Abstract
Adipose tissue contains a vast variety of cell types. Amongst the more abundant cell types present are adipocytes, pre-adipocytes, immune cells and endothelial cells. During times of excess caloric intake, these cells have to adjust and remodel in order to accommodate the increased demand for triglyceride storage. Based on a comprehensive analysis of the total adipose tissue secretome, this review focuses on three areas of adipokine biology: A) what changes does the extracellular matrix of adipose tissue during the development of obesity; B) does the adipocyte per se play a role in the innate immune response; and C) how is the angiogenic profile of adipose tissue linked to the development of insulin resistance?
The adipocyte is a very active endocrine cell. While there is a wealth of information on the transcriptional regulation of the different adipokines, there is only limited data available on how post-translational processes affect the protein levels of these secretory molecules in the extracellular environment. How is this process regulated and to what extend does it contribute to adipokine dysregulation in the context of obesity? To achieve that, we present a comprehensive overview of all of the currently available secreted adipose tissue products that have been identified at the protein level.
Introduction
It is widely appreciated that adipose tissue has important endocrine functions under both physiological and pathophysiological conditions (1–4). This review will focus on areas of adipokine biology that have so far received little attention, but at the same time represent areas that we believe have great implication for our understanding of adipocytes and adipokine physiology. In particular, a comprehensive “metaanalysis” of proteomics efforts on adipocytes is lacking to date. Here, we summarize what we consider to be the key findings in the area of secretion of adipocyte-derived factors.
The Adipocyte Secretome
So far, much attention in the study of adipocyte-derived factors (so called “adipokines”) has been devoted to the study of a relatively small number of proteins with important physiological functions. Notably, these include leptin, adiponectin, resistin, interleukin 6 (IL-6), monocyte chemtractant protein 1 (MCP-1), and tumor necrosis factor alpha (TNFα). On the other hand several proteomic approaches in both primary and tissue-culture derived 3T3-L1 adipocytes have emphasized the complex nature of the adipocyte secretome, highlighting that the adipocyte secretes a large number of different proteins (5–12). Although the analysis of primary adipocyte isolates and tissue culture-derived adipocytes have limitations due to the non-physiolopgical nature of the culture conditions, they help us elucidate aspects of the adipocyte secretome that are under-appreciated and define functional clusters of adipokines that have not yet been the focus of a systematic analysis.
We have collected information from all available published studies and generated a list of proteins that have been reported to be secreted from adipocytes (Table 1). We have clustered these proteins according to their postulated function(s). These adipokines fall into several groups, including adipokines contributing to the extracellular matrix, involved in metabolism or the immune response and other categories. This highlights the important regulatory role of the adipocyte with respect to extracellular matrix components, inflammatory pathways, and angiogenesis. The total number of confirmed proteins identified as secrtetory components of the adipocyte approaches 100 distinct proteins, and that list is likely to grow as the methods for the analysis of supernatants become more sensitive (Table I).
TABLE 1.
| Extracellular Matrix | Metabolism | Immune System | Others |
|---|---|---|---|
| Alpha 2 Macroglobulin1 | Adipsin | Alpha 1 acid glycoprotein | Angiopoietin 1 |
| Cathepsin B | Adiponectin | Colony Stimulating factor-1 | Angiopoietin 2 |
| Cathepsin D | Apelin | Complement component inhibitor | Angiotensinogen |
| Cathepsin L | Apo E | C1 | Calcitonin |
| Cathepsin S | Cortisol | Complement C1 | Chemerin |
| Collagen Alpha 1 (I) | Insulin like growth factor-1 | Complement C2 | Cyclophilin A |
| Collagen Alpha 1 (III) | Insulin like growth factor | Complement C3 | Cyclophilin C |
| Collagen Alpha 1 (IV) | binding protein 7 | Complement C4 | Extra cellular SOD |
| Collagen Alpha 1 (VI) | Lipoprotein lipase | Complement C7 | Galectin 1 |
| Collagen Alpha 1 (XV) | Leptin | Complement factor B | Firbroblast growth factor |
| Collagen Alpha 1 (XIV) | Fasting induced adipose factor | Complement factor C | Hepatic growth factor |
| Collagen Alpha 1 (XVII) | Plasminogen activated | Complement factor D | Mineralocorticoid releasing |
| Collagen Alpha 2 (I) | inhibitor-1 | C reactive protein | factor |
| Collagen Alpha 2 (IV) | Resistin | Haptoglobin | Nerve growth factor |
| Collagen Alpha 2 (VI) | Retinol binding protein 4 | Interleukin 1beta | Pigment epithelium derived |
| Collagen Alpha 3 (VI) | Vaspin | Interleukin 4 | factor |
| Dystroglycan | Visfatin | Interleukin 6 | Prostaglandin E2 |
| Entactin | Interleukin 7 | Prostaglandin I2 | |
| Fibulin-2 | Interleukin 8 | Prostaglandin 2alpha | |
| Fibulin-3 | Interleukin 10 | Serum transferrin | |
| Fibronectin | Interleukin 12 | Stromal derived factor 1 | |
| Galectin-3-binding protein | Interleukin 18 | TGF beta | |
| Gelsolin | Lipocalin 24p3 | Tissue Factor | |
| Laminin alpha 4 | Macrophage migration inhibitory | Vascular endothelial growth | |
| Laminin beta 1 | factor 1 | factor | |
| Laminin gamma | Serum Amyloid A3 | ||
| Lysyl oxidase | Tumor necrosis factor alpha9 | ||
| Matrilin-2 | |||
| Matrix Metalloproteinase 1 | |||
| Matrix Metalloproteinase 2 | |||
| Matrix Metalloproteinase 3 | |||
| Matrix Metalloproteinase 7 | |||
| Matrix Metalloproteinase 9 | |||
| Matrix Metalloproteinase 10 | |||
| Matrix Metalloproteinase 11 | |||
| Matrix Metalloproteinase 12 | |||
| Matrix Metalloproteinase 13 | |||
| Matrix Metalloproteinase 14 | |||
| Matrix Metalloproteinase 15 | |||
| Matrix Metalloproteinase 16 | |||
| Matrix Metalloproteinase 17 | |||
| Matrix Metalloproteinase 19 | |||
| Matrix Metalloproteinase 23 | |||
| Matrix Metalloproteinase 24 | |||
| Tissue inhibitors of metalloprotease 1 | |||
| Tissue inhibitors of metalloprotease 2 | |||
| Tissue inhibitors of metalloprotease 3 | |||
| Tissue inhibitors of metalloprotease 4 | |||
| Osteonectin (SPARC) | |||
| Perlecan | |||
| Procollagen C-proteinase enhancer | |||
| protein | |||
| Protein-Lysine 6-oxidase | |||
| Spondin-1 | |||
| Tenacin | |||
| Thrombospondin-1 | |||
| Thrombospondin-2 | |||
The extracellular matrix of adipose tissue
During the progression from the lean to the obese state, adipose tissue undergoes hyperplasia as well as hypertrophy in an attempt to cope with the increased demand for triglyceride storage. Therefore, the extracellular matrix of adipose tissue faces unique challenges with respect to adjusting to the need for remodeling and expansion.
The changes in cell morphology seen during differentiation in cell culture of pre-adipocytes to adipocytes and the accompanying impact on the expression of various extracellular matrix proteins have been appreciated for some time. For instance, a decrease in the key extracellular matrix protein fibronectin is an absolute prerequisite for differentiation (13, 14). In the extracellular matrix compartment, fibronectin can be degraded by another adipokine, cathepsin S. This cysteine protease is secreted at increasing levels in the obese state, and is able to drive the differentiation of primary human pre-adipocytes (15).
Another protease, Cathepsin K is also up regulated in the obese state and is required for the induction of lipid storage program during 3T3-L1 differentiation (16). Cathepsin K can degrade several matrix constituents, such as collagens type 1 and 2, but its primary target is the protein SPARC. SPARC does not serve as a structural extracellular matrix protein, but as mediator of cell-matrix interactions (17). Mice with a genetic deletion at the SPARC locus display an interesting phenotype. Even though there is no apparent difference in body weight, these mice have a significant increase in adipose tissue volume, associated with an increased number of adipocytes. This is also accompanied by an altered composition of other extracellular matrix components, such as a decrease in the levels of collagen 1 relative to the wild type controls (18). SPARC expression is dysregulated in adipocytes from ob/ob mice that have higher expression and secretion of SPARC (19).
Matrix metalloproteases (MMPs) are of a family of proteins that carry a zinc dependent protease domain, with specificity to a number of extracellular matrix proteins. The activity of these MMPs is controlled by another family of regulatory proteins, the tissue inhibitors of matrix metalloproteinases (TIMPs) that neutralize MMP activity in a 1:1 stoichiometric manner. Several MMPs and TIMPs are secreted from the adipocyte (Table I). This large array of proteases and protease inhibitors is a reflection of the critical need of the adipose tissue ECM to undergo remodeling during times of expansion. In obese mouse models (e.g. ob/ob and db/db mice), a large number of MMPs, including MMP-2, -3, -12, -14, and -19 are up-regulated, whereas MMP-7 is down regulated, and the expression of MMP-9, -11, and -13 are maintained at similar levels between lean and obese mice (20).
To date, it is not known whether the modulation of extracellular matrix constituents are a primary player in the development of obesity or whether changes in these proteins are simply downstream effectors that respond secondarily respond to changes in adipocyte size and number. There is however at least one example where the activity of a MMP has been shown to be critical. The membrane-associated MT1-MMP has been critically implicated in adipocyte differentiation in vivo. A mouse model with a genetic disruption of the MT1-MMP locus shows a significant impairment in white adipose tissue differentiation, resulting in adipose tissue that is populated with very small adipocytes, giving rise to a lipodystrophic animal. Also, of note, is the fact that the proteolytic activity of MMP-2 and -9, two of the most abundant MMPs further are induced during differentiation. This upregulation is significant since direct inhibition of these MMPs by neutralizing antibodies prevents differentiation (21). Similar to cathepsin S, a critical MMP-2 substrate is fibronectin. When the action of MMP2 is blocked, cathepsin S fails to fully compensate for the loss of MMP-2 activity, and adipogenesis is inhibited due to a failure to degrade the fibronectin network surrounding the adipocyte, while leaving the expression of other critical adipogenesis factors such as PPARγ and C/EBPβ unaffected (22).
Given the importance of these factors, it is not surprising that MMPs, the TIMPs are tightly and differentially regulated in the obese state. TIMP1 is strongly induced, whereas TIMP3 is down regulated in the expanding adipose tissue (20). After exposure to a high fat diet, TIMP1−/− mice have smaller adipocytes, less subcutaneous fat, and a lower body weight than their wild type littermates (23). Indeed TIMP-1 expression decreases with adipogenesis, and in vivo over expression leads to adipocyte hypertrophy and accelerated adipogenesis (24). TIMP-1 is circulating in plasma in the nanogram/ml range, and has been postulated to be a predictor of adiposity (but not insulin resistance) in humans (25).
Although our insights into the physiological relevance of the extracellular matrix of adipose tissue during obesity remains rather rudimentary, all the studies discussed above clearly highlight that a partial breakdown of the extracellular matrix is required for adipocyte differentiation, both in vitro as well as in vivo. As such, the expanding adipose tissue needs to modulate/degrade some of the extracellular matrix constituents in order to prevent the extracellular matrix from restraining the expanding adipocytes. Failure to do so may cause stress at the level of the individual adipocyte, leading to local inflammation and a significant impairment to accommodate the increased triglyceride load (figure 1). As a result of these local problems in adipose tissue, systemic effects occur, such as ectopic lipid deposition in organs such as the liver, muscle and pancreas, triggering a significant degree of lipotoxicity (26). In one of our recent studies by Kim et al. a modest over expression of adiponectin enabled ob/ob mice to expand their fat pad further, relieving other tissues from triglyceride deposition and resulting in a normalization of the metabolic phenotype. One of the notable differences reported in these mice is that a host of extracellular matrix proteins were down-regulated, including a number of collagens, MMPs and TIMPs (27). Combined, this strongly suggests that the dysregulation of extracellular matrix components in adipose tissue goes hand in hand with a systemic dysregulation of metabolism. Whether this is cause or effect will need to be shown on a case by case basis for the major components of the extracellular matrix in adipose tissue.
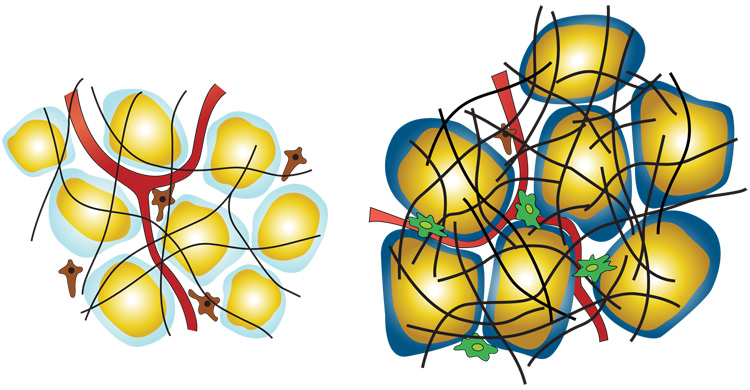
Figure 1. Cartoon representation of white adipose tissue in the lean (left) vs. obese (right) state.
Adipocytes are shown with yellow triglyceride droplets and blue cytoplasm. In the lean state the light blue cytoplasm represent a state of normoxia, whereas the dark blue in the obese state represents a hypoxic state. Pre adipocytes are shown in brown, macrophages in green, blood vessels/endothelial cells in red, and the extracellular matrix as black.
The immune-regulatory role of the adipocyte
A significant number of the adipocyte-derived factors play an intricate role in various aspects of the innate and adaptive immune response (Table I). They may be directly involved as chemokines or cytokines, or they may play a regulatory role (28). It is therefore not surprising that local, obesity-driven changes in adipokine secretion have a systemic impact on a number of branches of the immune system. The best described phenomenon relates to changes in the inflammatory status. Many aspects of the interactions between the immune system and metabolic function have been intensely studied, and many manifestations of the metabolic syndrome have been blamed on macrophage infiltration and chronic low grade inflammation in adipose tissue as it is expanding. These phenomena remain under intense study in many laboratories and have been revisited in numerous reviews and will not be further discussed here (29–34)(figure 1).
In contrast to the chronic subclinical inflammatory state, our insight of the role of adipokines in regular sepsis remains vastly unexplored. The liver is traditionally thought of as the major site for release of most mediators of the innate immune response. But since the adipocyte expresses and secretes many acute phase reactants at high levels (35), it is highly likely that the adipocyte also plays a role in the acute septic state. We have shown that the adipocyte expresses the lipopolysacharide (LPS)-activated toll like receptor 4 (TLR-4) and that it reacts to LPS exposure by increasing the expression and activity of NFκB, which in turn induces the high level production of serum amyloid A3 (SAA3), interleukin-6 (IL-6) and the lipocalin 24p3 (35–37). The important systemic role that the adipocyte plays in the context of pro-inflammatory signals is evidenced in mice where the adipocytes undergo a triggered, rapid adipocyte apoptosis through activation of a caspase-8 construct provided through transgenic expression in adipose tissue. When these fatless mice are challenged with LPS, they have significantly reduced systemic level of SAA3 and IL-6 relative to their control mice with normal adipocytes (38). These observations along with reports from a number of other laboratories suggest that the adipocyte can contribute to the systemic LPS-induced immune response, in part on its own, or more significantly, in concert with local paracrine interactions with adipose tissue macrophages.
The peroxisome proliferator-activated receptor gamma (PPARγ) agonists serve as potent anti-diabetic drugs. Much needs to be learned about the complex set of responses triggered by PPARγ activation in various tissues. Both the adipocyte and the macrophage serve as important targets for the anti-diabetic effects of PPARγ agonists, but additional cell types are clearly affected as well. Adipocytes respond to PPARγ agonist treatment by initiating an anti-inflammatory program with increased secretion of the anti-inflammatory adipokine adiponectin and decreased secretion of pro-inflammatory adipokines, such as tumor necrosis factor alpha (TNFα) and haptoglobin (39–41). A similar response is seen in the macrophages, where PPARγ activation suppresses the LPS induced production of nitric oxide, interleukin-6, and TNFα (42). Anti-inflammatory properties have been reported for PPARγ agonists under additional conditions, such as an impairment colonic inflammation in models of colitis (43) and an attenuation of local liver inflammation in models of non-alcoholic liver disease (44).
Aside from the direct inflammatory aspects of sepsis, septic patients also experience profound insulin resistance. Insulin can not only control hyperglycemia, but also has anti-inflammatory roles under some circumstances. In fact, insulin treatment of patients undergoing sepsis is now widely used (45). Whether adipose tissue constitutes a major target for the anti-inflammatory actions of insulin under these conditions remains to be seen.
Another area where the adipocyte may play a major role in local inflammatory processes is in the context of rheumatoid arthritis and osteoarthritis. Adipocytes not only serve as a storage depot for energy, but can also provide mechanical support within joints. These adipocytes and their pathophysiological changes in arthritis remain extremely poor characterized, but given their potent pro-inflammatory potential, it could easily be envisioned that they make a significant contribution to the local inflammation in the context of an arthritic joint. Adipokines such as leptin, adiponectin and resistin can be measured in synovial fluid of arthritic patients (46). Other investigators have suggested that adiponectin in particular plays a protective role in synovial fluid and may modulate cartilage destruction in chondrocytes (47). These structural fat pads deserve further investigation and it remains to be seen whether strategies specifically targeting anti-inflammatory aspects of these cells may contribute productively towards a reduction of the degenerative aspects of the disease.
Angiogenesis
While adipocytes occupy the bulk of the volume of adipose tissue, the adipose tissue stroma contains many more cell types that at least equal the adipocytes in number. Non-adipocytic cell types in adipose tissue include fibroblastic pre-adipocytes that can be triggered to embark on the adipogenic differentiation pathway. Other cell types include immune cells and, importantly, endothelial cells. New endothelium can be made by sprouting from existing endothelial cells or by maturation of new endothelial cells from circulating endothelial progenitor cells. Whereas the latter does not seem to play as prominent a role, the sprouting of the endothelium indeed seems to be a highly active and coordinated event where sprouting is especially active in the vicinity of differentiating adipocytes (48–50). In fact there is a critical dependency on angiogenesis for the developing adipose tissue development. Ongoing neovascularization is required for healthy adipose tissue, since exposure to angiogenesis inhibitors triggers a reduction in fat mass (51–53). There is even evidence suggesting that the vascular network forms before the mature lipid carrying adipocytes resides in the area (50).
The adipocyte secretes several factors that modulate the production of new blood vessels e.g. angiopoietin-1, angiopoietin-2, vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), hepatic growth factor (HGF), stromal derived factor 1 (SDF-1), TNF-α, resistin, leptin, tissue factor, placental growth factor (PGF), insulin-like growth factors (IGF) as well as lower molecular weight lipid factor monobutyrin (reviewed in (54)). Despite this great angiogenic potential, a rapidly expanding fat pad still experiences hypoxia, whereas other tissues in the same animal (such as muscle) do not (55–57). As a consequence the master regulator of hypoxia, hypoxia induced factor α (HIF-1α), and several of its downstream targets e.g. glucose transporter 1 (GLUT1), VEGF, pyruvate dehydrogenase-1, and hemeoxygenase-1 are induced. Besides VEGF, hypoxic isolated adipocytes secrete higher levels of a number of pro-angiogenic factors, such as leptin, IL-6, MIF, leptin, and PAI-1 (11). In addition to these hypoxia-induced angiogenic factors, it is well established that the obese state is associated with increased levels of plasma levels of TNFα, resistin, angiopoietin-1, and HGF (58). Even though the adipocyte remains at the center of these pro-angiogenic processes, a large amount of evidence points towards the macrophage as an extremely important source for angiogenic factors as well. The macrophages that reside in the adipose tissue in the lean state increase sharply in number as an individual becomes more obese. These macrophages are very active players in the course of the new vascularization associated with post-natal outgrowth of epidydimal adipose tissue (50) and have a potent pro-angiogenic profile under normoxic conditions and even more active when subjected to hypoxia (57). The adipose tissue macrophages polarizes into to populations. M1 is involved in the killing of intracellular parasites and are characterized by a type 1 inflammatory response releasing factors as IL6 and TNFα. M2 macrophages on the other hand are recruited for tissue remodeling including a pro-angiogenic potential, and a type 2 inflammatory response (59). Recently it has become apparent that obesity induces a phenotypic switch from the M2 to M1 macropohages (60), thereby seemingly decreasing the angiogenic potential of the residing macrophages.
There is also an important connection between the extra cellular matrix and angiogenesis. Hypoxia induces MMP-2 and -9 in adipocytes (61). Especially MMP-9 deserves attention in this sense, in that it is a known tumor angiogenic factor that works through extracellular matrix bound VEGF (62). Anti-VEGF treatment of rodents demonstrates that VEGF is an important player not only in adipose tissue vascularization but also in the development of new adipocytes (49, 50).
Despite the seemingly massive induction of a pro-angiogenic response, it is apparently not sufficient to prevent the development of hypoxia in the expanding adipose tissue. This could be partly due to the synthesis of additional signals that send a rather ambivalent message, since the increase in pro-angiogenic factors may be accompanied by an even larger increase in inhibitors of neovascularization, For instance, circulating levels of angiopoietin-2 and endostatin correlate well with body mass index (58). The underlying reasons for the upregulation of these anti-angiogenic factors are unknown. Another factor may be that whilst many pro-angiogenic factors may be induced, some critical downstream mediators of these signals may fail to be produced at appropriate levels.
TNP-470 is an angiogenesis inhibitor that has also been tested in the context of obesity. Ablation of the vasculature in the adipose tissue with this compound leads to a significant loss of body weight and fat mass, even though it is difficult to interpret since TNP-470 may also have effects on satiety (63). However, these results have certainly raised the speculation whether angiogenesis inhibitors may be used as anti-obesity drugs. The results presented by Koloni et al. are certainly intriguing (52). In this case, the targeted disruption of the adipose tissue vasculature through the use of a phage peptide–directed toxin destroying the adipose tissue endothelium leads to a reduction of adipose tissue, a phenotype reminiscent of other lipodystrophic mice models. Surprisingly, and in contrast to most lipodystrophic models, partial loss of fat leads to a decreased accumulation of liver lipids, lower fasting glucose, and fasting insulin levels. Presumably this is due to a lower energy intake, and higher energy expenditure. Given what we know about this model to date, it is difficult to speculate about the underlying mechanisms of this phenotype.
An important question could be raised in this context. While it is clear that obese adipose tissue suffers from hypoxia, is this hypoxia truly rate limiting for further expansion of fat mass? Is it the hypoxia which is at the source of local inflammation in adipose tissue? We do not yet know the answers to these questions, but this can clearly be addressed with mouse models that display a constitutive activation of the hypoxia-associated factors in adipose tissue.
Post translational regulation of adipokine secretion
We have described above the tremendous versatility of the adipose tissue proteome, and have focused our summary mostly on the local effects of adipokines in adipose tissue proper. These effects of course also translate into systemic physiological changes, which in turn are further modified by adipokines that have endocrine functions and exert their effects on target cells not directly exposed to adipose tissue. There is a wealth of information available on the transcriptional regulation of many of these adipokines. However, the adipocyte also seems to be remarkably versatile when it comes to post-translational ways of controlling protein export. A good example can be seen in the context of adiponectin. PPARγ agonist treatment increases circulating levels of adiponectin up to 4-fold, while leaving the mRNA levels relatively unchanged (64), suggesting potent mechanism are in place to regulate the release of adiponectin at the post-translational level. Research on trafficking through the ER and Golgi in adipocytes has for many years been biased towards studying the movements of the insulin-responsive GLUT4 transporter (65). GLUT4 vesicles are indeed fusing with the plasma membrane at an increased rate in the presence of insulin. However, these GLUT4 vesicles are not well suited to carry soluble cargo molecules since there is extensive cycling of these vesicles to and from the plasma membrane even in the unstimulated state. Therefore other mechanisms must be in place in the adipocyte that regulates the release of adiponectin. This is even more relevant in light of the fact that the adipocyte releases several different forms of adiponectin, and the processing of each of these forms seems to be differentially regulated. A novel mechanism has been suggested in two recent studies that demonstrate that adiponectin is assembled and processed in a similar manner as IgM molecules. Adiponectin is retained in the ER by the chaperone ERp44 and released by the action of the oxidoreductase Ero-Lα (66, 67). This process is highly regulated, particularly at the level of these chaperones. It is in fact these chaperones that are the primary transcriptional target for PPARγ agonists, leading to a more efficient release of the high molecular weight form of adiponectin from the adipocyte.
The adipocyte secretory pathway
Is this Erp44/Ero1-mediated pathway a widely used mechanism to shunt proteins through later stages of the secretory pathway? Is it utilized by other adipokines as well? At least one other adipokine, resistin, does not get retained in the ER by ERp44 (67), arguing that this mechanism is at least partially specific for adiponectin. However, we still know very little about the mechanisms by which the adipocyte handles the differential release of factors to the extracellular environment. Is there such a thing as a triggered release of factors from the adipocyte? There is little evidence that a rapidly releasable pool of proteins exists similar to what can be seen in neuroendocrine cells. The exposure of adipocytes to β3 adrenergic agonists can trigger a rapid release of insulin from cells (68). However, conventional wisdom holds that this is primarily caused by lipolysis, resulting in the release of free fatty acids rather than a triggered release of a protein constituent. Other than the insulin-induced translocation of GLUT4 vesicles, there is very little additional evidence that the adipocyte can release proteins in a shorter time scale. Lipoprotein lipase may be another example of a protein whose release from the ER is regulated (69). Insulin triggers a profound reshuffling of intracellular membranes, but with limited consequences on the release of soluble proteins (70).
However, the adipocyte is a tremendously active secretory cell whose mechanisms have yet to be revealed. The high level production of abundant secretory constituents such as adiponectin that is present at high levels in plasma and that turns over rapidly requires a secretory machinery that can keep up with the very high demands on the production of these proteins. Complement factor C3 is another component that is highly abundantly produced. Exposure to pro-inflammatory stimuli such as cytokines and LPS can rapidly transform the adipocyte into a cell devoted to the massive production of acute phase reactants, such as serum amyloid A3 and many others, while maintaining the production and release of the conventional adipokines. Under these conditions, factors such as macrophage migration inhibitory factor (MIF) are also produced a high levels. This is a protein whose mechanism of release is still not yet well defined and unlikely to involve the conventional secretory pathway (71).
An additional unusual feature of the adipocyte is the high abundance of caveolar structures throughout the entire cell. The presence of the flask-shaped structures gives the adipocyte plasma membrane it characteristic structure where up to 25% of the cell surface is occupied by these raft structures (70) that display a unique lipid composition compared to the remainder of the plasma membrane. While these caveolae are involved in signaling events (72–74), they also play a critical role in lipolysis. However, to date, we do not know whether these structures or their structural components, caveolin-1 and caveolin-2, are involved in vesicular trafficking of proteins. The abundant presence of these proteins throughout the secretory pathway, with high levels in the ER and Golgi, makes these proteins prime candidates as mediators for additional trafficking events within the realm of the classical secretory pathway and/or as mediators of trafficking of lipids and proteins directly from the ER to other subcellular compartments. Future efforts will have to be directed towards probing the existence and potential relevance of these additional trafficking events once appropriate cargo molecules have been identified whose secretion is critically affected by these pathways.
Concluding remarks
The role of adipose tissue as an important source of local mediators in the stroma of a host of organs as well as its role as an endocrine gland is now widely appreciated. As a whole, adipose tissue can make up a significant proportion of total body weight. So by sheer mass action, it is difficult to ignore the contribution it makes to plasma protein. In addition, since fat pads are interspersed in many different places systemically, it is important to note that these pads constitute different “miniorgans” with unique characteristics depending on their location and a differential proteomics fingerprint. We have attempted here to provide a comprehensive overview of protein factors that have been described in the literature. We have devoted much of the discussion on aspects of the adipocyte secretome that usually get less attention. It is not clear in all instances to what extent it is the adipocyte that serves as the primary site of production or whether another adipose tissue cell type in concert with the adipocyte is the major production site. In either case, if we have to treat the whole tissue as an entity. Thus much remains to be understood, both about the cross talk between the different cell types within the adipose tissue, as well as the secretory pathway.
Acknowledgements
The authors would like to thank Nancy Heard for help with the cartoon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 2.Nawrocki AR, Scherer PE. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today. 2005;10:1219–1230. doi: 10.1016/S1359-6446(05)03569-5. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 4.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Llamas G, Szalowska E, de Vries MP, Weening D, Landman K, Hoek A, Wolffenbuttel BH, Roelofsen H, Vonk RJ. Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics. 2007;6:589–600. doi: 10.1074/mcp.M600265-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Cushman SW, Pannell LK, Hess S. Quantitative proteomic analysis of the secretory proteins from rat adipose cells using a 2D liquid chromatography-MS/MS approach. J Proteome Res. 2005;4:570–577. doi: 10.1021/pr049772a. [DOI] [PubMed] [Google Scholar]
- 7.Klimcakova E, Moro C, Mazzucotelli A, Lolmede K, Viguerie N, Galitzky J, Stich V, Langin D. Profiling of adipokines secreted from human subcutaneous adipose tissue in response to PPAR agonists. Biochem Biophys Res Commun. 2007;358:897–902. doi: 10.1016/j.bbrc.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS, Fernandez MM, Bunkenborg J, Roepstorff P, Kristiansen K, Lodish HF, Mann M, Pandey A. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002;1:213–222. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 9.Scherer PE, Bickel PE, Kotler M, Lodish HF. Cloning of cell-specific secreted and surface proteins by subtractive antibody screening. Nat Biotechnol. 1998;16:581–586. doi: 10.1038/nbt0698-581. [DOI] [PubMed] [Google Scholar]
- 10.Tsuruga H, Kumagai H, Kojima T, Kitamura T. Identification of novel membrane and secreted proteins upregulated during adipocyte differentiation. Biochem Biophys Res Commun. 2000;272:293–297. doi: 10.1006/bbrc.2000.2759. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Mariman E, Keijer J, Bouwman F, Noben JP, Robben J, Renes J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell Mol Life Sci. 2004;61:2405–2417. doi: 10.1007/s00018-004-4256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 13.Antras J, Hilliou F, Redziniak G, Pairault J. Decreased biosynthesis of actin and cellular fibronectin during adipose conversion of 3T3-F442A cells. Reorganization of the cytoarchitecture and extracellular matrix fibronectin. Biol Cell. 1989;66:247–254. [PubMed] [Google Scholar]
- 14.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3t3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 15.Taleb S, Cancello R, Clement K, Lacasa D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y, Junfeng H, Tianhong L, Lu W, Shulin C, Yu Z, Xiaohua L, Weixia J, Sheng Z, Yanyun G, Guo L, Min L. Cathepsin K in adipocyte differentiation and its potential role in the pathogenesis of obesity. J Clin Endocrinol Metab. 2006;91:4520–4527. doi: 10.1210/jc.2005-2486. [DOI] [PubMed] [Google Scholar]
- 17.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, Van Obberghen E. The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem. 2001;276:22231–22237. doi: 10.1074/jbc.M010634200. [DOI] [PubMed] [Google Scholar]
- 20.Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 21.Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 22.Croissandeau G, Chretien M, Mbikay M. Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem J. 2002;364:739–746. doi: 10.1042/BJ20011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lijnen HR, Demeulemeester D, Van Hoef B, Collen D, Maquoi E. Deficiency of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) impairs nutritionally induced obesity in mice. Thromb Haemost. 2003;89:249–255. [PubMed] [Google Scholar]
- 24.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 Regulates Adipogenesis during Mammary Gland Involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kralisch S, Bluher M, Tonjes A, Lossner U, Paschke R, Stumvoll M, Fasshauer M. Tissue inhibitor of metalloproteinase-1 predicts adiposity in humans. Eur J Endocrinol. 2007;156:257–261. doi: 10.1530/eje.1.02328. [DOI] [PubMed] [Google Scholar]
- 26.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Mooreq I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 34.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–42083. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- 36.Ajuwon KM, Jacobi SK, Kuske JL, Spurlock ME. Interleukin-6 and interleukin-15 are selectively regulated by lipopolysaccharide and interferon-gamma in primary pig adipocytes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R547–R553. doi: 10.1152/ajpregu.00585.2003. [DOI] [PubMed] [Google Scholar]
- 37.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab. 2004;287:E1178–E1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 38.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358–3370. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 40.Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S17–S21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- 41.do Nascimento CO, Hunter L, Trayhurn P. Regulation of haptoglobin gene expression in 3T3-L1 adipocytes by cytokines, catecholamines, and PPARgamma. Biochem Biophys Res Commun. 2004;313:702–708. doi: 10.1016/j.bbrc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Alleva DG, Johnson EB, Lio FM, Boehme SA, Conlon PJ, Crowe PD. Regulation of murine macrophage proinflammatory and anti-inflammatory cytokines by ligands for peroxisome proliferator-activated receptor-gamma: counter-regulatory activity by IFN-gamma. J Leukoc Biol. 2002;71:677–685. [PubMed] [Google Scholar]
- 43.Ramakers JD, Verstege MI, Thuijls G, Te Velde AA, Mensink RP, Plat J. The PPARgamma agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J Clin Immunol. 2007;27:275–283. doi: 10.1007/s10875-007-9074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahan V, Eren F, Avsar E, Yavuz D, Yuksel M, Emekli E, Imeryuz N, Celikel C, Uzun H, Haklar G, Tozun N. Rosiglitazone Attenuates Liver Inflammation in a Rat Model of Nonalcoholic Steatohepatitis. Dig Dis Sci. 2007 doi: 10.1007/s10620-007-9756-x. [DOI] [PubMed] [Google Scholar]
- 45.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 46.Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14:1095–1100. doi: 10.2174/092986707780362826. [DOI] [PubMed] [Google Scholar]
- 47.Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762:711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 50.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 51.Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 52.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 53.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 56.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 57.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 58.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 60.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 62.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim YM, An JJ, Jin YJ, Rhee Y, Cha BS, Lee HC, Lim SK. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol. 2007;38:455–465. doi: 10.1677/jme.1.02165. [DOI] [PubMed] [Google Scholar]
- 64.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 65.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 66.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang ZV, Schraw TD, KimZ JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem. 1997;272:17686–17693. doi: 10.1074/jbc.272.28.17686. [DOI] [PubMed] [Google Scholar]
- 69.Roh C, Roduit R, Thorens B, Fried S, Kandror KV. Lipoprotein lipase and leptin are accumulated in different secretory compartments in rat adipocytes. J Biol Chem. 2001;276:35990–35994. doi: 10.1074/jbc.M102791200. [DOI] [PubMed] [Google Scholar]
- 70.Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Mastick CC, Lodish HF. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flieger O, Engling A, Bucala R, Lue H, Nickel W, Bernhagen J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 2003;551:78–86. doi: 10.1016/s0014-5793(03)00900-1. [DOI] [PubMed] [Google Scholar]
- 72.Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 74.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]