.
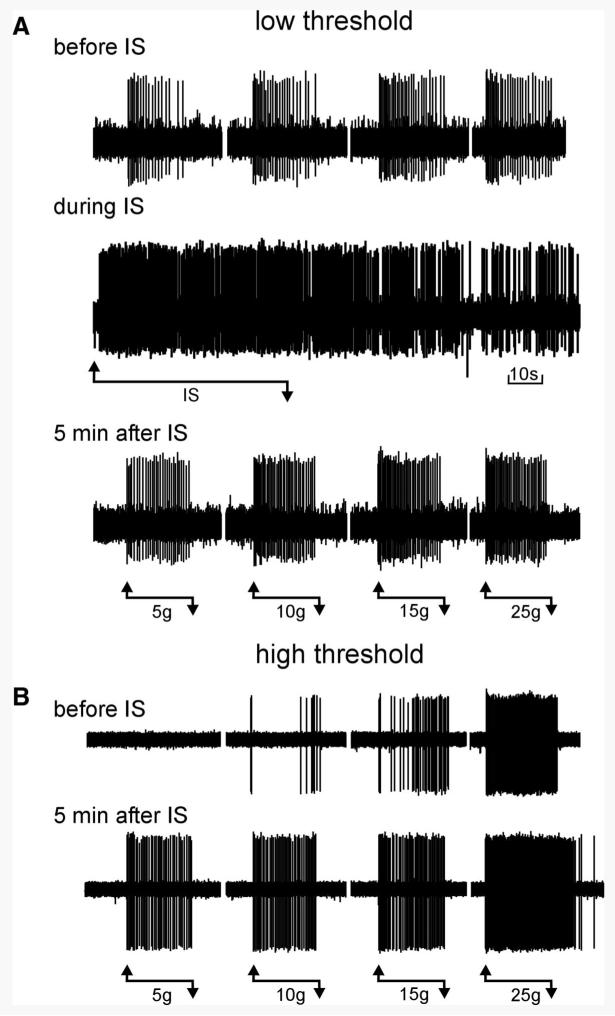
Sensitization of muscular pelvic nerve afferent fiber responses to stretch. A: responses to stretch of a low-threshold muscular afferent are shown before, during (1 min), and 5 min after application of inflammatory soup (IS, pH 6.0) to its receptive field. In this example, the afferent responded vigorously to the IS (note that activity returned to control before stretch was tested). B: responses of a high-threshold muscular afferent are shown before and 5 min after application of IS (pH 6.0) to its receptive field. In this example, the afferent did not respond to the IS. The horizontal lines beneath recordings indicate the application of IS (1 min) or stretch (20 s) and the loads applied.
As suggested by the variability of responses of low-threshold PN muscular fibers to stretch at greater loads (Fig. 4A), there are fibers within the low-threshold sample with different encoding properties (Fig. 4D) (see also Shea et al. 2000; Zagorodnyuk et al. 2006). The majority of low-threshold fibers (16/25) encoded the applied load only at low, physiological intensities of stretch (up to ∼10 g) after which response magnitude plateaus. In contrast, 9 of the 25 low-threshold fibers not only encoded the applied load throughout the range of loads applied, but also gave mean maximum responses (206 ± 68 total spikes in 20 s at 25 g) fourfold greater than the mean maximum response of high-threshold PN muscular afferents (49 ± 14 total spikes in 20 s at 25 g).
Muscular afferents are not present in the same proportion in the LSN pathway as in the PN pathway and only 10 were quantitatively studied. One of the 10 LSN muscular fibers had a high threshold for response and all of the 9 low-threshold fibers were of the “low encoding” type.
Sensitization of muscular PN bladder afferent fibers
We further examined the properties of muscular PN bladder afferents that responded to stretch to determine whether their mechanosensory properties sensitized after exposure to an inflammatory soup (IS, pH 6.0). Figure 5 shows examples of sensitization of low-threshold (Fig. 5A) and high-threshold (Fig. 5B) PN muscular afferents. Responses to stretch are clearly increased when tested 5 min after a 1-min exposure to IS and high-threshold fibers tested in this manner also exhibited a decrease in response threshold to 5 g (Fig. 5B). Figure 5A also illustrates activation of a fiber by IS (i.e., response “during IS”) and in Fig. 5B after-discharge at high intensities of stretch (25 g), an observation not noted in the absence of sensitization. In four experiments, vehicle (pH 6.0) was applied to the receptive fields of two low-threshold and two high-threshold PN muscle afferents. Responses to stretch (1–25 g) were not sensitized when tested either 5 or 20 min after exposure to pH 6.0 vehicle; mean responses were 94 and 95% of prevehicle baseline. Summary data for 17 muscular PN afferents are given in Fig. 6. Data for low-threshold (11) and high-threshold (6) fibers are presented in Fig. 6, A and B, respectively, revealing significant sensitization to stretch 5 and 20 min after exposure to IS (P < 0.01 for low- and high-threshold afferents at both times of testing). Ten of the 17 PN muscular fibers responded to application of IS, revealing chemosensitivity in addition to mechanosensitivity. Interestingly, no sensitization to von Frey probing was apparent at any force tested (Fig. 6C).
FIG. 6.
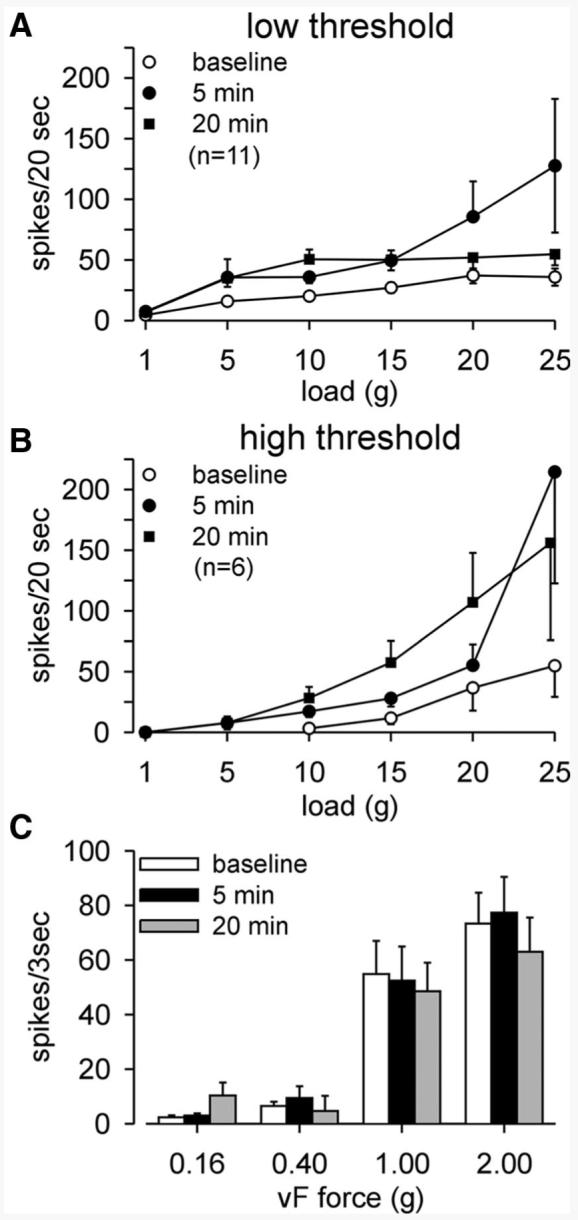
Summary of low- (A) and high-threshold (B) pelvic nerve (PN) muscular afferent fiber responses to stretch before (baseline) and 5 and 20 min after a 1-min application of IS to the receptive field. Responses to stretch were significantly sensitized by IS. In A,F = 9.23 (P = 0.002) and F = 23.22 (P < 0.0001) vs. baseline at 5 and 20 min, respectively, after exposure to IS. In B, F = 4.73 and F = 6.53 (P = 0.003 for both) vs. baseline at 5 and 20 min, respectively, after exposure to IS. Not only is response magnitude increased after exposure to IS, all high-threshold fibers responded at both times of testing to the 5-g load, representing a reduction in response threshold. C: responses to von Frey probing of PN muscular afferent receptive endings were not sensitized by IS.
DISCUSSION
The present report characterizes mechanosensitive properties of afferent fibers in the LSN and PN innervation of the mouse urinary bladder. We identified four classes of afferent fiber, three of which were common to both pathways. The fourth class of fiber, urothelial fibers that responded to gentle stroking of the urothelium (10 mg), was present only in the PN pathway. The principal findings include clear evidence of differences between the LSN and PN innervation of the urinary bladder with respect to location of receptive fields and proportions of classes of mechanosensitive fibers contained in each pathway. Serosal and muscular afferents represented 97% of the sample of LSN mechanosensitive afferents studied and their receptive endings were located principally near the base of the bladder. Muscular afferents and, overall, stretch sensitivity dominated response properties of PN mechanosensitive afferents; three quarters of the sample responded to stretch and receptive fields were distributed throughout the bladder. These findings suggest significant functional differences between mechanosensitive afferents contained in these pathways.
The difference in distribution of LSN and PN receptive endings in the bladder is consistent with the anatomical distribution of LSN and PN axons in the bladder. In a series of studies in the cat, Uemura and colleagues describe the distributions of sacral (PN) and lumbar (LSN) axons in the urinary bladder. They report that PN axons were distributed equally to all areas of the bladder (Uemura et al. 1973), consistent with the widespread distribution of PN receptive fields in the mouse urinary bladder reported here. They note that terminal axons were more numerous outside muscle fascicles, which they suggested appeared to be tension receptors, a finding not different from the present report where most of the PN afferent fibers responded to stretch/tension. In a subsequent report (Uemura et al. 1974), axons from the lumbar innervation were most numerous in the trigone region, followed by the ventral neck of the bladder. A third report (Uemura et al. 1975) found that 3.7-fold more lumbar than sacral afferent axons innervated the submucosa of the bladder, commenting that this was the reverse ratio when the muscle coat of the bladder was examined. They also confirmed in this study that lumbar axons were concentrated in the bladder neck, whereas sacral afferent axons were evenly distributed throughout the bladder. Their findings in the cat are consistent with the results reported here, with respect to both the distributions of mechanosensitive receptive endings in the mouse bladder (widespread for the PN and concentrated at the base of the bladder for the LSN) and innervation of bladder muscle by the PN and sensitivity to stretch/tension. In the rat, Vera and Nadelhaft (1992) confirmed that the urinary bladder is innervated by axons with cell bodies in the upper lumbar (L1–L3) and lumbosacral (L6–S1) dorsal root ganglia. Interestingly, using two different tracers they found very few double-labeled cells and concluded that the dome and base of the bladder are innervated by separate and distinct neuronal populations.
The function of serosal afferents, which constituted 67% of the LSN pathway, is unclear. Serosal afferents responded optimally and in a graded fashion to punctate (perpendicular) von Frey probing, a decidedly unphysiological stimulus, and not to stretch/tension. We do not consider the probing stimulus to be noxious when applied for 3 s; it was used in the present experiments principally to locate the most sensitive (“hot spot”) part of the receptive field and to characterize receptive endings that did not also respond to either of the other mechanical stimuli tested. One might speculate that activation of multiple serosal afferents during bladder filling or muscle contraction or spasm could contribute to a sharp, transient unpleasant or painful sensation, but the urinary bladder does not typically experience rapid filling or spasm. Perhaps these punctate receptive fields are located on or near vascular elements in the bladder wall (e.g., see Floyd et al. 1976), either within the muscle layers or serosa, but we were unable in this preparation to determine their precise location within the tissue.
We did not examine either sensitivity to or sensitization by inflammatory soup of responses of serosal fibers to probing (or LSN muscular fibers to stretch), and doing so may have revealed chemosensitivity and/or a role of LSN afferents in bladder reflexes altered by bladder irritation. Two reports suggest that the hypogastric nerve, a component of the LSN pathway, is important to chemosensitivity and perhaps responses to noxious distension of the bladder (Moss et al. 1997) as well as changes in urinary frequency caused by chemical bladder inflammation (Mitsui et al. 2001). It should be noted, however, that the hypogastric nerve contains axons from both dorsal root ganglia and sympathetic chain ganglia that reach the hypogastric nerve through the pelvic nerve as well as from postganglionic neurons in the major pelvic ganglion (Nadelhaft and Vera 1991). Accordingly, assignment of nerve function on the basis of interpretation of multiunit recordings or transection of the hypogastric nerve is imprecise.
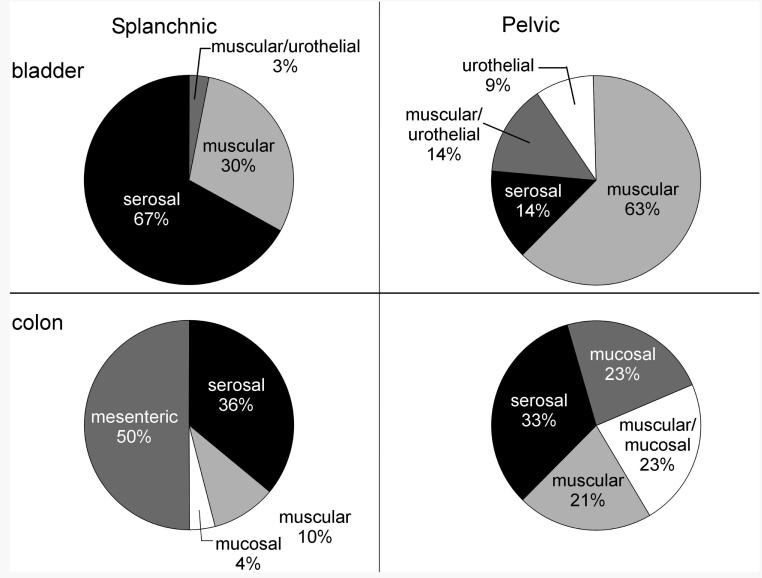
We are aware of no other report studying mechanosensitive bladder afferents in the splanchnic/hypogastric pathway in the mouse. A multiunit study in the rat (Moss et al. 1997) and single-unit studies in the cat (Bahns et al. 1986; Floyd et al. 1976; Winter 1971) have been reported. In the cat, Floyd et al. (1976) probed the bladder in situ from the serosal side and found that most hypogastric nerve afferents had multiple, punctate receptive fields that included sites on both the vasculature and serosa, most of which were restricted to the base of the bladder. In contrast, Bahns et al. (1986) found only single mechanosensitive sites on the surface of the serosal side of the bladder. Both sets of investigators also distended the cat bladder, Floyd et al. (1976) reporting that hypogastric bladder afferents exhibited a wide range of response thresholds (a conclusion arrived at by Winter 1971 as well), whereas Bahns et al. (1986) reported a narrower range of response thresholds, concluding that there were no nociceptive visceral afferents in the hypogastric pathway. The in vitro preparation used here precluded bladder distension as a stimulus, but 30% of the LSN innervation of the mouse bladder was responsive to stretch/tension, suggesting correspondence with results in the cat. We cannot comment, however, on how representative is the finding of a high proportion (67%) of serosal afferents in this pathway in the mouse, although there are clearly hypogastric–LSN receptive endings that innervate the serosa in cats as well. In a recent study of colon mechanosensitive afferents in the mouse LSN pathway, 36% were classed as serosal. In the PN bladder pathway, serosal afferents represented only 14% of the sample studied here, a proportion approximately one half the 33% of serosal afferents described in the mouse PN colon pathway (Brierley et al. 2004; see Fig. 7 for summary). Serosal bladder afferents in the LSN pathway may be more sensitive to lower intensities of stimulation than their PN counterparts, perhaps suggesting a functional role in the sensation of bladder filling. The data presented here, however, cannot confirm this supposition.
FIG. 7.
Summary of proportions of afferent fiber types in the mouse splanchnic (left column) and pelvic (right column) pathways for urinary bladder (top row) and colon (bottom row). Data for mouse colonic afferents were adopted from Brierley et al. (2004).
The relative proportions of muscular afferents in the bladder LSN and PN pathways are near the opposite of the proportions of serosal afferents; muscular afferents constituted 63% of the PN pathway and 30% of the LSN pathway. Muscular afferents in both pathways exhibited indistinguishable responses to von Frey probing with the exception that PN afferents exhibited dynamic responses at the onset of the 1-g von Frey stimulus (which were absent in LSN afferents). Muscular afferents in hollow organs are widely considered to be functionally important to sensations of organ filling (low-threshold afferents) and discomfort and pain (high-threshold afferents) when organs are overfilled/overdistended. Normally, high-threshold afferents likely function as nociceptors in response to acute stimuli, but given that both low- and high-threshold afferents sensitize (a characteristic of nonvisceral nociceptors) and both can encode into the noxious range, it is likely that both low- and high-threshold stretch-sensitive endings in hollow organs contribute to discomfort and pain in the presence of organ inflammation (see subsequent discussion). It has been generally held that both innocuous, physiological filling of the urinary bladder and bladder pain are signaled mainly by pelvic afferents and principally by those that respond to stretch (Kuru 1965). This interpretation, first, discounts the significant proportion of muscular afferents contained in the LSN pathway and, second, is not consistent with other literature cited here.
As has been described for other hollow organs, we found stretch-sensitive muscular afferents with low and with high thresholds for response in both the LSN and PN bladder innervation, although the proportions differed between the two pathways. In a related in vitro study of stretch-sensitive PN bladder afferents in the guinea pig, Zagorodnyuk et al. (2006) described stretch-sensitive mechanoreceptors that they termed muscle mechanoreceptors, three quarters of which had low thresholds for response and one quarter of which had response thresholds approximately eightfold greater and were considered by them to be high-threshold muscle mechanoreceptors. They also described bladder PN tension–mucosal mechanoreceptors similar in characteristics to the muscular/urothelial class of afferents described in the present report and mucosal mechanoreceptors that were stretch insensitive, findings consistent with the present report. Recently, Daly et al. (2007) reported that 80% of mechanosensitive PN afferent fibers innervating the mouse urinary bladder were of the low-threshold type. They similarly report both dynamic and static/stable components to the response to filling pressure. Interestingly they also observed that the response magnitude of low-threshold fibers was on average greater than the response magnitude of high-threshold fibers. In the present study, 35% of the 39 fibers studied had high thresholds for response, a proportion of the sample greater than that reported in studies in the rat (Sengupta and Gebhart 1994; Shea et al. 2000) and greater than that in the recent study by Daly et al. (2007) in the mouse. This may reflect differences between bladder filling as a stimulus as opposed to stretch in vitro and remains to be established.
Sensitization is a characteristic of nociceptors defined originally as an increase in response magnitude to a suprathreshold noxious intensity of stimulation after skin insult and a reduction in response threshold (Bessou and Perl 1969). Afferent fiber sensitization reflects an increase in the excitability of the receptive ending and has been demonstrated as important to the development of hyperalgesia/hypersensitivity, including visceral hypersensitivity. In previous reports (e.g., Handwerker and Reeh 1991; Jones et al. 2005; Su and Gebhart 1998) and in the present report, we used an inflammatory soup applied to bladder receptive endings to study chemosensitivity and sensitization of PN muscular afferents. It should be acknowledged that bradykinin, a component of the inflammatory soup, can cause smooth muscle contraction and thus confound interpretation of studies such as described here. We believe this to be unlikely, however, because the inflammatory soup did not reliably excite receptive endings and responses to probing were unaffected. As previously documented for PN afferents innervating the mouse (Jones et al. 2005) and rat (Su and Gebhart 1998) colon, the inflammatory soup reliably sensitized responses to bladder stretch, as reflected in the increase in response magnitude of both low- and high-threshold PN muscle afferents to stretch and the reduction in response threshold for high-threshold afferents. Rong et al. (2002) also documented sensitization of both low- and high-threshold PN bladder afferents responsive to bladder filling by the P2X3 receptor agonist α,β-methylene adenosine triphosphate (ATP), supporting a role for endogenous ATP in increasing excitability of bladder afferents.
The significance of these outcomes to bladder sensation is several-fold. First, bladder muscular afferents that normally respond at a low rate of discharge to filling in the physiological range discharge when sensitized at greater rates to the same low-intensity stimuli. Significantly, it appears that some of the low-encoding, low-threshold muscular afferents exhibit high encoding properties after exposure to inflammatory mediators. Second, high-threshold afferents that previously did not respond to intensities in the low physiological range exhibited a reduction in response threshold to 5 g when sensitized and continued to exhibit increased sensitivity for the duration of testing, which was terminated after 20 min. Finally, given that low- and high-threshold bladder afferents can encode stretch/distending stimuli into the noxious range and also sensitize, stretch/distension sensitive afferents appear to be able to contribute to discomfort and pain. Together, these data confirm previous reports of sensitization of visceral afferents innervating hollow organs and suggest, for the bladder, that discomfort and pain can be generated by normal bladder filling in circumstances of interstitial cystitis/painful bladder syndrome.
ACKNOWLEDGMENTS
We thank M. Burcham for assistance in preparation of the figures.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-35790.
REFERENCES
- Bahns E, Ernsberger U, Jänig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pfluegers Arch. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl E. Responses of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterol. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Daly DM, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wildtype and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005a;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielfeldt K, Lamb K, Gebhart GF. Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol. 2005b;93:3112–3119. doi: 10.1152/jn.01127.2004. [DOI] [PubMed] [Google Scholar]
- Floyd K, Hick VE, Morrison JFB. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976;259:457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler H-J, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler H-J, Jänig W, Koltzenburg M. Receptive properties of myelinated primary afferents innervating the inflamed urinary bladder of the cat. J Neurophysiol. 1993;69:395–405. doi: 10.1152/jn.1993.69.2.395. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Reeh PW. Pain and inflammation. In: Bond MR, Charlton JE, Woolf CJ, editors. Progress in Pain Research and Clinical Management. Vol. 4. Elsevier; Amsterdam: 1991. pp. 59–75. [Google Scholar]
- Jones RCW, 3rd, Otsuka J, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Jones RCW, 3rd, Xu L, Gebhart GF. Mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require TRPV1 and ASIC3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiol Rev. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J Neurophysiol. 2001;86:2276–2284. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS. Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R695–R703. doi: 10.1152/ajpregu.1997.272.2.R695. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons labelled after the application of tracer to the distal stump of the transected hypogastric nerve in the rat. J Autonom Nerv Syst. 1991;36:87–96. doi: 10.1016/0165-1838(91)90104-b. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the rat's urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Bradley WE. Distribution of the lumbar afferent axons in muscle coat of cat urinary bladder. Am J Anat. 1974;139:389–398. [Google Scholar]
- Uemura E, Fletcher TF, Bradley WE. Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anat Rec. 1975;183:579–588. doi: 10.1002/ar.1091830408. [DOI] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Dirks VA, Bradley WE. Distribution of sacral afferent axons in cat urinary bladder. Am J Anat. 1973;136:305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- Vera P, Nadelhaft I. Afferent and sympathetic innervation of the dome and the base of the urinary bladder of the female rat. Brain Res Bull. 1992;29:651–658. doi: 10.1016/0361-9230(92)90134-j. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Costa M, Brookes SJH. Major classes of sensory neurons to the urinary bladder. Autonom Neurosci. 2006;126–127:390–397. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]