Abstract
Objective
Determination whether common variation at the CHGA locus increases susceptibility to hypertension.
Background
Chromogranin A (CHGA) regulates catecholamine storage and release. Previously we systematically identified genetic variants across CHGA.
Methods
Dense genotyping across the CHGA locus in >1000 individuals with the most extreme BPs in the population, as well twin pairs with autonomic phenotypes. Characterizing function of a trait-associated 3'-UTR variant with transfected CHGA 3'-UTR/luciferase reporter plasmids.
Results
CHGA was overexpressed in patients with hypertension, especially hypertensive men, and CHGA predicted catecholamines. In individuals with extreme BPs, CHGA genetic variants predicted BP, especially in men, with a peak association occurred in the 3'-UTR at C+87T, accounting for up to ~12/~9 mmHg. The C+87T genotype predicted CHGA secretion in vivo, with the +87T allele (associated with lower BP) also diminishing plasma CHGA by ~10%. The C+87T 3'-UTR variant also predicted the BP response to environmental (cold) stress; the same allele (+87T) that diminished basal BP in the population also decreased the SBP response to stress by ~12 mmHg, and the response was smaller in women (by ~6 mmHg). In a chromaffin cell-transfected CHGA 3'-UTR/luciferase reporter plasmid, the +87T allele associated with lower BP also decreased reporter expression by ~30%. In cultured chromaffin cells, reducing endogenous Chga expression by si-RNA caused ~2/3 depletion of catecholamine storage vesicles.
Conclusions
Common variant C+87T in the CHGA 3'-UTR is a functional polymorphism causally associated with hypertension especially in men of the population, and propose steps ("intermediate phenotypes") whereby in sex-dependent fashion this genetic variant influences the ultimate disease trait. These observations suggest new molecular strategies to probe the pathophysiology, risk, and rational treatment of hypertension.
Keywords: hypertension, chromaffin, catecholamine, adrenal, sympathetic
INTRODUCTION
Chromogranin A (CHGA), a 48-kDa acidic polypeptide (1, 2), is the major protein co-stored and co-released with catecholamines from secretory vesicles in adrenal medulla and postganglionic sympathetic axons (3). Catecholamine storage vesicles (or chromaffin granules) of the adrenal medulla contain remarkably high concentrations of CHGA, catecholamines, ATP, and Ca2+, and CHGA seems to bind and store both catecholamines and Ca2+ (4). CHGA also binds to the vesicle membrane, where it may influence the release of calcium from secretory granules to the cytosolic exocytotic machinery through the inositol 1, 4, 5-trisphosphate receptor/Ca2+ channel (5). CHGA is required for formation of catecholamine secretory vesicles in chromaffin cells and its expression may be sufficient to induce a regulated secretory system in nonsecretory cells (6). CHGA is also a pro-hormone that gives rise to biologically active peptides such as the dysglycemic peptide pancreastatin (7, 8); the antimicrobial peptide chromacin (9); the vasodilator vasostatin (10); and catestatin that acts to inhibit catecholamine release (11, 12).
Essential hypertension is complex trait (13), with contributions from multiple factors: cardiovascular, neuronal, renal, and adrenal. Over the past ~20 years, phenotypic links between CHGA and essential (idiopathic, genetic) human (14–17) and rodent (18) hypertension have been repeatedly observed. Plasma CHGA concentration correlates with catecholamine release rates (19), and increases in blood pressure caused by the action of catecholamines are likely to be coupled to the formation of dense-core secretory granules, whose biogenesis is regulated in vivo by CHGA (20). Recently we systematically identified common genetic variation in human CHGA by resequencing the gene in several human populations (21); here we explored whether common genetic variation at the CHGA locus is associated with to blood pressure, beginning with a large population-based sample of extreme blood pressures, in which we found that a 3’-UTR polymorphism (C+87T) is associated with substantially to both DBP and SBP. We then established its influence on an earlier pathogenic phenotype (environmental stress-evoked change in BP), and finally documented its effect on gene expression in a transfected reporter system.
MATERIALS AND METHODS
Subjects and clinical characterization
Hypertension
Diagnosis of hypertension
Since hypertension is part of a larger syndrome, all individuals of diverse ancestries were phenotyped for not only blood pressure, but also associated traits, both metabolic and renal (on-line Table I and on-line Table II).
Phenotype (CHGA, catecholamine) and BP study
In the first (purely phenotypic) study, we measured the plasma concentrations of CHGA, norepinephrine, lipids, and creatinine (see below) in n=724 individuals with normal renal function (serum creatinine ≤1.5 mg/dl), stratified by blood pressure status: normal blood pressure (documented at <135/<85 mmHg, on no medications), versus a diagnosis of essential hypertension (documented at DBP ≥90 mmHg). Of those with hypertension, 75% were treated with antihypertensive medications. Blood pressures were determined in triplicate (and then averaged) in seated subjects with an oscillometric device (DynaPulse; PulseMetric, Vista, CA), validated and calibrated as described previously (22). During the same visit, the same subjects also underwent prolonged (5-minute, ~400 beat), non-invasive monitoring of BP with a radial artery applanation tonometer (Colin Pilot; Colin Instruments, San Antonio, TX); such prolonged radial arterial readings correlated with both SBP (Spearman rho = 0.57, p<0.001) and DBP (Spearman rho = 0.53, p<0.001) obtained by the DynaPulse brachial cuff.
CHGA genotype and BP study
In the second (genotype/phenotype) study, a population cohort with extreme blood pressures consisted of 470 male and 558 female white (European ancestry, by self-identification) subjects. These participants were selected based on DBP in the upper or lower most extreme (5th) percentiles of DBP distribution in 25,599 men and 27,479 women in a primary care practice at Kaiser-Permanente of Southern California medical group (23,24) Subjects were ascertained on the DBP trait, because twin and family studies provide evidence that DBP is substantially heritable (25), and SBP correlates highly with DBP. BP was measured in seated individuals with an aneroid sphygmomanometer in a single health appraisal clinic site by trained, long-term personnel, and BP measurement was repeated if elevated on initial reading. The health appraisal visit included measurement of vital signs, extended questionnaire, and clinical laboratory evaluation, including hemogram, chemistry panel, glucose, and lipids. Individuals in the upper DBP percentiles were age-matched to subjects in the lower extreme percentiles. We ascertained 189 men (age 58.5±10.4 [SD] years) with DBP ≥96 mmHg and 281 men (age 57.7±15.8 years) with DBP ≤61 mmHg. Among the women, 175 were ascertained with high DBP (≥92 mmHg; age 61.4±11.2 years), along with 383 age-matched women with low DBP (DBP ≤59 mmHg; age 53.9±14.0 years). BP was treated by antihypertensive medications in 48% of subjects with hypertension. Thus, the DBP group separation for men was >35 mmHg, while that for women was >33 mmHg. Power calculations were performed using the on-line genetic power calculator for quantitative trait loci (http://statgen.iop.kcl.ac.uk/gpc/)(26) according to the method described by Schork et al(27). The power of an association study on the extreme samples was computed under varying disease allele frequencies for type I error rates of 0.05, 0.001, or 0.00000001 (“genome-wide” level), for recessive, additive, and dominant models of inheritance, using the proportion of variance of BP explained by the locus at 0.5%, 1%, 2.5%, and 5%, and assuming the marker locus is the actual trait locus (D’=1.0). At a genome-wide alpha of p<10−8, we determined that this sample has >90% power to detect genotype association with BP, if the genotype at minor allele frequency ≥15% contributes ≥2.5% of total variance.
Twin pairs
Twin subject characteristics are described in our previous reports (28). Twin pairs, aged 15–84 years (median, 40 years), were 69% monozygotic (MZ) and 31% dizygotic (DZ). Twin zygosity was confirmed by single nucleotide and microsatellite polymorphisms, as previously described (25, 29, 30). 9.9% of the twins were hypertensive (8.8% treated with antihypertensive medications). Twin phenotyping is described below.
Environmental (cold) stress in twin pairs
N=149 twin pairs were studied; each subject was self-identified as being of white (European) ancestry. Blood pressure was recorded continuously and non-invasively in seated subjects with a radial artery applanation tonometer and dedicated sensor hardware (Colin Pilot; Colin Instruments, San Antonio, TX) and software (ATLAS, WR Medical, Stillwater, MN; TDA [Tonometric Data Analysis], Colin Instruments, San Antonio, TX). During the CPT (cold pressor test), after a 10-minute equilibration period the left hand was immersed in ice water (at 0°C) for 60 seconds, as previously described (28,31). The device was periodically calibrated against an automated cuff blood pressure in the contralateral arm. Heart rate was similarly recorded with thoracic EKG electrodes. We identified at least 3 beats with consistent (within 10%) values for BP and HR just before and at the end of the CPT, and resulting changes in blood pressure and heart rate were recorded. Even though BP may continue to rise at 2–3 minutes of cold exposure (32), we chose the 1 minute time-point since in longitudinal studies this 1-minute BP increment has been shown to predict the development of hypertension decades later (33).
Biochemical assays
EDTA-anticoagulated plasma was frozen and stored at −70°C prior to assay. The region-specific radioimmunoassay for CHGA116–439 (precursor) was based on a polyclonal rabbit antiserum (34). 125I-radiolabeling of the protein was enabled by endogenous Tyr residues, as described in detail elsewhere (35, 36). Catecholamines were measured by radioenzymatic assay, as previously described (37).
Genomics
Genomic DNA was prepared from leukocytes in EDTA-anticoagulated blood, using PureGene extraction columns (Gentra Biosystems, Minnesota) as described (38).
The reference sequence (RefSeq) for human CHGA was obtained from the UCSC Genome Browser (http://genome.ucsc.edu). We resequenced the CHGA locus (8 exons, intron/exon borders, UTRs, and proximal promoter) for exhaustive variant discovery in n=180 subjects (2n=360 chromosomes), as previously described (21). These subjects included n=51 white (European ancestry) subjects.
SNP diploid genotypes were scored by either of two base-extension systems: the MALDI-TOF system of Sequenom (39) or the luminescent system of Pyrosequencing (40). In each case, initial PCR amplification of the template was followed by primer-mediated base extension across the variant position. Pyrosequencing primers were designed using the dedicated software provided by Pyrosequencing (Uppsala, Sweden). Target sequences were amplified by PCR from 15 ng genomic DNA in a final volume of 10 ul. To ensure accurate assignment, genotypes were verified by visual inspection and artifactual data were excluded from further statistical analysis.
Chromaffin cell culture
PC12 rat pheochromocytoma cells (41) were obtained from David Schubert, Salk Institute, La Jolla, CA. They were cultured in high-glucose Dulbecco’s modification of Eagle’s medium with 10% heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum, and penicillin/streptomycin. Cell passage number (since initiation of the line) was between 10 and 25 in these experiments.
Function of CHGA 3’-UTR variants: 3’-UTR/luciferase reporter activity assays
The full-length human CHGA cDNA previously subcloned into pET21a(+)-hCHGA-His was used as a template. The entire 407 bp of human CHGA 3’-UTR was PCR-amplified from this plasmid, and then ligated into the XbaI site of the pGL3-Promoter vector (Promega, Madison, WI), just downstream (3’) of the luciferase open reading frame. Single nucleotide polymorphisms in the 3’-UTR were recreated by site-directed mutagenesis (QuikChange, Stratagene) to reproduce the three different naturally occurring variants in the CHGA 3’-UTR: C+87T (C11825T), C+96T (C11834T), and C+274T (C12012T). Inserts were sequence-verified (for both orientation and the correct point mutation) before use. Plasmids were purified on columns (Qiagen, Valencia, CA) prior to transfection of supercoiled DNA. PC12 rat pheochromocytoma cells were transfected (at 50–60% confluence, 1 day after 1:4 splitting) with 1 ug of each construct mentioned above, as well as 10 ng of the Renilla luciferase expression plasmid pRL-TK (Promega, Madison, WI), as an internal transfection efficiency control in each well, by the cationic liposome method (Superfect, Qiagen, Valencia, CA). The firefly and renilla luciferase activities in the cell lysates were measured 24 hours after transfection and the results were expressed as the ratio of firefly/renilla luciferase activity (“Stop &Glow”, Promega, Madison, WI). Each experiment was repeated a minimum of three times.
Role of CHGA in catecholamine storage vesicle formation
siRNA and transfection
Rat Chga-siRNA oligonucleotides (sense CAACAACAACACAGCACUdTdT, and antisense AGCUGCUGUGUUGUUGUUGdTdT) were synthesized by Dharmacon (Lafayette, CO) according to the AA (N19) TT pattern (siRNA selection software, Whitehead Institute, Cambridge, MA). Annealed siRNA duplexes were resuspended in RNAse-free solution buffered to pH 7.4. PC12 cells were transfected with the indicated amount of siRNA-Chga duplex using RNAiFect transfection reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. Silencing of Chga expression was evaluated by immunoblotting, and the effect of reduced expression of Chga on dense-core secretory granule cellular content was examined by transmission electron microscopy.
Immunoblotting analysis
Whole cell lysates were prepared and expression of Chga and actin was evaluated by SDS-PAGE followed by immunoblotting, using a rabbit polyclonal anti-catestatin (rat Chga352–372) or a monoclonal anti-actin (anti-actin I-19, Santa Cruz Biotechnology) primary antibody, followed by horseradish peroxidase conjugate secondary antibodies. Immunoreactivity was visualized by chemiluminescence (Pierce Chemical Company) and protein expression was quantified by densitometry (NIH image 1.6).
Electron microscopy
Cells were incubated in modified Karnovsky's fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer, pH 7.4) overnight at 4° C, followed by 1% OsO4 in 0.1 M Na cacodylate buffer, pH 7.4 and subsequently dehydrated using a graded series of ethanol solutions followed by propylene oxide and infiltration with epoxy resin. After polymerization at 65°C overnight, thin sections were cut and stained with uranyl acetate (4% uranyl acetate in 50% ethanol) followed by bismuth subnitrate. Sections were examined at an accelerating voltage of 60 kV using a Zeiss EM10B electron microscope.
Statistical analyses
Results are presented as the mean value ± SEM.
Haplotypes and population BP extremes
Pairwise linkage disequilibrium (LD) between each common SNP pair across the CHGA locus was quantified as D’ by the GOLD (Graphical Observation of Linkage Disequilibrium) software (42). Comparison of haplotype frequencies between population BP extremes (hypertensive cases versus controls) was performed using the SNPEM (SNP Expectation Maximation) algorithm (43). SNPEM estimates haplotype frequencies for each group using the EM algorithm, taking into account the probability of all possible haplotype pairs, and calculates a likelihood statistic to compare haplotype frequencies between two groups (cases vs. controls) and a permutation test to determine significance in the face of multiple comparisons (set at 10,000 permutations). SNPEM was used to perform a “sliding window” analysis to identify associated haplotype lengths (from 1–4 SNPs) across the locus (44), thus evaluating all possible haplotypes across the four SNPs, thereby interrogating genetic variation at the locus in an unbiased, hypothesis-free way. Potential shortcomings of this method include limitation of analysis to dichotomous traits; a focus on the chromosome, rather than the individual, as the unit of analysis; no measure of direction or magnitude of a genetic effect (other than p value); and lack of adjustability for potential confounding covariates. The second approach utilized haplotype assignment to individuals. HAP (version 3.0) was used to impute haplotypes from diplotype genotypes (45). This two-step analysis approach was implemented to avoid limitations of the first method and potential inflation of Type I (false positive) errors introduced by haplotype inference and assignment in the second method (44). The two highest probability haplotypes were assigned to each individual and used in marker-on-trait analyses; haplotype assignment was not further modeled for probability or uncertainty. Haplotypes were then used as independent variables in general linear model tests, such as 1- or 2-way ANOVA. Additional permutation tests (46) on 3×2 contingency tables (diploid genotype versus BP status), implemented at <http://www.physics.csbsju.edu/stats/exact.html>, were used to confirm genotype effect on the dichotomous BP trait.
To account for multiple comparisons resulting from 4 SNP loci typed across the CHGA locus in the population BP extremes, three methods were used. First, a conservative Bonferroni correction was employed, assuming independence of genotypes across the 4 positions, yielding a modified alpha threshold of 0.05/4=0.0125. Second, the method of SNPSpD (SNP Spectral Decompositon) was used (47), to account for correlations among the SNPs. The third method was use of the principles of False Discovery Rate (FDR), using the Simes procedure (48) as outlined by Benjamini and Hochberg (49), that assumes independence of the 4 SNP genotypes, yielding a modified alpha threshold of alpha = (m+1)/2m, where m= the number of independent tests.
Twin pairs
Estimates of heritability (h2) in twin pairs were obtained using the variance-component methodology implemented in the SOLAR (Sequential Oligogenic Linkage Analysis Routines) package (50), available at the SOLAR web site (http://www.sfbr.org/solar/). Some of the h2 values (e.g., cold stress) in twin pairs have been reported previously (28). Descriptive statistics (means ± SEM) were computed for genotype groups across both members of each twin pair, using generalized estimating equations (GEE), in SAS (Statistical Analysis System, Cary, NC), establishing an exchangeable correlation matrix to take into account intra-twin-pair correlations (51). Data were stored in Microsoft Access, and analyses were conducted in SPSS (Statistical Package for the Social Sciences, Chicago, IL), SAS, or SOLAR. If traits were not normally distributed, values were log10-transformed to decrease skewness, or tested by non-parametric methods (Kruskal-Wallis or median tests).
3’-UTR motifs
mRNA (3’-UTR) motifs differing between SNP variants were examined at RegRNA, an integrated web server for identifying regulatory RNA motifs and elements, including miRNA motifs (52) implemented at <http://regrna.mbc.nctu.edu.tw/>. Likely mRNA stabilities (from energy-minimized 3’-UTRs) between 3’-UTR variants were estimated with the algorithm RNAfold (53) implemented at <http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi>. One-way ANOVA with LSD post hoc correction was performed on in vitro SNP-specific 3’-UTR activities.
RESULTS
Genetic case/control study in blood pressure extremes from the population
Here we studied whether allelic or haplotypic variation at CHGA predicts blood pressure elevation.
Sliding window haplotype analysis: the dichotomous BP trait (hypertension; on-line Table I)
After systematic variant discovery at CHGA, we first evaluated 20 common SNPs (each with minor allele frequency >10%) distributed across ~13 kbp at the locus, to probe patterns of pair-wise linkage disequilibrium in subjects of European (2n=102 chromosomes) ancestry. Within this population, the 20 common SNPs were generally tightly linked, with D’>0.9 across the entire locus (on-line Figure I).
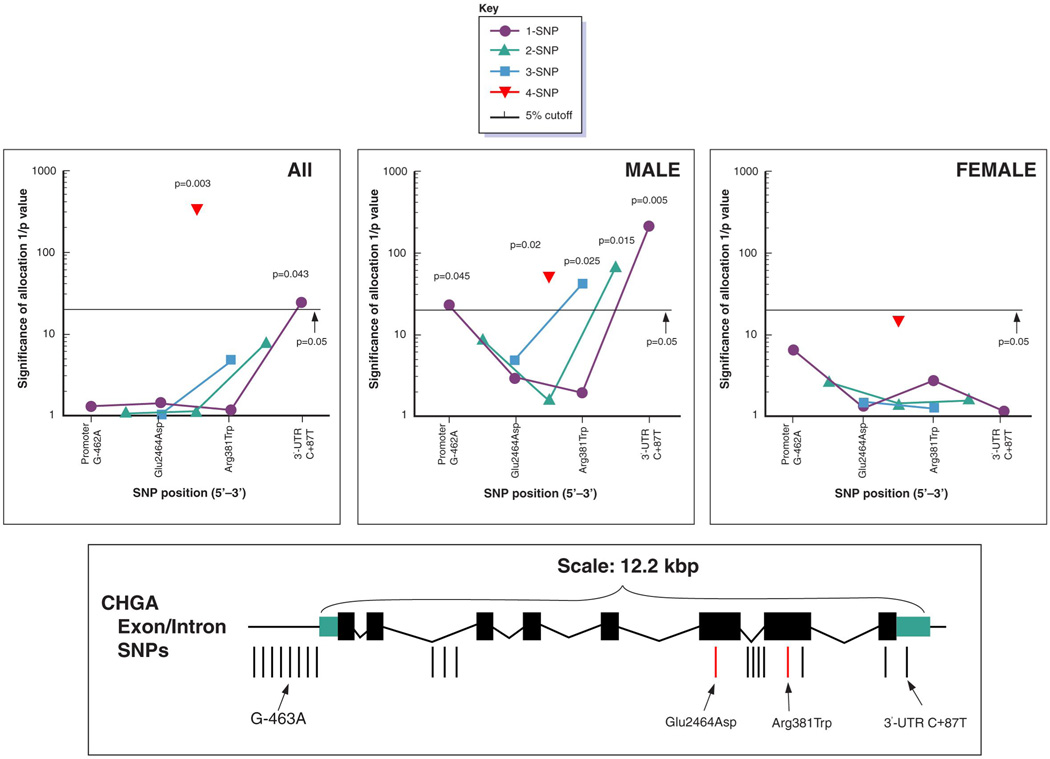
Since pair-wise linkage disequilibrium (LD) analysis demonstrated high LD across the CHGA locus in the white population, we used SNP-EM to impute the most likely haplotypes across the entire locus. We selected four common (minor allele frequency 8–26%) SNPs in several functional domains (promoter, coding, and UTR) to span the locus (Table 1): promoter G-462A, coding/exon-6 Glu246Asp, coding/exon-7 Arg381Trp and 3’-UTR/exon-8 C+87T (C11825T). 1- (single) SNP, 2-SNP, 3-SNP, or 4-SNP haplotype associations were then tested for association with hypertension, in the population BP extreme individuals (on-line Table I). We report p values from omnibus permutation tests in SNP-EM (54). CHGA SNPs and haplotypes displayed sex-dependent effects on blood pressure status (Figure 1A). In the entire group (men and women), significant associations were found for the 4-SNP haplotype (p=0.003) as well as the 3’-UTR alone (p=0.043). Associations of CHGA SNPs and haplotypes were substantially more prominent in men than women. In men, 1-, 2-, 3-, and 4-SNP haplotypes associated with blood pressure (all p<0.05), with the most prominent associations clustering toward the 3’-end of the gene. For single SNPs, the peak association was at the 3’-UTR (C+87T, p=0.005xxx).
Table 1. CHGA haplotypes across the locus in subjects with extreme BP values in the population.
Characteristics of the individual variants. Base positions are numbered (−/+) with respect to the cap site (exon-1, or transcriptional start site).
| Variant | Position to cap site (−/+) | Domain | Major allele (%) | Minor allele (%) | Hardy-Weinberg equilibrium | |
|---|---|---|---|---|---|---|
| χ2 | p | |||||
| G-462A | −462 | Promoter | G (76.7%) | A (23.3%) | 9.20 | 0.002 |
| Glu246Asp | +8540 | Coding, exon 6 | Glu (92.0%) | Asp (8.0%) | 0.76 | 0.384 |
| Arg381Trp | +9610 | Coding, exon 7 | Arg (86.3%) | Trp (13.7%) | 1.39 | 0.238 |
| C+87T | +11825 | 3’-UTR, exon 8 | C (73.5%) | T (26.5%) | 0.260 | 0.610 |
Figure 1. CHGA common haplotypes and blood pressure in population.
Panel A. CHGA polymorphisms in individuals from population blood pressure extremes: Haplotype sliding-window analysis. Four common variants (each minor allele frequency ≥8%; Table 1) were scored to span the CHGA locus. The exon/intron structure of the locus, as well as the positions for all common SNPs (21), is depicted in the schematic at the bottom. Results for haplotypes composed of 1, 2, 3 or 4 SNPs were computed by the SNP-EM (SNP-Expectation Maximization) algorithm for the dichotomous BP trait, and significance is plotted as reciprocal p values for each group: all subjects (left), men only (center), and women only (right). The p values were derived from omnibus permutation tests. Significant (<0.05) p values are shown at the appropriate point.
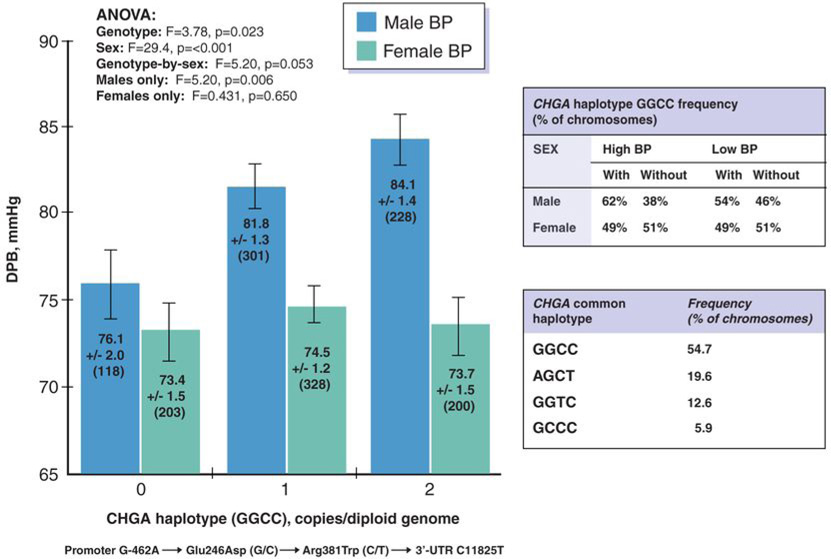
Panel B. CHGA common extended haplotype GGCC: Sex-dependent effect on blood pressure as a quantitative trait in population blood pressure extremes. Extended 4-SNP haplotype GGCC is the most common haplotype in this population (at 57.4% of chromosomes; see inset). The effect of haplotype GGCC on the quantitative trait DBP is illustrated separately for men and women]. There is a significant overall effect for genotype (p=0.023), as well as an effect in men alone (p=0.006).
An overly conservative Bonferroni correction for 4 SNP loci yields a target alpha of (0.05/5)=0.0125, while a False Discovery Rate Simes-adjusted alpha for 4 tests would be 0.05(4+1)/2*4=0.03125, and by SNPSpD (47) the experiment-wide significance threshold required to keep Type I error rate at 5% is 0.0134. Each of these thresholds is exceeded in men. In women, none of the haplotypes (or single SNPs) offered significant prediction of blood pressure status (all p>0.05).
Characteristics of the 4 SNP genotypes are presented in Table 1. Although promoter G-462A deviated from Hardy-Weinberg Equilibrium, all 3 genotype classes were represented in the data (G/G=715 [60.4%], G/A=386 [32.6%], A/A=83 [7.0%]), and visual inspection of the Pyrosequencing base-extension luminescence tracings revealed robust/unequivocal evidence of incorporation of each base (G versus A, on the antisense [−] strand).
CHGA haplotypes and the quantitative BP trait
Haplotype GGCC (promoter - 462G→246Glu/G→381Arg/C→3’-UTR/+87C), the most common haplotype at the locus (Figure 1B), dose-dependently predicted higher DBP in men (p=0.006) though not women (p=0.650). Thus, in the context of haplotype GGCC, the C (major) allele at 3’-UTR C+87T progressively elevated DBP in men.
CHGA 3’-UTR C+87T and the dichotomous as well as continuous BP trait (hypertension)
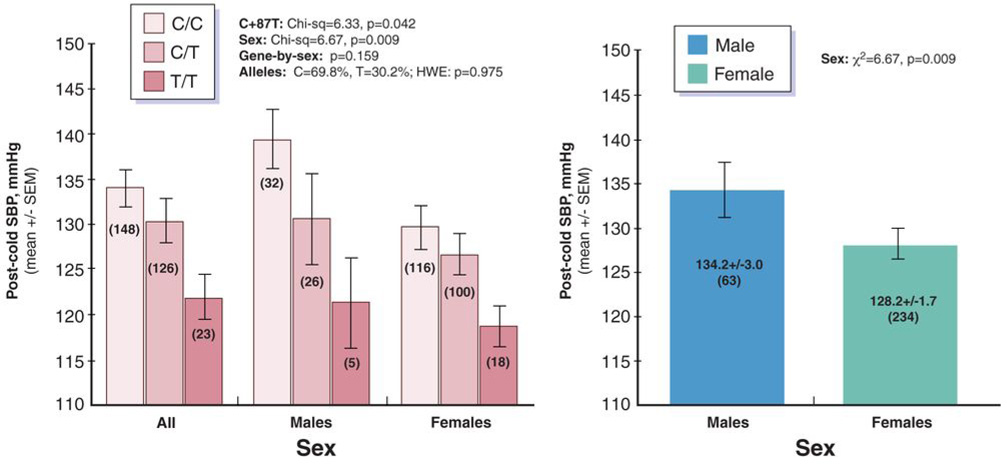
During sliding-window analysis, the 3’-UTR variant displayed the most prominent effect upon blood pressure status. In men considered alone, hypertensive and normotensive individuals differed significantly in C+87T (C11825T) diploid genotype frequencies (p=0.005), though a genotype effect was not seen in women alone (p=0.158)(Table 2A & on-line Figure IIa). Permutation tests (on 3×2 contingency tables, diploid genotype versus BP status) confirmed the genotype effect in men (p=0.015), and lack of effect in women (p=0.363).
Table 2. CHGA 3’-UTR common variant C+87T: Effect of the T allele on blood pressure status and BP values.
Subjects are grouped by absence (C/C) or presence (T/T or C/T) of the T (minor) allele.
| 2a : CHGA C+87T genotype frequencies and BP status (dichotomize). Results evaluated by chi-square test. | ||||
|---|---|---|---|---|
| Sex | Genotype | BP-high (n) | BP-low (n) | p (2-sided) |
| Male | C/C | 156 | 107 | |
| C/T or T/T | 95 | 112 | 0.004 | |
| Female | C/C | 112 | 170 | |
| C/T or T/T | 119 | 148 | 0.262 | |
| 2b: CHGA C+87T and BP as a continuous trait. Allelic association (presence or absence of the T allele) evaluated by univariate ANOVA. | |||
|---|---|---|---|
| Sex | Genotype | SBP (mmHg) | DBP (mmHg) |
| Male | C/C | 137.2+/−1.6 | 83.7+/−1.3 |
| C/T or T/T | 131.2+/−1.8 | 77.6+/−1.5 | |
| ANOVA p value | 0.016 | 0.003 | |
| Female | C/C | 123.8+/−1.7 | 71.8+/−1.2 |
| C/T or T/T | 126.9+/−1.7 | 73.8+/−1.3 | |
| ANOVA p value | 0.206 | 0.272 | |
In Table 2B (on-line Figure IIb), we show the effects of C+87T on SBP and DBP as continuous traits (in mmHg). Even though these subjects were ascertained from the population on a DBP criterion, the hypertensive individuals exhibited elevations of both DBP and SBP (on-line Table II), and there were significant effects of C+87T genotype on both SBP (p=0.028) and DBP (p=0.037). Likewise, there were significant genotype-by-sex interactions on both SBP (p=0.015) and DBP (p=0.010). In men, BP differed substantially between homozygote (C/C, T/T) classes: by ~12 mmHg for SBP and ~9 mmHg for DBP. By ANOVA (adjusted) R2 in men, C+87T accounted for ~1.9% of the population variance in SBP (or ~13.7 mmHg), and ~1.2% of the variance for DBP (or ~5.8 mmHg). In women, C+87T did not affect either SBP or DBP.
To focus on the role of the minor (T) allele, we combined the minor allele homozygotes (T/T) with heterozygotes (C/T) categories, and compared them to C/C homozygotes. The C allele retained its significant effect on blood pressure in men (p=0.003–0.016), though not women.
Because women in the BP extreme samples spanned a range of ages, we then divided the women about the approximate age of human menopause (±50 years); there was no effect of C+87T on blood pressure in either the older (≥50 years; p=0.067 for SBP and p=0.066 for DBP) or younger (<50 years; p=0.187 for SBP and p=0.379 for DBP) women, nor was there an age-by-sex interaction (p=0.879 for SBP and p=0.815 for DBP).
Human CHGA expression in vivo
Heritability (h2) in twin-pairs
We used a phenotyped twin-pair cohort to estimate the influence of heredity on CHGA secretion (Table 3). Plasma CHGA concentration showed significant (p<0.0001) heritability in twins, at 45±12% for the precursor (epitope: CHGA116–439).
Table 3. Heritability of “intermediate” traits in twin pair.
Heritability (h2=VG/VP, or the fraction of phenotypic variance accounted for by genetic variance) was determined by variance components analysis in SOLAR
| Trait | h2 as % (± SEM) | P value |
|---|---|---|
| Biochemical | ||
| Plasma CHGA116–439 (precursor) | 45±12% | p<0.0001 |
| Physiological | ||
| Cold stress final SBP | 29±8% | p<0.0001 |
CHGA and norepinephrine secretion: Effects of hypertension and sex
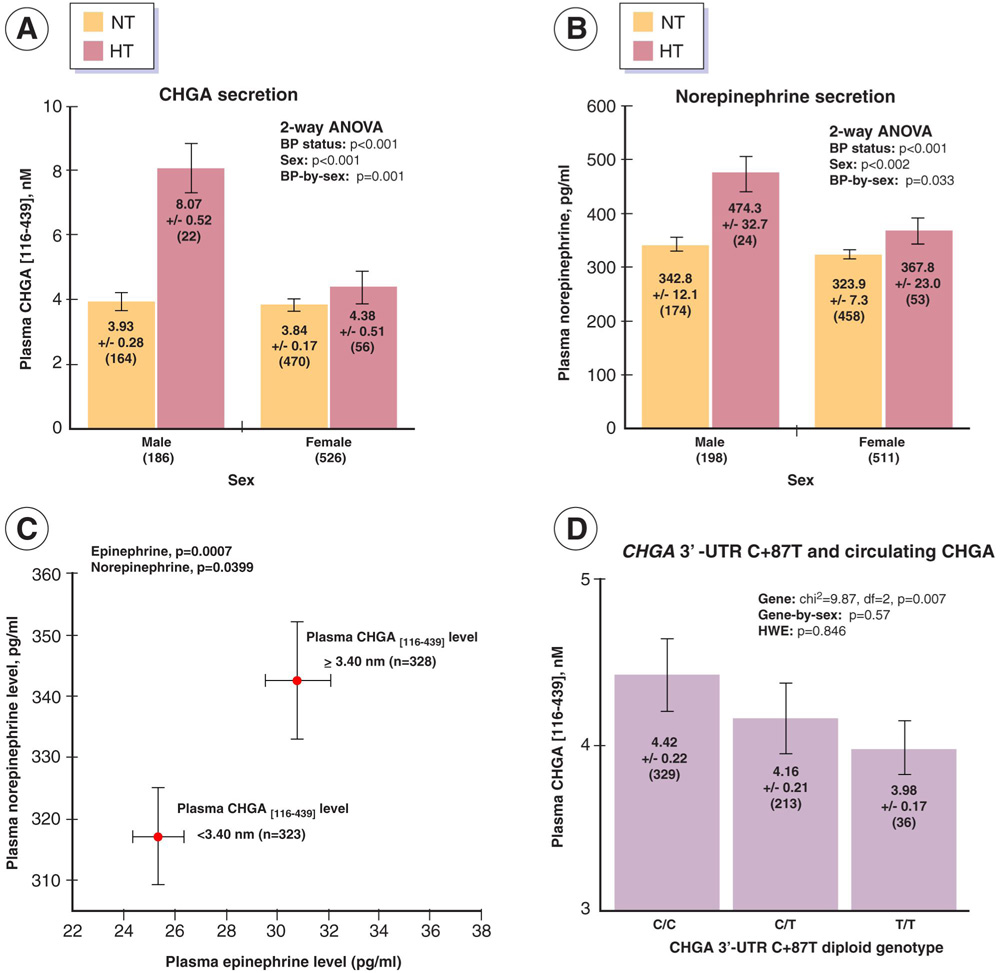
We tested whether CHGA gene expression varied in patients with essential hypertension, evaluating the plasma CHGA precursor (epitope: human CHGA116–439). Plasma CHGA was substantially higher in hypertension (p<0.001; on-line Table II, Figure 2A), but a significant (p<0.001) BP status-by-sex interaction largely confined the increase to male hypertensives. The results suggest increased CHGA biosynthesis and exocytotic secretion especially in men with hypertension. Plasma norepinephrine was also elevated in hypertension (p<0.001; on-line Table II, Figure 2B), but once again a significant BP status-by-gene interaction (p=0.002) restricted the effect mainly to men.
Figure 2. CHGA and catecholamine secretion: BP, sex, and the 3’-UTR.
Panel A. Hypertension, sex, and CHGA secretion. Resting plasma concentration of the CHGA precursor (epitope: CHGA116–439) was measured in plasma from seated human subjects with normal renal function (serum creatinine ≤1.5 mg/dl). We studied subjects with a diagnosis of essential hypertension (HT), versus unmedicated controls (NT) with normal blood pressure.
Panel B. Hypertension, sex and catecholamine secretion. The same individuals were studied for plasma norepinephrine concentration (CHGA and norepinephrine were measured in the same plasma sample).
Panel C. CHGA as a predictor of catecholamine secretion. Individuals were divided into quantiles above and below the median value for plasma CHGA concentration, and plasma catecholamine concentrations were calculated in the two groups (CHGA and catecholamine were measured in the same plasma sample).
Panel D. CHGA 3’-UTR variant C+87T: Influence on circulating CHGA.Resting plasma concentration of the CHGA precursor (epitope: CHGA116–439) was measured in plasma from 578 genotyped white subjects (187 men, 391 women). Each subject had normal renal function (serum creatinine ≤1.5 mg/dl). Since the distribution of plasma CHGA116–439 concentration in this sample deviated substantially from normality (skewness=6.03±0.10, kurtosis=55.2±0.20), we used a non-parametric median test (evaluating whether two or more independent samples [defined by diploid genotype] are drawn from populations with the same median, using the chi-square statistic). There was no gene-by-sex interaction on the CHGA trait (p=0.57).
CHGA: Predictor of catecholamine secretion
When we subdivided individuals into two (upper and lower) CHGA quantiles by dividing about the median value (Figure 2C), individuals with greater CHGA secretion also exhibited increased plasma norepinephrine (p=0.0399) and epinephrine (p=0.0007) secretion.
CHGA 3’-UTR C+87T (C11825T) cis-QTL and plasma CHGA
Since CHGA C+87 affected blood pressure, and the CHGA plasma level is substantially higher in hypertensive than normotensive individuals (Figure 2A), the relationship between C+87T genotype and CHGA plasma level was explored. Figure 2D shows that common variant CHGA C+87T predicts human plasma CHGA116–439 concentrations; increasing numbers of the T allele (0→1→2) progressively reduced CHGA by ~10% (p=0.007). No gene-by-sex effect was found (p=0.57).
CHGA genotype and environmental (cold) stress: Studies in twin pairs
Since systemic hypertension may result from the cumulative effects of transient adverse blood pressure responses to environmental stress in genetically predisposed individuals (55), we probed the blood pressure response to systematic cold stress (28) in a series of predominantly normotensive twin pairs. The stress BP trait is significantly heritable (h2=29±8%, p<0.0001), as estimated by twin pair variance components (28) (Table 3). The common CHGA 3’-UTR variant C+87T (C11825T) predicted final (post-cold) SBP (p=0.042) during this environmental stressor (Figure 3A): increasing numbers of the minor (T) allele seemed to blunt the final SBP response, while the basal SBP pre cold stress were almost the same between the three genotype groups (C/C group: 119.4+/−1.6, C/T group: 119.8+/−1.6, and T/T group: 114.3+/−2.7 mmHg, p=0.21). Sex also influenced post-stress SBP: men had ~6 mmHg higher values for post-stress SBP than women (p=0.009, Figure 3B), though there was no gene-by-sex interaction on this BP trait (p=0.185). While initial (pre-cold) SBP also predicted final (post-cold) SBP (Spearman ρ=0.713, p<0.001), it did not predict the cold-induced change in SBP (Δ=[final-initial]; Spearman ρ=0.074, p=0.156).
Figure 3. Blood pressure response to environmental (cold) stress in twin pairs: Effect of CHGA common allelic variant C+87T in the 3’-UTR, as well as sex.
Provocation of efferent sympathetic outflow was undertaken in each subject by immersion of one hand in ice water (at 0°C) for one minute, with continuous blood pressure monitoring. Results are shown for final (post-cold) SBP, and analyzed by generalized estimating equations, establishing an exchangeable correlation matrix to take into account intra-twin-pair correlations.
Panel A: CHGA C+87T genotype effect on post-stress SBP; data are shown in all persons, as well as men and women separately. Genotype affected the trait (p=0.042), and there was no gene-by-sex interaction (p=0.159) on trait.
Panel B: Sex effect on post-stress SBP (p=0.009).
Function of CHGA 3’-UTR variants
Sequence conservation and phylogeny at CHGA 3’-UTR common variant C+87T
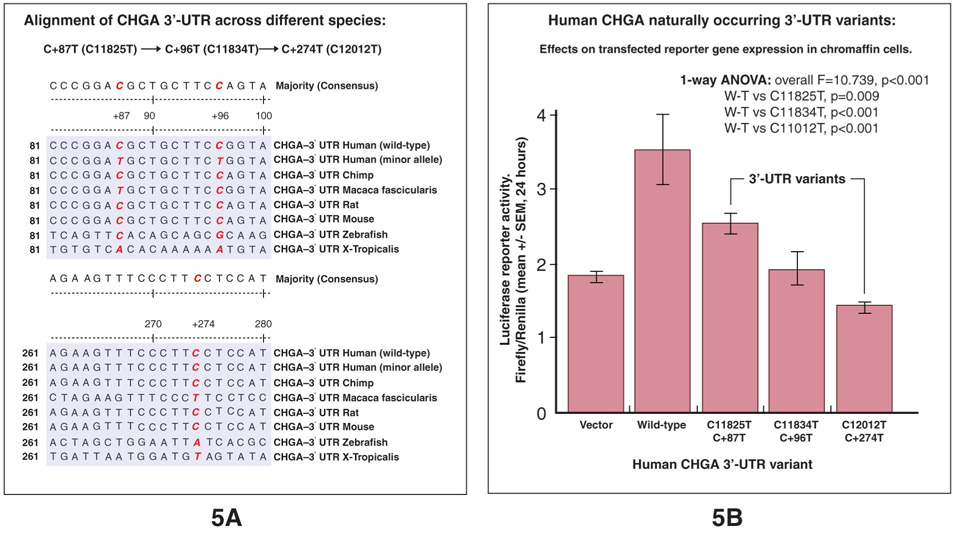
Figure 4A depicts the CHGA 3’-UTR sequence across species in the region of 3 naturally occurring variants (21): common variant C+87T (C11825T, global minor allele frequency across populations, 17.7%), as well as the less common variants C+96T (C11834T, MAF=0.6%), and C+274T (C12012T, MAF=0.6%). The local genomic region at and around C+87T is highly conserved across species; at 3’-UTR position +87, C or T are the only alleles found in mammals. Similar local sequence conservation across mammals is found around the positions of the more unusual 3’-UTR variants C+96T and C+274T. Based on the chimpanzee sequence, C+87 is likely to be the ancestral +87 allele in the human population (56) though another primate (Macaca) also displays the +87T allele.
Figure 4. Function of CHGA 3’-UTR variants.
Panel A. Sequence alignment of the CHGA 3’-UTR across different species. 3’-UTR sequences are aligned across 7 species, to illustrate conservation at positions of three naturally occurring human polymorphisms: C+87T (C11825T), C+96T (C11834T), and C+274T (C12012T).
Panel B. Human CHGA naturally occurring 3’-UTR variants: Effects on transfected reporter gene expression in chromaffin cells. The 3’-UTR variants tested were: C+87T (C11825T), C+96T (C11834T), and C+274T (C12012T). Results of luciferase activity measurements 24 hours after transfection of the 4 versions of the 407-bp CHGA 3’-UTR (wild type and 3 variants) into chromaffin cells. Results are expressed as the ratio of firefly luciferase/Renilla luciferase (encoded by the transfection efficiency plasmid pRL-TK). Each experiment was performed in triplicate, and such experiments were repeated at least twice. Wild-type contains the major (C) allele at all 3 positions. Variant constructs contain the minor (T) allele at the indicated position, but the major (C) at the two other positions.
In cella reporter gene activity assay
To directly investigate the role of natural genetic variation of the CHGA 3’-UTR in CHGA expression, we constructed plasmids in which we monitor the influence of the 3’-UTR on a luciferase reporter. The full 407 kbp human CHGA 3’-UTR, containing one of four variant (wild-type versus one common or two uncommon SNPs) sites, was inserted downstream (3’) of the luciferase gene in the pGL3 promoter vector. After transfection into PC12 chromaffin cells, reporter gene activity was measured at 24 hours. The wild-type 3’-UTR haplotype (C+87→C+96→C+274) increased reporter gene activity (compared to the vector without the 3’-UTR insert), while the T allele at any of these three positions decreased reporter gene activity (as compared to the wild-type haplotype); the common +87T variant alone decreased expression by ~30% (p=0.009; Figure 4B). These in vitro results document that common SNP C+87T is a functional polymorphism, and the in vitro effect of the +87T allele to diminish expression is consistent with the in vivo observations that +87T is associated with diminished plasma CHGA, stress BP response, and basal BP in the population.
CHGA 3’-UTR variants: Computational biology
C+87T is not located within a known motif that influences mRNA stability, such as an A/U (A/T) -rich region (Figure 4A), and does not create or abolish other motifs such as micro-RNA recognition sites (52), nor were there changes in folding energy (ΔG°= −147.58 kcal/mol for C+87 versus −147.42 kcal/mol for +87T), and the two mRNAs did not differ in overall stem/loop structure (53).
Disease mechanisms: Role of CHGA in catecholamine storage vesicle formation
To probe how quantitative alterations in CHGA gene expression might affect sympathoadrenal structure or function, we "silenced" Chga expression in chromaffin (rat PC12) cells using the specific technique of siRNA targeting the rat Chga mRNA. The Chga siRNA reagent dose-dependently suppressed Chga protein expression by up to ~95%, without affecting expression of the control (structural or "housekeeping") protein actin (Figure 5A & 5B). In control cells (treated with "mock" siRNA reagent), ultrastructural examination revealed numerous chromaffin granules, largely "docked" at the cytoplasmic surface of the plasma membrane (Figure 5C). Application of the Chga siRNA resulted in the disappearance of the majority of the chromaffin granules from the cell (Figure 5D); on a quantitative basis, the number of dense-core granules per cell X–Y cell plane declined by ~2/3 (p<0.0001, Figure 5E). Ultrastructural morphology of the chromaffin cells (nuclei, mitochondria, endoplasmic reticulum) was not otherwise disturbed, reinforcing the specificity of the change in chromaffin granules.
Figure 5. Disease mechanisms: Role of CHGA in catecholamine storage vesicle formation. Effect of small interfering RNA (siRNA) to "silence" expression of Chga protein in sympathoadrenal PC12 cells.
PC12 cells were grown after transfection with the indicated amount of 22 bp siRNA-rat Chga duplexes at 4 mcg/well for 72h.
Panel A. Visualization of Chga immunoreactivity by chemiluminescent immunoblot. Whole cell lysates were prepared, and expression of Chga and actin (control, "housekeeping" protein) was evaluated by SDS-PAGE followed by immunoblotting using a rabbit polyclonal anti-catestatin (rat Chga367–387) antibody or a monoclonal anti-actin primary antibody.
Panel B. Densitometry to quantify Chga and actin protein expression, using NIH image v1.6 software.
Panels C&D. Electron microscopy. Ultrastructural examination of the effect of siRNA Chga "silencing" on dense-core granule biogenesis in sympathoadrenal PC12 cells. Cells were grown in the presence of 22 bp siRNA-rat Chga duplexes (Panel D), or mock/control (Panel C) at 4 mcg/well for 72h. Aldehyde-fixed cells were processed for electron microscopy as described in the materials and methods section. Dense core granules (arrowheads) are seen either "docked" to, or in the vicinity of the plasma membrane. Note that fewer secretory granules are present in the cytoplasm of siRNA-rat Chga-treated cells. Panel C: Control (mock-treated) PC12 cells. Panel D: Chga si-RNA-treated PC12 cells. Abbreviations: n, nucleus; m, mitochondria; er, endoplasmic reticulum. Scale bar: 500 nm.
Panel E. Quantification of the abundance of dense-core secretory granules reveals a decrease number of granules per cell planes, as defined by the number of granules found in an XY section of the mid-cell body. n=40 for both populations of cells. ***p< 0.0001, by t test.
DISCUSSION
Overview
CHGA plays a pivotal role in the sympathochromaffin system, both in the formation of catecholamine secretory vesicles and in the regulation of transmitter release (1,20). In this report we approach the impact of common human variation at the CHGA locus for autonomic physiology and disease. We found that CHGA is overexpressed in hypertension, and that a common (~27% frequency) genetic variant in the CHGA 3’-UTR (C+87T) is strongly associated with human essential hypertension, accounting for up to ~12/~9 mmHg of BP variation within the population. The 3’-UTR variant also predicts environmental stress-induced increments in blood pressure, suggesting a mechanism for early effects of the gene on a pathogenic series of events eventuating in sustained blood pressure elevation. The 3’-UTR variant is in a region of sequence conservation across species, and acts to change CHGA gene expression in chromaffin cells, perhaps eventuating in diminution of catecholamine secretory granules; thus, CHGA C+87T fulfills many criteria for a functional variant contributing to disease predisposition. At multiple levels (CHGA expression, heritable circulatory response to environmental stress, and finally basal BP in the population), sex seemed to play an important role in mediating the effect of genetic variation on phenotype.
Sex: Role in hypertension and intermediate phenotypes
In the wake of the sex dependent effect of CHGA genetic variation on BP, we performed a series of studies to explore the interaction of gene and sex, and found that sex played a role at each of several steps. At a biochemical level, CHGA and norepinephrine secretion were elevated in hypertension, but the increase seemed to be confined to men. At a physiological level, the pressor response to environmental stress was influenced by both sex and C+87T genotype. Finally, at a disease level, sex was again crucial: in individuals with the most extreme BPs in the population, associations with C+87T were substantially more impressive in men than in women, effectively confining the effect of C+87T to men.
Why might adrenergic genetic variation yield such different consequences in men and women? Acute vascular responses to adrenergic stimuli are sex-dependent (57,58), and the long-term consequences of repeated stressors on resting blood pressure or the late appearance hypertension differ by sex; for example, in the longitudinal CARDIA study of cardiovascular risk in young adults (59), reactivity to cold stress predicted 5-year rise in BP and earlier development of hypertension, but only among men and not women. Since sex steroids may mediate such differences in blood pressure regulation (33,60–65), though we did not observe an effect of C+87T in either pre- or post-menopausal women.
Haplotypes versus individual variants
Haplotypes are a useful tool for scanning large genomic regions in the search for disease predisposition variants (66). Once a contributory genetic locus has been identified, systematic variant discovery may then yield up the underlying polymorphism. At CHGA, resequencing (21) identified one common variant in the 3’-UTR: C+87T (minor allele frequency, 26.5%). During the initial hypertension association, the peak association for BP in men was far more significant for C+87T (p=0.005) than for haplotypes extending across the entire locus (p=0.045); even haplotype associations tended to peak towards the 3’-end of the locus. When haplotypes were associated with the BP quantitative traits, common haplotype GGCC accounted for ~8 mmHg of DBP variation in men; by contrast, 3’-UTR variant C+87T accounted for ~11 mmHg of DBP variation in men. Thus, after systematic polymorphism at a locus, an individual SNP explained a greater proportion of BP variation, and at higher significance, than did haplotypes. Later we established the functional significance of the 3’-UTR SNP in vitro.
Intermediate phenotypes
Essential hypertension is a complex trait (13), with multiple contributory factors derived from both genes and environment. In the setting of late penetrance of the ultimate disease trait (such as hypertension), as well as likely genetic heterogeneity, the “intermediate phenotype” (67) strategy may be a useful approach in the search for disease predisposition loci. Autonomic traits with heritable determination may be of particular value in investigation of the genetic underpinnings of hypertension. In accordance with this pathway concept, we pursued intermediate traits in this study. Secretion of CHGA, estimated by its plasma concentration, is not only elevated in hypertension (p<0.001), but also influenced by C+87T genotype (p=0.007). The hemodynamic response to environmental (cold) stress may be a predictor of the development of later cardiovascular events, such as hypertension (33,62,64,65,68). Such a response, occurring even prior to the onset of disease, would be a useful physiological “intermediate phenotype” in probing the genetic determinants of hypertension (31,67,69). We report the cold stress response in our twins, indicating that both change in SBP and final (post-stress SBP) are heritable, and may be valuable intermediate phenotypic anchor points for hypertension (28).
In a group of predominantly normotensive twin pairs subjected to autonomic phenotyping, CHGA 3’-UTR variant C+87T predicted post-stress SBP (p=0.042). The independent sex effect on this trait (p=0.009) suggests that the intermediate phenotype mechanism for this trait may operate differently in men and women; indeed, cold stress-induced rises in BP seem to be more effective predictors of long-term changes in BP in men than in women (59).
CHGA 3’-UTR polymorphism: In cella functional assay
The 3’-untranslated regions (3’-UTRs) of genes may contain elements that govern mRNA stability, localization and translatability, which are crucial steps in the pathway from gene to protein (52,70). We found three SNPs in the CHGA 3’-UTR, and C11825T as the only common one, was relatively conservative among different vertebrate species. Our in vitro reporter gene activity assay provides direct evidence of the functionality of 3’-UTR common variant C+87T, as well as two other less common 3’-UTR variants, C+96T and C+274T. At C+87T, the T allele diminished expression of the reporter; the T allele was also associated with lower stress blood pressures in twin pairs, as well as lower basal SBP and DBP in the population.
What is the mechanism by which C+87T influences CHGA expression? C+87T does not lie in an A/U (A/T) -rich region of the 3’-UTR, and a computational survey of the 3’-UTR (52) did not reveal evidence of other well-known mRNA stability elements at/near C+87T, such as microRNA recognition motifs. Nor did the C+87T transition influence the likely stem/loop secondary structure of the message, or its folding energy (53). Thus, C+897T may lie in a novel, previously unexplored motif. Future studies of cytoplasmic or nuclear protein binding by the motif may better elucidate the nature of its action.
Catecholamine storage vesicles
siRNA knockdown of CHGA expression in chromaffin cells in cella substantially diminished catecholamine storage vesicle formation, perhaps because CHGA serves to initiate binding and condensation events within the core of the chromaffin granule (4). This suggests a possible mechanism whereby alterations in CHGA expression in vivo might influence autonomic function; a decrement in secretory vesicles would result in diminished releasable transmitter stores in response to acute stimulation, such as cold stress. Indeed, basal CHGA secretion predicted the plasma concentrations of norepinephrine and epinephrine in vivo. Since repeated adverse pressor responses (55) may ultimately predispose to fixed elevations in BP (33,62,64,65,68), the cold stress results may provide a mechanistic physiological link between genotype and the ultimate disease phenotype.
The changes in chromaffin granule morphology we noted are consistent with previous observations on CHGA depletion in vitro using Chga antisense RNA (6) or si-RNA (71), as well as results in chromaffin cells in vivo after targeted ablation of the CHGA locus (20).
Study limitations and advantages
Catecholamine storage vesicles store and release other active peptides besides the chromogranins/secretogranins, including the potent vasoconstrictor neuropeptide Y (72) and the enkephalins (73); the genes encoding these other peptides might also harbor alleles that influence BP, but additional genetic loci were beyond the scope of this study. Although our blood pressure extreme groups were ascertained on a DBP criterion, recent evidence indicates that SBP is at least as important a risk factor for target organ damage; we plan future studies to explore the potential effect of CHGA polymorphism in isolated systolic hypertension. The prevalence of hypertension varies substantially across ethnic groups; since these initial studies in hypertension on dealt primarily with subjects of European descent, it will be important to extend future observations to different biogeographic ancestry groups. CHGA is present in the core of secretory vesicles throughout the neuroendocrine system (1); here we explored the significance of CHGA polymorphism only for hypertension, and have not yet considered its influence on other endocrine or neurological disease syndromes. Finally, although we have noted sex differences in CHGA polymorphism effects on both basal and stress-inducible BP, we do not have a clear molecular understanding of how such gene-by-sex interactions arise.
Genetic studies of complex traits have been plagued by false-positive conclusions. Here we used several complementary experimental and statistical approaches to guard against this possibility. We studied the effects of CHGA variation upon not only a disease trait but also earlier “intermediate” phenotypes, confirming the effects of CHGA 3’-UTR variation upon BP traits in two independent groups (population BP extremes and twin pairs). Statistically, we employed haplotype, correction for non-independance of SNPs in LD with each other, and permutation approaches to ensure that the conclusions would be robust. Finally, we established a biological role for the trait-associated genetic variant.
Conclusions and perspectives
Our data suggest that remarkably common (~27% frequency) functional variation in the 3’-UTR of the catecholamine secretory vesicle protein CHGA confers a change in heritable environmental stress-induced change in blood pressure, as well as sex-dependent risk for hypertension, both diastolic and systolic. Our observations are consistent with the “intermediate phenotype” (67) framework for complex traits. Common variation at CHGA alters gene expression, initially changing autonomic tone as evidenced by changes in the heritable response of blood pressure to environmental stress, eventuating over decades in fixed alterations in basal blood pressure.
At several stages, sex seems to be a critical factor in this cascade of events: expression of CHGA, the pressor response to environmental stress, and the effect of the 3’-UTR C+87T on the ultimate population profile of BP.
These observations are consistent with the “common disease/common allele” hypothesis for frequent traits in the population (74), and suggest new molecular strategies for probing the pathophysiology, risk, and rational treatment of hypertension.
Supplementary Material
Acknowledgments
Support: National Institutes of Health, Department of Veterans Affairs, International Society of Nephrology.
List of abbreviations
- CHGA
chromogranin A
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- SNP
single nucleotide polymorphism
- ATP
adenosine triphosphate
- BP
blood pressure
- MZ
monozygotic
- DZ
dizygotic
- CPT
cold pressor test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by an International Society of Nephrology fellowship.
REFERENCES
- 1.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 2.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takiyyuddin MA, Cervenka JH, Sullivan PA, et al. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Videen JS, Mezger MS, Chang YM, O'Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 5.Yoo SH, So SH, Huh YH, Park HY. Inositol 1,4,5-trisphosphate receptor/Ca(2+) channel modulatory role of chromogranins A and B. Ann N Y Acad Sci. 2002;971:300–310. doi: 10.1111/j.1749-6632.2002.tb04484.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an "on/off" switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 7.Cadman PE, Rao F, Mahata SK, O'Connor DT. Studies of the dysglycemic peptide, pancreastatin, using a human forearm model. Ann N Y Acad Sci. 2002;971:528–529. doi: 10.1111/j.1749-6632.2002.tb04518.x. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- 9.Strub JM, Goumon Y, Lugardon K, et al. Antibacterial activity of glycosylated and phosphorylated chromogranin A-derived peptide 173–194 from bovine adrenal medullary chromaffin granules. J Biol Chem. 1996;271:28533–28540. doi: 10.1074/jbc.271.45.28533. [DOI] [PubMed] [Google Scholar]
- 10.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin a344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999;274:2920–2928. doi: 10.1074/jbc.274.5.2920. [DOI] [PubMed] [Google Scholar]
- 12.Mahata SK, O'Connor DT, Mahata M, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao RJ, Parmer RJ, Takiyyuddin MA, O'Connor DT. Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore) 1991;70:33–45. [PubMed] [Google Scholar]
- 15.O'Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985;7:I76–179. doi: 10.1161/01.hyp.7.3_pt_2.i76. [DOI] [PubMed] [Google Scholar]
- 16.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O'Connor DT. Chromogranin A. Storage and release in hypertension. Hypertension. 1990;15:237–246. doi: 10.1161/01.hyp.15.3.237. [DOI] [PubMed] [Google Scholar]
- 17.Takiyyuddin MA, Parmer RJ, Kailasam MT, et al. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor DT, Takiyyuddin MA, Printz MP, et al. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 19.Dimsdale JE, O'Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. 1992;51:519–525. doi: 10.1016/0024-3205(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 20.Mahapatra NR, O'Connor DT, Vaingankar SM, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen G, Mahata SK, Cadman P, et al. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinton TJ, Cotter B, Kailasam MT, et al. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80:323–330. doi: 10.1016/s0002-9149(97)00353-6. [DOI] [PubMed] [Google Scholar]
- 23.Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 24.Waalen J, Felitti V, Gelbart T, Ho NJ, Beutler E. Prevalence of coronary heart disease associated with HFE mutations in adults attending a health appraisal center. Am J Med. 2002;113:472–479. doi: 10.1016/s0002-9343(02)01249-4. [DOI] [PubMed] [Google Scholar]
- 25.Seasholtz TM, Wessel J, Rao F, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 26.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 27.Schork NJ, Nath SK, Fallin D, Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Rao F, Wessel J, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood TA, Rao F, Stridsberg M, et al. Pleiotropic effects of novel trans-acting loci influencing human sympathochromaffin secretion. Physiol Genomics. 2006;25:470–479. doi: 10.1152/physiolgenomics.00295.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wessel J, Moratorio G, Rao F, et al. C-reactive protein, an 'intermediate phenotype' for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Lafleche AB, Pannier BM, Laloux B, Safar ME. Arterial response during cold pressor test in borderline hypertension. Am J Physiol. 1998;275:H409–H415. doi: 10.1152/ajpheart.1998.275.2.H409. [DOI] [PubMed] [Google Scholar]
- 33.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–76. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Stridsberg M. Measurements of chromogranins and chromogranin-related peptides by immunological methods. Adv Exp Med Biol. 2000;482:319–327. doi: 10.1007/0-306-46837-9_25. [DOI] [PubMed] [Google Scholar]
- 35.Stridsberg M, Angeletti RH, Helle KB. Characterisation of N-terminal chromogranin A and chromogranin B in mammals by region-specific radioimmunoassays and chromatographic separation methods. J Endocrinol. 2000;165:703–714. doi: 10.1677/joe.0.1650703. [DOI] [PubMed] [Google Scholar]
- 36.Stridsberg M, Oberg K, Li Q, Engstrom U, Lundqvist G. Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol. 1995;144:49–59. doi: 10.1677/joe.0.1440049. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann V, Buscher R, Go MM, et al. Beta2-adrenergic receptor polymorphisms at codon 16, cardiovascular phenotypes and essential hypertension in whites and African Americans. Am J Hypertens. 2000;13:1021–1026. doi: 10.1016/s0895-7061(00)01188-2. [DOI] [PubMed] [Google Scholar]
- 39.Buetow KH, Edmonson M, MacDonald R, et al. High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci U S A. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta. 2006;363:83–94. doi: 10.1016/j.cccn.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 41.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 43.Fallin D, Cohen A, Essioux L, et al. Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer's disease. Genome Res. 2001;11:143–151. doi: 10.1101/gr.148401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 45.Halperin E, Eskin E. Haplotype reconstruction from genotype data using Imperfect Phylogeny. Bioinformatics. 2004;20:1842–1849. doi: 10.1093/bioinformatics/bth149. [DOI] [PubMed] [Google Scholar]
- 46.Clarkson D, Fan Y-A, Joe H. A remark on algorithm 643: FEXACT: An algorithm for performing Fisher's exact test in RxC contingency tables. ACM Transactions on Mathematical Software. 1993;19:484–488. [Google Scholar]
- 47.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simes RJ. An improved Bonferroni procedure for multiple test of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 50.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Do KA, Broom BM, Kuhnert P, et al. Genetic analysis of the age at menopause by using estimating equations and Bayesian random effects models. Stat Med. 2000;19:1217–1235. doi: 10.1002/(sici)1097-0258(20000515)19:9<1217::aid-sim421>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 52.Huang HY, Chien CH, Jen KH, Huang HD. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 2006;34:W429–W434. doi: 10.1093/nar/gkl333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 54.Schork NJ, Gardner JP, Zhang L, et al. Genomic association/linkage of sodium lithium countertransport in CEPH pedigrees. Hypertension. 2002;40:619–628. doi: 10.1161/01.hyp.0000037131.41957.a8. [DOI] [PubMed] [Google Scholar]
- 55.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 56.Excoffier L, Novembre J, Schneider S. SIMCOAL: a general coalescent program for the simulation of molecular data in interconnected populations with arbitrary demography. J Hered. 2000;91:506–509. doi: 10.1093/jhered/91.6.506. [DOI] [PubMed] [Google Scholar]
- 57.King D, Etzel JP, Chopra S, et al. Human response to alpha2-adrenergic agonist stimulation studied in an isolated vascular bed in vivo: Biphasic influence of dose, age, gender, and receptor genotype. Clin Pharmacol Ther. 2005;77:388–403. doi: 10.1016/j.clpt.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 59.Markovitz JH, Raczynski JM, Wallace D, Chettur V, Chesney MA. Cardiovascular reactivity to video game predicts subsequent blood pressure increases in young men: The CARDIA study. Psychosom Med. 1998;60:186–191. doi: 10.1097/00006842-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 61.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 32.Menkes MS, Matthews KA, Krantz DS, et al. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–530. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 63.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 64.Schneider GM, Jacobs DW, Gevirtz RN, O'Connor DT. Cardiovascular haemodynamic response to repeated mental stress in normotensive subjects at genetic risk of hypertension: evidence of enhanced reactivity, blunted adaptation, and delayed recovery. J Hum Hypertens. 2003;17:829–840. doi: 10.1038/sj.jhh.1001624. [DOI] [PubMed] [Google Scholar]
- 65.Snieder H, Harshfield GA, Barbeau P, Pollock DM, Pollock JS, Treiber FA. Dissecting the genetic architecture of the cardiovascular and renal stress response. Biol Psychol. 2002;61:73–95. doi: 10.1016/s0301-0511(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 66.Schork NJ, Fallin D, Thiel B, et al. The future of genetic case-control studies. Adv Genet. 2001;42:191–212. doi: 10.1016/s0065-2660(01)42023-2. [DOI] [PubMed] [Google Scholar]
- 67.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 68.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 69.O'Connor DT, Insel PA, Ziegler MG, et al. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 70.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- 72.Takiyyuddin MA, Brown MR, Dinh TQ, et al. Sympatho-adrenal secretion in humans: factors governing catecholamine and storage vesicle peptide co-release. J Auton Pharmacol. 1994;14:187–200. doi: 10.1111/j.1474-8673.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 73.Parmer RJ, O'Connor DT. Enkephalins in human phaeochromocytomas: localization in immunoreactive, high molecular weight form to the soluble core of chromaffin granules. J Hypertens. 1988;6:187–198. doi: 10.1097/00004872-198803000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;17:502–510. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.