Abstract
The activity of 5'-adenosine monophosphate–activated protein kinase, a negative regulator of cell size, is up-regulated with age in resting and overloaded fast-twitch skeletal muscle but not slow-twitch muscle. Here, we provide evidence to support the hypothesis that elevated 5'-adenosine monophosphate–activated protein kinase activity plays a potentially important integrative role in the age-related atrophy and diminished capacity for growth specific to fast-twitch skeletal muscle.
Keywords: sarcopenia, aging, loading, hypertrophy, fiber type, translation, protein synthesis
INTRODUCTION
Skeletal Muscle Aging
Skeletal muscle wasting with age, or “sarcopenia,” is a significant clinical problem and results in a loss of functional independence and quality of life in the elderly. Overall skeletal muscle atrophy with age is due to a reduction in muscle fiber size and number (29), and it is well established that individual fiber atrophy with age is specific to the fast-twitch fiber, and not slow-twitch fiber, population (29). Although interventions such as resistance exercise training can produce clinically important gains in muscle strength and fiber size in the elderly (14), fast-twitch fiber hypertrophy with resistance training is reduced with age, even when slow-twitch fibers significantly enlarge (14,27). Impaired growth has also been noted in chronically overloaded fast-twitch, but not slow-twitch, skeletal muscle of old rats (25). The cause(s) of fast-twitch fiber-specific atrophy and decreased growth capacity in aged skeletal muscle are still being elucidated. Based on our work (9,24–26,30) and that of others (7), we propose that elevated 5'-adenosine monophosphate (AMP)–activated protein kinase (AMPK) activity plays a potentially important integrative role in the age-related atrophy and diminished capacity for growth specific to fast-twitch skeletal muscle.
AMPK and Control of Cell Size
5'-Adenosine monophosphate–activated protein kinase has been called an intracellular “fuel-gauge” that is activated during metabolic stresses (energy depletion), when AMP/adenosine-5'-triphosphate (ATP) and inorganic phosphate (Pi)/phosphocreatine (PCr) ratios are high (11). Activation of AMPK occurs by both covalent (Thr172 phosphorylation via upstream kinases such as LKB1) and allosteric means (direct binding via AMP) (11). In skeletal muscle, AMPK activation results in metabolic alterations geared toward restoring and maintaining short- and long-term energy balance within the cell. In general, glucose/lipid catabolism, glycogen storage, and mitochondrial biogenesis are stimulated, whereas energy-consuming anabolic processes such as the synthesis of fatty acids and proteins are inhibited (11). The widely accepted paradigm in most cell types is that AMPK regulates the homeostatic balance between cell growth and cell atrophy, allowing the cell to either focus on ATP production to support cellular metabolic needs or ATP use for maintenance/growth of cell size depending upon the cell's energy state.
Compared with young muscle, aged skeletal muscle displays higher AMP and lower PCr concentrations at rest and after exercise (3). 5'-Adenosine monophosphate–activated protein kinase, a negative regulator of cell size, is normally activated by such high-energy phosphate disturbances (11). Thus, the central hypothesis of this review is that AMPK activity is elevated in aged skeletal muscle and plays a potentially important integrative role in the atrophy and decreased growth capacity of aged fast-twitch skeletal muscle. Specifically, we provide evidence that AMPK negatively mediates overload-induced growth in aged fast-twitch muscle, in part by suppression of translational signaling. We further provide evidence that chronic AMPK activation in resting muscle leads to basal fiber atrophy and death, a phenomenon seen in sarcopenic muscle.
Potential mechanisms by which AMPK may exert its effects are discussed under the premise that cellular mechanisms affecting muscle fiber size must influence the balance between protein synthesis versus protein degradation and/or the balance between myonuclear addition (via myogenic precursor cells, including satellite cells) versus nuclear apoptosis. All four of these processes are altered in aging muscle, leaning the balance toward fiber atrophy and diminished growth (5,21,28,29). Interestingly, AMPK plays a major integrative role in protein synthesis/degradation balance in young skeletal muscle (4,15,17,24), and support is provided that elevated AMPK activity may alter this balance with age. Although little to no data exist as to whether AMPK mediates myonuclear addition and/or apoptosis in young or aged muscle, the very intriguing possibility of AMPK's proapoptotic and/or antiapoptotic role in skeletal muscle is nevertheless discussed by drawing parallels from other cell types.
AMPK AND LOADING-INDUCED MUSCLE GROWTH
Chronic Overload
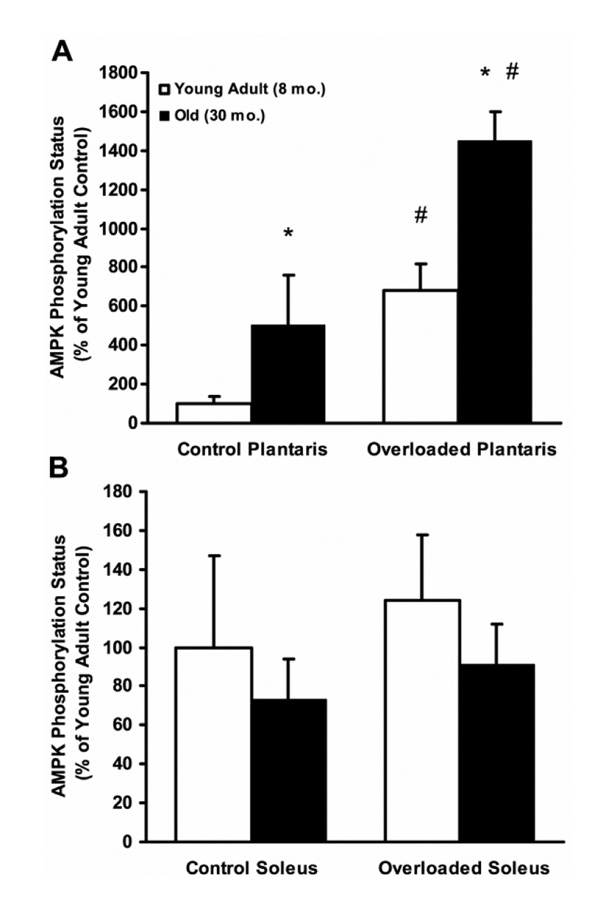
Because age-related atrophy and decreased growth capacity is specific to fast-twitch skeletal muscle, we postulated that 1) AMPK activity would be elevated with age in resting and chronically overloaded fast-twitch muscle but not in slow-twitch muscle, and 2) this elevated AMPK activity would correspond with atrophy and diminished overload-induced growth in fast-twitch muscle but not in slow-twitch muscle. We indeed found that AMPK phosphorylation exhibits a five-fold elevation with age in resting fast-twitch plantaris muscles of Fisher344 × Brown Norway F1 hybrid male rats, but not in slow-twitch soleus muscles, which corresponded to significantly greater atrophy in the fast-twitch muscles ((25); Fig. 1). Moreover, this age-related AMPK hyperphosphorylation was maintained in 7-d overloaded fast-twitch muscles (after unilateral surgical ablation of the synergistic gastrocnemius muscle), whereas still no differences were found in overloaded slow-twitch soleus muscles ((25); Fig. 1). As expected, fast-twitch plantaris muscle hypertrophy was significantly attenuated by greater than 70% with age, whereas slow-twitch hypertrophy was not diminished with age ((25); Fig. 1). All AMPK phosphorylation data were also confirmed by acetyl coenzyme A carboxylase phosphorylation as an index of in vivo AMPK activity.
Figure 1.
5'-Adenosine monophosphate–activated protein kinase (AMPK) phosphorylation status (phospho-AMPK integrated optical density (IOD)/pan-AMPK IOD) at Thr172 on the alpha subunit is greater in old (30 mo) than in young adult (8 mo) fast-twitch plantaris muscles (A), but not in slow-twitch soleus muscles (B), of male Fisher344 × Brown Norway F1 hybrid rats under control (sham-operated) and 7-d overloaded conditions (after unilateral gastrocnemius ablation). Overload-induced fast-twitch plantaris growth was significantly reduced by 75% with age, whereas slow-twitch soleus growth was not significantly reduced with age. Data are expressed as mean (+SE) (n = 7 per group). *Significant main effect (P ≤ 0.05) for age, regardless of loading status. †Significant main effect (P ≤ 0.05) for overload, regardless of age. The AMPK activity as assessed by acetyl coenzyme A carboxylase phosphorylation was highly correlated with AMPK phosphorylation status. Representative blots are omitted in the interest of space. (Reprinted from Thomson, D.M., and S.E. Gordon. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J. Appl. Physiol. 98:557–564, 2005. Copyright © 2005 American Physiological Society. Used with permission.)
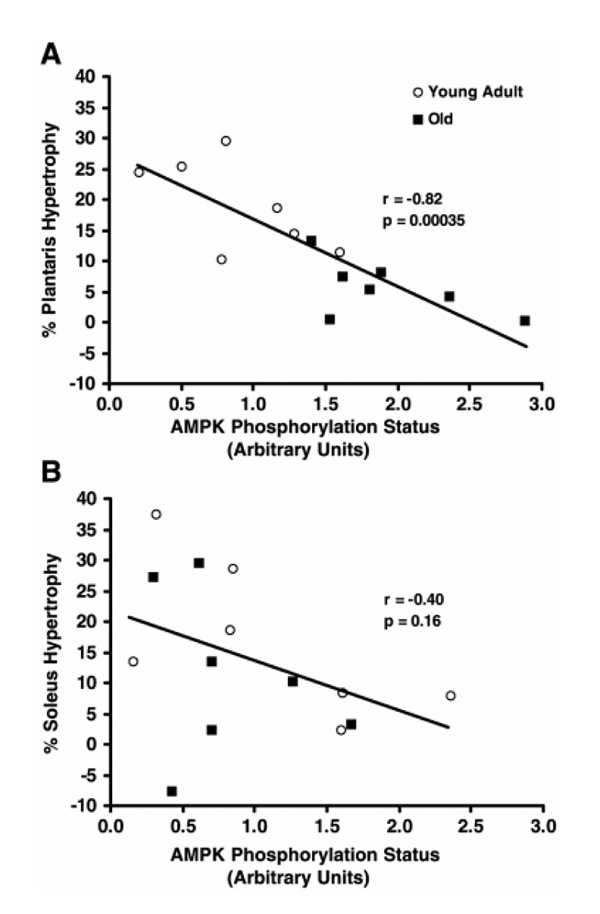
The data in Figure 1 may seem paradoxical, whereby AMPK (known to inhibit protein synthesis and cell growth) is activated in fast-twitch muscle in response to the overloading stimulus (known to promote protein synthesis and fiber growth). However, this likely emphasizes the importance of AMPK in controlling a homeostatic balance between energy production needs (loading activity) and energy-consuming anabolic requirements stimulated by adaptive growth signals. Thus, the degree to which AMPK may negatively mediate muscle growth likely depends upon its “set point” as determined by energy balance, and this set point is elevated in aged fast-twitch muscle. In fact, there was a tight negative correlation between AMPK phosphorylation status and percent hypertrophy in the overloaded fast-twitch plantaris muscles, a correlation that was not significant in the slow-twitch soleus muscle ((25); Fig. 2). Still, it is evident that, even in the slow-twitch soleus muscle, there is a definitive threshold for AMPK phosphorylation above which hypertrophy seemed limited.
Figure 2.
5'-Adenosine monophosphate–activated protein kinase (AMPK) phosphorylation status (phospho-AMPK integrated optical density (IOD)/pan-AMPK IOD) at Thr172 on the alpha subunit is negatively correlated with percent increase in total protein content in 7-d overloaded fast-twitch plantaris muscles (A), but not in overloaded slow-twitch soleus muscles (B), after unilateral gastrocnemius ablation in young adult (8 mo; n = 7) and old (30 mo; n = 7) Fisher344 × Brown Norway F1 hybrid rats. Note that there is still a threshold for AMPK phosphorylation status in soleus muscles above which hypertrophy was noticeably reduced. (Reprinted from Thomson, D.M., and S.E. Gordon. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J. Appl. Physiol. 98:557–564, 2005. Copyright © 2005 American Physiological Society. Used with permission.)
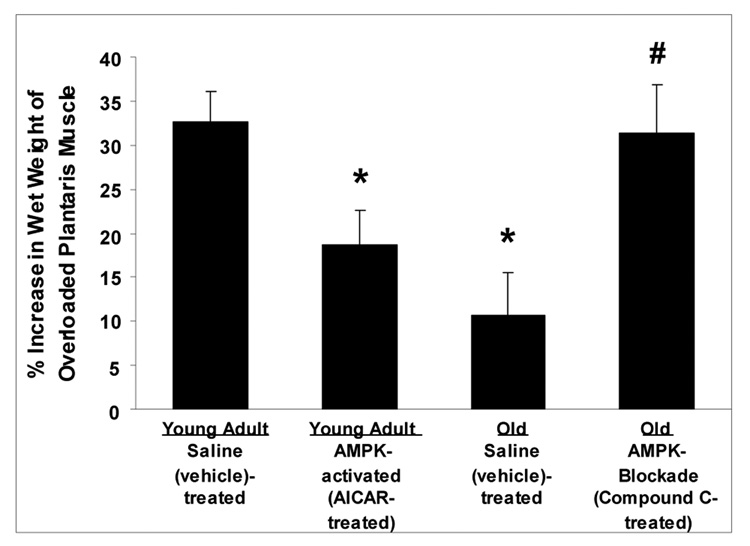
To further establish whether AMPK hyperactivation negatively mediates fast-twitch skeletal muscle growth in aged animals, we performed two more chronic overload experiments (30). In young adult rats, we accentuated AMPK activation by continuous local perfusion (via osmotic pump and catheter) of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR; an AMPK activator) onto fast-twitch plantaris muscles during 7 d of overload. Conversely, in aged rats, we inhibited AMPK activation by continuous local perfusion of Compound C (an AMPK inhibitor) onto fast-twitch plantaris muscles during 7 d of overload. 5'-Adenosine monophosphate–activated protein kinase manipulation was confirmed by AMPK and acetyl coenzyme A carboxylase Western blotting. As hypothesized, stimulation of AMPK attenuated overload-induced growth in young muscles, and inhibition of AMPK restored the impaired growth in old muscles to as level similar to that seen in young saline-treated muscles (Fig. 3). These data confirm that AMPK hyperactivation may at least partly underlie the diminished muscle growth observed in aged fast-twitch muscle with overload and indicate that AMPK inhibition may be a potential target for restoration of this growth in fast-twitch muscles of aged animals.
Figure 3.
5'-Adenosine monophosphate–activated protein kinase (AMPK) activation suppresses fast-twitch plantaris muscle growth during 1 wk of overload (after unilateral gastrocnemius ablation) in young adult (8 mo) Fisher344 × Brown Norway F1 hybrid rats, whereas AMPK blockade restores the lost growth observed in old (30 mo) rats. Overloaded muscles were locally and continuously perfused (osmotic pump and catheter) with either 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR; an AMPK activator) at 0.5 mg00B7h−1, Compound C (an AMPK inhibitor) at 0.05 mg00B7h−1, or vehicle (saline) during the 1-wk overload period. Data are expressed as mean (+SE), n = 7 per group. *Significantly different (P ≤ 0.05) than young adult saline. †Significant different (P ≤ 0.05) than old saline. (Data from (30).)
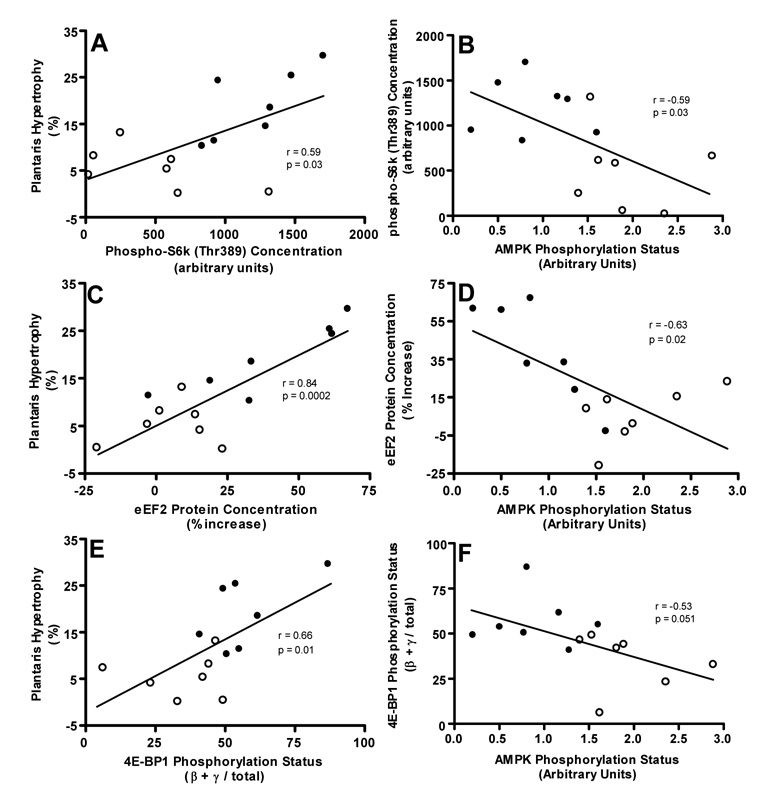
We also found evidence that elevated AMPK activity may inhibit growth of aged fast-twitch muscle by inhibiting mammalian target of rapamycin (mTOR) and its downstream translational signaling intermediates, 70-kd ribosomal protein S6 kinase (S6K), ribosomal protein S6, eukaryotic elongation factor 2, and eukaryotic initiation factor 4E-binding protein 1. All of these intermediates were suppressed with age in overloaded fast-twitch muscle (26). Moreover, phospho-S6K at Thr389 (an mTOR-dependent site), 4E-binding protein 1 phosphorylation status, and total eukaryotic elongation factor 2 accretion were all positively correlated with percent muscle hypertrophy and negatively correlated with AMPK phosphorylation ((26); Fig. 4). This agrees with findings that the protein synthesis response to chronic overload is diminished in aged fast-twitch muscle (20) and that AMPK inhibits skeletal muscle protein synthesis in young muscle by suppressing mTOR-mediated translational signaling (4,24), which is vital for overload-induced skeletal muscle hypertrophy (2). Collectively, these data lead us to speculate that the severely limited growth response to overload in aged fast-twitch muscle is at least partly a result of elevated AMPK activation and a resultant inhibition of protein translational signaling and protein synthesis. The potential role that AMPK may play with respect to other factors influencing muscle mass (e.g., protein degradation, myonuclear addition, or apoptosis) in chronically overloaded muscle remains to be examined.
Figure 4.
Phospho-70-kd ribosomal protein S6 kinase (phospho-S6k; Thr389) content (A and B), eukaryotic elongation factor 2 (eEF2) protein accretion (C and D), and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) phosphorylation (percentage of 4E-BP1 signal encompassed in the β and γ bands; E and F) are positively correlated with percent hypertrophy and negatively correlated with 5'-AMP–activated protein kinase (AMPK) phosphorylation status (Thr172) in overloaded fast-twitch plantaris muscles of young adult (YA; 8 mo; closed circles) and old (O; 30 mo; open circles) rats (n = 7 per group). (Reprinted from Thomson, D.M., and S.E. Gordon. Impaired overload-induced muscle growth is associated with diminished translational signaling in aged rat fast-twitch skeletal muscle. J. Physiol. 574:291–305, 2006. Copyright © 2006 Wiley Blackwell. Used with permission.)
Resistance Exercise
Similar to chronic overload, mTOR and downstream translational signaling intermediates respond to an acute resistance exercise stimulus, and this mTOR signaling is essential for further translational signaling and muscle protein synthesis 16 h after exercise (2). As expected from an acute exercise energy disturbance, AMPK is activated for 1–2 h after resistance exercise in humans and may partly suppress early translational signaling and protein synthesis (6). Indeed, we found that accentuating contraction-induced AMPK activity suppresses translational signaling in rat fast-twitch muscle in response to resisted contractions induced by high-frequency electrical stimulation (a model of resistance exercise that produces significant hypertrophy when performed on a repetitive basis) (24). Thus, after resisted muscle contractions, AMPK activation again seems to act as a homeostatic balance to determine the extent to which protein translation is allowed under the energetic circumstances. Importantly, we have observed a higher AMPK response to high-frequency electrical stimulation in aged rat fast-twitch muscle compared with young (9), and skeletal muscle AMPK phosphorylation demonstrates a prolonged elevation in response to resistance exercise in old versus young men as well (7). Such results could explain the attenuated translational signaling (19) and the diminished myofibrillar protein synthesis response (23) to resistance exercise in aged fast-twitch muscle.
In addition to potentially suppressing protein synthesis, it is possible that the elevated AMPK response to resistance exercise accelerates protein degradation in aged muscle. In resting muscle, AMPK stimulates myotube myofibrillar protein degradation and induces in vivo and in vitro expression of atrophy-related forkhead box (FOXO) genes and muscle-specific ubiquitin ligase genes such as muscle atrophy F-box (MAFbx, or Atrogin-1) and muscle RING finger 1 (MuRF1) (15,17). It is normal for some of these proteolytic genes to respond early after resistance exercise in young muscle (16); proteolysis of mixed muscle protein is elevated during the 24 hr after resistance exercise in young muscle but to a lesser degree than protein synthesis (2). The fact that MAFbx expression responds three- to four-fold more to resistance exercise in the muscles of older women compared with young (21) raises the possibility that greater AMPK activation in aged muscle may be responsible for this accentuated response. Still, it remains to be determined whether this results in muscle proteolysis that is truly greater in aged versus young individuals after resistance exercise because one study observed no age-related increase in muscle interstitial 3-methylhistidine (a marker of myofibrillar degradation) after resistance exercise in men, although resting muscle myofibrillar degradation was greatly elevated with age in that study (28).
DOES AMPK PLAY A ROLE IN SARCOPENIA?
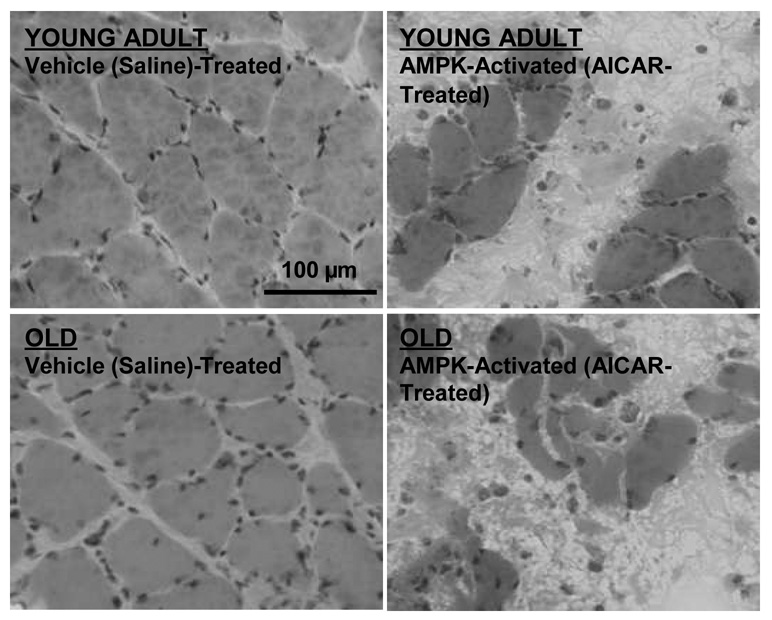
Gradual basal skeletal muscle atrophy with age (sarcopenia) is perhaps even more clinically significant than diminished overload-induced growth. The fact that AMPK activity displays a five-fold increase with age in resting fast-twitch, but not slow-twitch, muscle ((25); Fig. 1) led us to speculate that AMPK may partly underlie basal fast-twitch–specific atrophy, an effect not testable by acute AMPK activation studies. Thus, we continuously activated AMPK in the resting muscles of young and old rats for 7 d (AICAR perfusion via osmotic pump and catheter). Importantly, we used slow-twitch soleus muscles for this experiment, hypothesizing that aged slow-twitch muscle (previously displaying neither elevated AMPK activity nor atrophy with age ((25); Fig. 1) would exhibit sarcopenia-like properties if AMPK were chronically activated similar to aged fast-twitch muscle. As hypothesized, continuous AMPK activation elicited significant fiber atrophy in both young adult and old muscles (Fig. 5). Moreover, an additional unexpected finding of continuously activating AMPK in resting muscle was a high frequency of fiber death (Fig. 5).
Figure 5.
One week of continuous 5'-AMP–activated protein kinase (AMPK) activation causes fiber atrophy and death in slow-twitch soleus muscles of young adult (YA; 8 mo) and old (O; 30 mo) Fisher344 × Brown Norway F1 hybrid rats. Muscles were locally and continuously perfused (osmotic pump and catheter) with either 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR; an AMPK activator) at 0.5 mg00B7h−1 or vehicle (saline) during the 1-wk period. Fiber cross-sectional area was significantly (P ≤ 0.05) reduced in both age groups (by 25% in YA muscles and by 24% in O muscles; n = 3 per group). Images are representative muscle cross-sections stained with hematoxylin and eosin.
The fiber atrophy induced by AMPK activation (Fig. 5) agrees with findings that cultured myotube diameter is reduced under conditions in which AMPK activity is up-regulated (1) and supports the possibility that chronically elevated AMPK activity may contribute to basal fast-twitch fiber atrophy with age (sarcopenia). Suppression of muscle protein translation and synthesis (4,24) may be one mechanism by which AMPK exerts this effect; however, it is highly doubtful that solely suppressing protein synthesis would elicit the striking fiber atrophy and death observed in just 7 d (Fig. 5). Thus, activation of protein degradation pathways is also a likely possibility. As previously mentioned, AMPK is known to stimulate muscle-specific lysosomal and proteasomal gene expression (FOXO, Atrogin-1, and MuRF1) as well as myofibrillar protein degradation (15,17). The increased FOXO3A and MuRF1 mRNA expression and increased myofibrillar degradation with age in resting human muscle of mixed fiber types (21,28) may therefore result in part from elevated AMPK activity in the fast-twitch population. Interestingly, some have observed elevated rates of mixed (10) and myofibrillar (13) protein synthesis, along with higher S6K activation (13), in aged rat fast-twitch muscle. Such a scenario would indicate a futile increase in protein synthesis rate in the face of an even faster degradation rate in these muscles, although still others have conversely observed lower basal protein synthesis in aged muscle (29). Regardless of protein synthesis differences, an elevated protein degradation rate in aged fast-twitch muscle fibers likely plays a role in subtly, and over a long period of time, altering the synthesis-degradation balance toward gradual atrophy. We propose that chronically elevated AMPK activity may play a role in this phenomenon.
The possibility that continuously elevated AMPK activity affects the balance of myonuclear addition versus nuclear apoptosis in aged fast-twitch muscle is intriguing. Unfortunately, few data exist concerning AMPK's potential involvement in either phenomenon in skeletal muscle. Certainly, suppression of basal myonuclear addition cannot account for the rapidity with which AICAR induced fiber atrophy and death (Fig. 5), and whether these effects were due to apoptotic mechanisms and/or more general fiber necrosis remains to be determined. However, AMPK does seem to play a key role in apoptotic signaling in mononucleated muscle cell types, including smooth muscle cells, cardiac myocytes, and immortalized (C2C12) skeletal myoblasts (8,18). It is currently unclear whether AMPK is proapoptotic or antiapoptotic in these cells because there is somewhat equivocal evidence to support both.
In contrast to mononucleated cells, apoptosis in mature multinucleated skeletal muscle fibers does not necessarily mean fiber death but is more associated with fiber atrophy as the myonuclear domain volume remains relatively constant. It is unknown whether nuclear apoptosis leads to protein degradation and fiber atrophy or vice versa (i.e., fiber atrophy leads to nuclear apoptosis, which maintains a constant myonuclear domain volume). As might be expected, the frequency of apoptotic nuclei is well known to be increased in aged atrophying muscle fibers (5). If AMPK is proapoptotic in mature muscle fibers similar to other muscle cell types under some conditions (8), then chronically elevated AMPK activity ((25); Fig. 1) may play a role in the age-related increase in apoptotic nuclei. In fact, we propose that any potential role of AMPK in aging multinucleated muscle fibers is more likely proapoptotic than antiapoptotic because of the following logic: 1) if myonuclear domain volume is to be kept constant, it is paradoxical to protect against nuclear death while selectively reducing protein synthesis and/or stimulating protein degradation; and 2) the potential antiapoptotic effects of AMPK in mononucleated cells is thought to enhance cell survival during times of brief energy deficit (8), whereas apoptosis in multinucleated muscle fibers is more associated with atrophy than a risk of cell death (and lost nuclei can be replenished from the myogenic precursor cell pool when energy balance is restored). Moreover, although the extent of atrophy and/or nuclear apoptosis necessary to eventually achieve a “threshold” precipitating complete muscle fiber death is unknown, our data indicate a role for continuous AMPK activation in fiber atrophy and eventual fiber death, not fiber survival (Fig. 5). Because fiber atrophy and death are also characteristics of sarcopenic muscle (29), the potential role of AMPK in mediating apoptosis in aged skeletal muscle fibers, either directly or indirectly (via fiber atrophy and the loss of myonuclear domains) demands further exploration.
SUMMARY AND FUTURE PERSPECTIVES
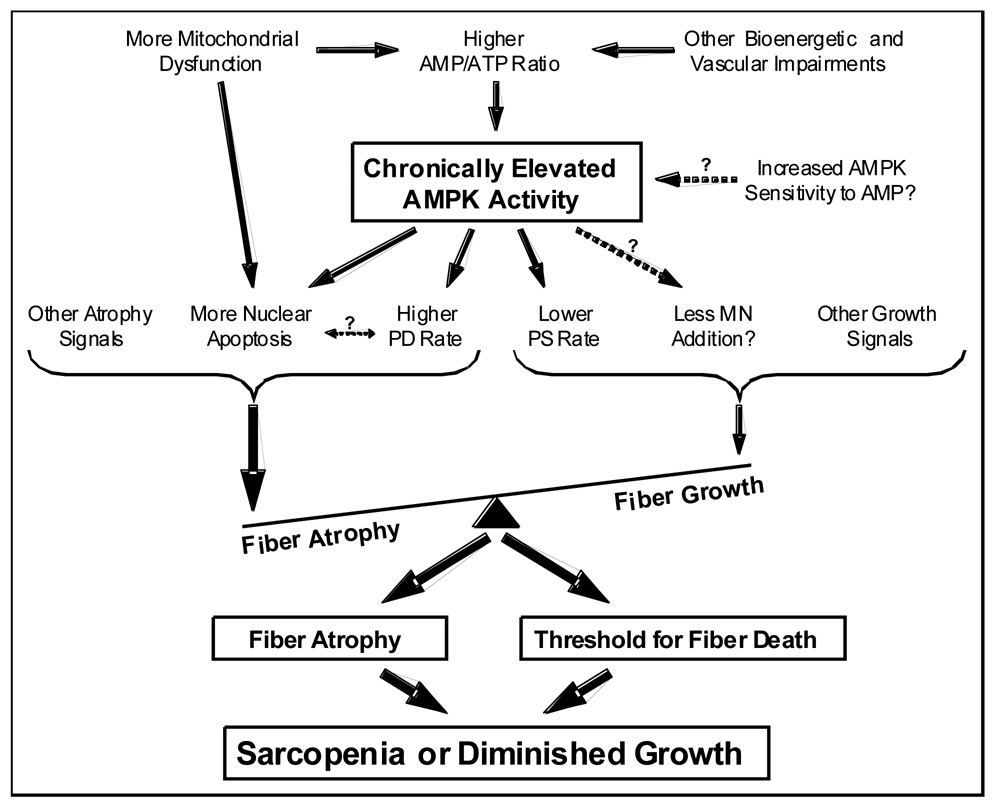
We have demonstrated that AMPK, a negative regulator of cell size that is sensitive to compromised energy balance, is up-regulated and negatively mediates overload-induced growth in aged fast-twitch muscle, likely in part by suppressing protein translation (25,26,30). 5'-Adenosine monophosphate–activated protein kinase is also up-regulated with age in resting fast-twitch muscle (25), and continuous stimulation of AMPK activity in resting muscle results in significant fiber atrophy and death (Fig. 5), a characteristic of sarcopenic muscle. Based on collective data from us and others, we propose a partly hypothetical paradigm (Fig. 6) in which age-related alterations in energy balance result in chronic AMPK activation, which in turn plays an integrative role in negatively mediating muscle fiber size in resting as well as overloaded fast-twitch muscle.
Figure 6.
Hypothetical paradigm of the potential causes and consequences of chronic 5'-AMP–activated protein kinase (AMPK) activation in aged fast-twitch muscle fibers (relative to young adult muscle). Note that the AMPK “set point” is elevated with age regardless of the balance of other loading/activity-related growth or atrophy signals and thus likely acts to accelerate atrophy in resting fast-twitch muscle and suppress growth in overloaded fast-twitch muscle. Because AMPK activity is only increased in resting and overloaded fast-twitch muscle, but not slow-twitch muscle (25), this paradigm is hypothesized to be absent in slow-twitch fibers. MN indicates myonuclear; PD, protein degradation; PS, protein synthesis.
The detrimental effect of chronic AMPK activation on fast-twitch muscle mass contrasts reported beneficial effects of repeated AMPK stimulation (via daily AICAR injections) such as increased mitochondrial enzyme and glucose transporter (GLUT4) expression (11). Nevertheless, these effects fit the paradigm wherein AMPK stimulates energy-producing processes while suppressing cell growth and size to a more metabolically sustainable level. We postulate that chronic AMPK activation in aged fast-twitch fibers is a compensatory (and partly futile) attempt to rectify the insulin resistance, mitochondrial dysfunction (5), and compromised energy balance (3) observed in aging skeletal muscle. However, fiber atrophy and diminished capacity for growth are likely simultaneous consequences of chronic AMPK activation in aged fast-twitch fibers. Such potentially detrimental effects must be considered if AMPK is to be examined as a pharmacological target for metabolic diseases.
Many questions remain, such as the extent to which AMPK may control protein synthesis versus degradation and/or myonuclear addition versus apoptosis in aged fast-twitch muscle under various conditions. Potential regional AMPK activation within individual muscle fibers must be explored because local fiber atrophy and breakage have been shown to be colocalized with mitochondrial DNA mutations at different locations along the lengths of aged muscle fibers (12). The local integration between mitochondrial dysfunction, mitochondrially regulated apoptotic signaling (5), energy balance, AMPK-induced atrophy, and potential AMPK-induced apoptotic signaling must also be teased out. Additionally, the potential for inherent age-related alterations in the AMPK molecule itself (such as AMPK subunit isoform switching leading to increased AMP sensitivity) and altered upstream AMPK kinase and phosphatase input (11) should be examined. Lastly, we must also note contrasting data from others who found exercise- and AICAR-induced AMPK activity to be unresponsive in aged fast-twitch muscles of Fisher344 rats (22). Because our findings are from Fisher344 × Brown Norway F1 hybrid rats (9,24–26,30), the effects of genetic differences may thus be important, and it is vital that the best animal model of skeletal muscle aging be determined.
Acknowledgments
This study was supported by an American Federation for Aging Grant (S.E.G.) and NIH AG025101 (S.E.G.).
Footnotes
We present evidence that elevated AMPK activity may partly underlie atrophy and diminished capacity for growth in aged fast-twitch muscle.
References
- 1.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- 3.Bastien C, Sanchez J. Phosphagens and glycogen content in skeletal muscle after treadmill training in young and old rats. Eur. J. Appl. Physiol. Occup. Physiol. 1984;52:291–295. doi: 10.1007/BF01015212. [DOI] [PubMed] [Google Scholar]
- 4.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 5.Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J. Appl. Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J. Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fick CA, Gordon SE. Age-related differences in the skeletal muscle protein synthesis response 24 hours after shortening but not lengthening contractions. In: Fick CA, editor. Regulation of Protein Synthesis: Singular and Combined Effects of Age, AMPK, and Resisted Contractions on Control of Protein Synthesis and Elongation Factors in Skeletal Muscle (Doctoral Dissertation) Greenville, NC: East Carolina University; 2007. [Google Scholar]
- 10.Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J. Gerontol. A. Biol. Sci. Med. Sci. 1996;51:B323–B330. doi: 10.1093/gerona/51a.5.b323. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med. Sci. Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 12.Herbst A, Pak JW, McKenzie D, Bua E, Bassiouni M, Aiken JM. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: evidence for a causal role in muscle fiber loss. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62:235–245. doi: 10.1093/gerona/62.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball SR, O'Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2004;287:E772–E780. doi: 10.1152/ajpendo.00535.2003. [DOI] [PubMed] [Google Scholar]
- 14.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 15.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1555–E1567. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 16.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J. Appl. Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima K, Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2007;71:1650–1656. doi: 10.1271/bbb.70057. [DOI] [PubMed] [Google Scholar]
- 18.Niesler CU, Myburgh KH, Moore F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp. Physiol. 2007;92:207–217. doi: 10.1113/expphysiol.2006.034736. [DOI] [PubMed] [Google Scholar]
- 19.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J. Appl. Physiol. 2004;97:243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 20.Pehme A, Alev K, Kaasik P, Seene T. Age-related changes in skeletal-muscle myosin heavy-chain composition: effect of mechanical loading. J. Aging Phys. Act. 2004;12:29–44. doi: 10.1123/japa.12.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J. Gerontol. Biol. Sci. Med. Sci. 2007;62:1407–1412. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 22.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR. Limited myogenic response to a single bout of weight-lifting exercise in old rats. Am. J. Physiol. Cell Physiol. 2000;278:C1143–C1152. doi: 10.1152/ajpcell.2000.278.6.C1143. [DOI] [PubMed] [Google Scholar]
- 24.Thomson DM, Fick CA, Gordon SE. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J. Appl. Physiol. 2008;104:625–632. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- 25.Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J. Appl. Physiol. 2005;98:557–564. doi: 10.1152/japplphysiol.00811.2004. [DOI] [PubMed] [Google Scholar]
- 26.Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J. Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am. J. Physiol. Cell Physiol. 2001;281:C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 28.Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J. Physiol. 2004;554:803–813. doi: 10.1113/jphysiol.2003.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welle S. Cellular and molecular basis of age-related sarcopenia. Can J. Appl. Physiol. 2002;27:19–41. doi: 10.1139/h02-002. [DOI] [PubMed] [Google Scholar]
- 30.Westerkamp CM, Gordon SE. 5'-AMP-activated protein kinase (AMPK) regulates overload-induced fast-twitch skeletal muscle hypertrophy over 7 days. In: Westerkamp CM, editor. The Role of 5'-AMP-Activated Protein Kinase in Skeletal Muscle Hypertrophy with Age and Overload (Doctoral Dissertation) Greenville, NC: East Carolina University; 2007. pp. 45–78. [Google Scholar]