Abstract
Background
HSP70 plays crucial roles in endothelial cell apoptosis, which is involved in the early phase and progress of coronary heart disease (CHD). However, the association between polymorphisms of HSP70 genes and the risk of CHD still remains unclear. Our aim was to determine whether genetic variants in the HSPA1A gene are associated with the risk of CHD.
Methodology/Principal Findings
By resequencing and genotyping, the associations of 2 single nucleotide polymorphisms (SNPs) +190G/C (rs1043618) and −110A/C (rs1008438) in the HSPA1A gene with risk of CHD were determined in a 1,003 pairs case-control study. The SNP function was further analyzed using a luciferase reporter assay in two cell lines. The results indicated that +190CC genotype was associated with significantly higher risk of CHD when compared with +190GG genotype (OR = 1.56, 95% CI: 1.10–2.20, P = 0.012), while association between −110A/C polymorphism and CHD was not statistically significant (P>0.05). However, the −110C/+190C haplotype had a significantly higher risk of CHD when compared with the −110A/+190G haplotype (OR = 1.17, 95% CI: 1.01–1.34, P = 0.031). Luciferase reporter assays showed that the +190C allele resulted in 14%∼45% reduction in luciferase expression in endothelial and non-endothelial cells when compared with the +190G allele.
Conclusions/Significance
The identified genetic variants in the HSPA1A gene combinatorially contribute towards the susceptibility to CHD likely by affecting the level of synthesis of HSP70. This study may provide useful markers for identification of subjects at risk for CHD.
Introduction
Coronary heart disease (CHD) is one of the leading causes of morbidity and mortality in China[1]. Each year there are about 2 million deaths from stroke- and CHD-related causes [2]. Gaining insights into the pathogenesis of CHD and identification of subjects at risk for CHD remains a tremendous challenge. It is well established that endothelial cells are the first protective barrier of vessel walls against various endogenous and exogenous stressors, and they also play crucial roles in controlling vascular tone, permeability, blood flow, coagulation, thrombolysis, inflammation and tissue repair[3]. Damage or apoptosis of endothelial cells is often considered as the initiating event of atherogenesis and also accelerates endothelialized plaque erosion and thrombosis[4], which will promote the development of acute cardiovascular incidents of CHD, such as unstable angina and myocardial infarction[5].
HSP70, as the main molecular chaperone, plays important roles in protecting against a wide variety of stress stimuli, including heavy metals, inflammation, and oxidative/ischemic injury[6]. Numerous evidences have corroborated the anti-oxidant and anti-apoptosis roles of HSP70[7]–[11]. HSP70 has also been found in the central portion of human atherosclerotic plaques[12]. Endothelial cells subjected to various stress conditions express increased amounts of HSP70 protein, which then functions as an antioxidant[8] and inhibits key processes of apoptosis pathways, to protect the integrity and functional activity of endothelial cells[13]. In addition, both in vivo and in vitro studies, indicated that the increased expression of HSP70 can protect the heart from stressful injury[14]–[17] and was associated with a reduction in myocardial apoptosis in ischemia-reperfusion injury[18]. It has been reported that the SNPs and haplotypes of HSP70 may contribute to certain disease susceptibility and stress tolerance [19]–[25]. HSPA1A is the key component of the 12 members of the HSP70 family being expressed both under normal conditions and substantively stimulated after different stresses[26], [27]. Based on the important role of HSP70 in protecting the integrity and functional activity of endothelial cells and its anti-oxidant properties, we hypothesized that genetic variations in the HSPA1A gene might affect HSP70 protein expression, thus conferring one's predisposition to CHD.
To comprehensively evaluate the potential implications of 2 single nucleotide polymorphisms (SNPs) of HSPA1A gene (−110A/C and +190G/C polymorphisms) in the etiology of CHD, we conducted a large-scale case-control study of 1,003 CHD cases and 1,003 age- and sex- frequency matched controls in a Chinese population. Using an in vivo reporter assay in endothelial cells (HUVEC) and non-endothelial cells (Hela), we found that the SNP +190G/C (dbSNP accession number rs1043618), located in the 5′ untranslated region (UTR) of HSPA1A gene, may affect the synthesis level of Hsp70 protein through translation efficiency or post-transcriptional regulation.
Results
SNPs Identification in the 5′ Flanking Region of HSPA1A Gene in Han Chinese
Resequencing of the HSPA1A gene in 60 unrelated Han Chinese revealed two SNPs in 5′ flanking region of HSPA1A gene. Referring the gene transcription start site as +1, one SNP is −110A/C (dbSNP accession number rs1008438) in the core promoter region and the other is +190G/C in the 5′UTR region, with minor allele frequency of 0.355 and 0.240 respectively. There were significant differences in allele frequencies of −110A/C and +190G/C across different ethnic groups (P<0.05) (Table 1).
Table 1. Comparison of Allele Frequencies of Polymorphisms in HSPA1A genes among Different Populations [36].
| SNPs | Population | Wild type | Mutation type | P Value |
| HSPA1A −110A/C | A | C | 0.000 | |
| Han Chinese | 0.645 | 0.355 | ||
| Sub-Saharan African | 0.083 | 0.917 | ||
| Hispanic | 0.477 | 0.523 | ||
| European | 0.886 | 0.114 | ||
| HSPA1A +190G/C | G | C | 0.001 | |
| Han Chinese | 0.760 | 0.240 | ||
| Japanese | 0.802 | 0.198 | ||
| CEU* | 0.658 | 0.342 | ||
| Yoruba | 0.902 | 0.098 |
CEU: Utah residents with ancestry from northern and western Europe.
General Characteristics of the Subjects
The clinical and demographic features of subjects have been described in our previous study[28]. In summary, CHD patients had a higher prevalence of conventional vascular risk factors, including smoking, non-drinking, history of hypertension and DM, family history of CHD and higher level of FBG, whereas TC level in patients were lower in cases than controls, probably due to cholesterol-lowering treatment in the cases.
HSPA1A Genotypes and CHD Risk
These two bi-allelic SNPs were investigated in 1,003 CHD patients and in controls. The distributions of SNPs +190G/C and −110A/C did not depart from the Hardy-Weinberg equilibrium in the control group (P = 0.18 and 0.32 respectively). Genotype frequencies of the two studied SNPs are summarized in Table 2. There was borderline significant difference in genotype distributions of SNP +190G/C between CHD cases and controls, but adjustment for the conventional risk factors such as age, sex, pack-year of smoking, drinking, activity, hypertension, DM, and family history of CHD yielded significant results (P = 0.012). Compared with GG genotype of +190G/C, subjects with CC genotype had a higher risk of CHD after adjusting for the conventional risk factors above (Crude OR = 1.33, 95% CI: 1.00–1.77, P = 0.052 and adjusted OR = 1.56, 95% CI: 1.10–2.20, P = 0.012 respectively). There was no significant difference between CHD cases and control group in −110A/C locus before or after adjusting for conventional risk factors (P>0.05). Haplotype analysis indicated that patients with −110C/+190C haplotype had a higher risk of CHD when compared with −110A/+190G haplotype (OR = 1.17, 95% CI: 1.01–1.34, P = 0.031) (Table 3).
Table 2. Analysis of Association between HSPA1A Polymorphisms and Risk of CHD in a Chinese population.
| Genotype | Cases | Controls | Crude OR (95% CI) | Adjusted OR (95% CI)* | ||
| n | % | n | % | |||
| +190G/C | ||||||
| GG | 434 | 44.2 | 479 | 47.8 | 1.00 | 1.00 |
| GC | 416 | 42.4 | 415 | 41.4 | 1.11(0.92–1.34) | 1.04(0.83–1.31) |
| CC | 131 | 13.4 | 109 | 10.8 | 1.33(1.00–1.77)† | 1.56(1.10–2.20)‡ |
| GC+CC | 547 | 55.8 | 524 | 52.2 | 1.15(0.97–1.38) | 1.13(0.91–1.40) |
| −110A/C | ||||||
| AA | 317 | 32.3 | 350 | 34.9 | 1.00 | 1.00 |
| AC | 476 | 48.6 | 472 | 47.1 | 1.11(0.91–1.36) | 1.15(0.90–1.46) |
| CC | 187 | 19.1 | 181 | 18.0 | 1.14(0.88–1.47) | 1.17(0.86–1.60) |
| AC+CC | 663 | 67.7 | 653 | 65.1 | 1.12(0.93–1.35) | 1.14(0.91–1.43) |
Adjusted for age, sex, pack-year of smoking, drinking, activity, hypertension, DM and family history of CHD. Compared with GG genotype.
P = 0.052 and
P = 0.012, respectively.
Table 3. Haplotype Distribution of HSPA1A in CHD and Control Group.
| Haplotype (−110/+190) | CHD | Controls | OR (95% CI) | ||
| n | % | n | % | ||
| −110A/+190G | 1092 | 54.44 | 1118 | 55.74 | 1.00 |
| −110C/+190C | 657 | 32.75 | 577 | 28.76 | 1.17 (1.01–1.34)* |
| −110C/+190G | 208 | 10.37 | 256 | 12.76 | 0.83 (0.68–1.02) |
| −110A/+190C | 49 | 2.44 | 55 | 2.74 | 0.91 (0.62–1.35) |
| Total | 2006 | 100 | 2006 | 100 | — |
Polymorphic bases were in 5′ to 3′ order and from left to right the order is −110A/C and +190G/C.
Compared with −110A/+190G haplotype, P = 0.031.
HSPA1A 5′ Flanking Region Carrying the +190C Polymorphisms leads to Lower HSP70 Expression
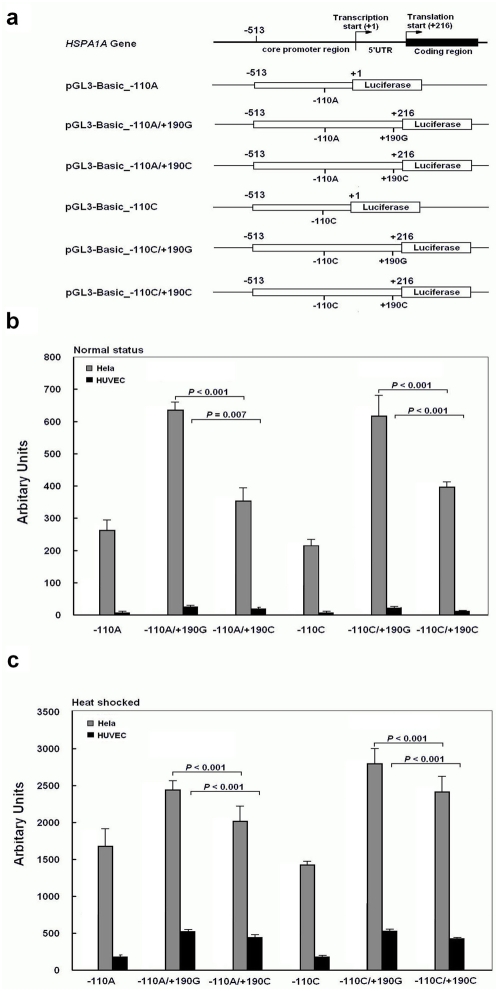
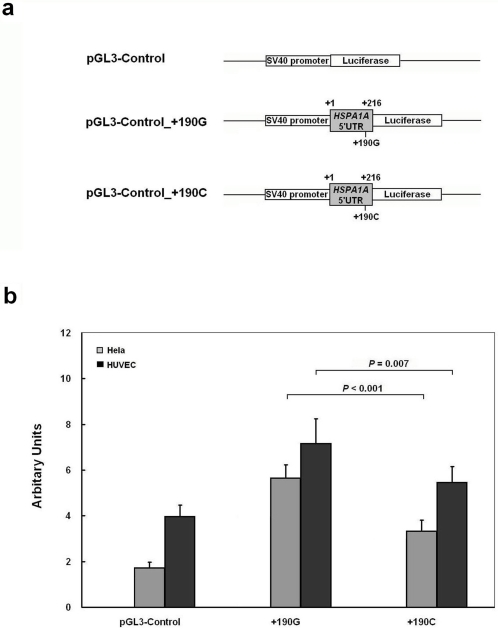
To investigate the possible functional significance of the SNPs in the HSPA1A gene, plasmids were constructed with luciferase as reporter gene and transfected in cultured cells. Under normal culture status, the RLA of all constructs containing fragments that include both core promoter region and 5′UTR of HSPA1A gene (−513 to +216) were remarkably higher than those carrying only the core promoter region (−513 to −1) in Hela, and HUVEC cells (P<0.0001) (Figure 1b). As for the four distinct haplotype plasmids, the RLA of −110A/+190G and −110C/+190G haplotype were significantly higher than that of −110A/+190C and −110C/+190C respectively (P<0.001), but no differences were found between −110A/+190G and −110C/+190G haplotype nor between −110A/+190C and −110C/+190C haplotype, suggesting that the reduction of RLA was due to the presence of the +190C allele (Figure 1b). To further explore the regulatory properties of the SNPs, transfected cells were submitted to a heat shock. The RLA recorded, were increased for about 6∼7 times in Hela cells and about 20 times in HUVEC cells. In the heat shock groups, the results were similar to those under normal conditions in all cells (Figure 1c). The HSPA1A 5′-flanking region bearing the −110C/+190C haplotype exhibited about 30%∼50% lower RLA than the −110A /+190G haplotype in endothelial (HUVEC) and non-endothelial cells (Hela). We also inserted the full-length 5′UTR containing either +190G or +190C directly following the SV40 promoter and before the translation start codon of the firefly luciferase gene of pGL3-Control vector (Figure 2a). The full-length 5′UTR of HSPA1A gene increased luciferase activity of the pGL3-Control vector (P<0.001), with the +190C allele resulting in 14%∼45% reduction in luciferase expression compared to the +190G allele in Hela and HUVEC cell lines (P<0.001, and P = 0.007, respectively) (Figure 2b).
Figure 1. Effects of −110A/C and +190G/C polymorphisms on luciferase activity.
(a), Schematic representation of HSPA1A gene 5′ flanking region and reporter constructs. (b) to (c), the six reporter constructs and empty pGL3-Basic was transfected into Hela and HUVEC cells under normal culture (b) or heat shocked conditions (c). All constructs were cotransfected with pRL-SV40 to standardize the transfection efficiency. Fold increase of luciferase activity was measured by defining the activity of the empty pGL3-Basic vector as 1. The RLA values are presented as means, and the T bars represent standard deviations (SD). Mean±SD from triplicates.
Figure 2. Effect of +190G/C polymorphism on the expression of luciferase.
(a), Schematic representation of reporter constructs. (b), These constructs and empty pGL3-Control plasmid were transfected into Hela and HUVEC cells. All constructs were cotransfected with pRL-SV40 to standardize the transfection efficiency. RLA was determined 24 hours later in normal culture condition. The RLA values are presented as means, and the T bars represent standard deviations (SD). Mean±SD from triplicates.
Discussion
Our study is among the few that examined the association between SNPs of HSPA1A gene and risk of CHD. Resequencing of this gene in 60 unrelated Han Chinese revealed two SNPs in 5′ flanking region of HSPA1A gene; one is −110A/C in the core promoter and the other is +190G/C located in the 5′UTR region. The allele frequencies of these two SNPs in the Han Chinese population were significantly different from Sub-Saharan African, Hispanic and European races. Our large case-control analysis showed no strong association between −110A/C polymorphism and CHD, which was consistent with the result of a previous European study that indicated no association between this polymorphism and myocardial infarction[28]. However, we found that the +190C allele and the haplotypes containing +190C were significantly associated with CHD risk. Since the two SNPs both reside in the highly conserved regulatory region of the gene, we further explored their putative effects on HSPA1A promoter activity and found that the HSPA1A 5′UTR enhanced expression of a luciferase reporter driven either by the HSPA1A or SV40 promoter; the allele-specific data of reporter assays in both endothelial (HUVEC) and non-endothelial (Hela) cells yielded consistent results that the +190C allele in the 5′UTR of HSPA1A gene leads to a reduction in promoter activity and probably lower synthesis level of HSP70 protein than +190G allele.
The function of HSPA1A 5′UTR sequence of HSPA1A mRNA was comprehensively analyzed and reported to act as a general enhancer of mRNA translation[29]. Our findings are consistent with the above study and the effect of HSPA1A 5′UTR was independent of the promoter used. The HSPA1A 5′UTR sequence has an internal ribosome entry site (IRES) for translation initiation by a cap-dependent mechanism, which depends on its 3-dimensional secondary structures of full-length 5′UTR, especially the base-pairing status and nucleotide context of the 3′-terminal AUG-proximal region of HSPA1A 5′UTR [30]. Theoretical secondary structure folding analysis by Mfold program suggested that the HSPA1A 5′UTR sequence containing +190G or +190C allele had distinct thermodynamic parameters and different stem-loop structure in its 3′-terminal region[31]. Albeit untested experimentally, the modulation caused by the +190 G/C polymorphism, which is just 26 bp upstream the initial codon, may arise from altered stem-loop structure of the 5′UTR of HSPA1A mRNA, thus confer different efficiency of HSPA1A mRNA translation. Since heat shock treatment is the typical model up-regulating expression of HSP70, we can postulate that such difference in 5′UTR may also be applicable under other stresses, such as inflammation, ischemic and oxidative injury, all of which play critical roles in the pathogenesis of CHD.
Thus we showed that the −110C/+190C haplotype, which was significantly associated with CHD risk had a 30%∼50% lower RLA than −110A /+190G haplotype in both endothelial and non-endothelial cells. The RLA of HSPA1A promoter plasmid driven by −110A was higher than that of −110C in Hela cell lines, but didn't reach the significant level in HUVEC cells (data not shown). We would like to speculate that the −110A/C polymorphism is also a potential functional SNP, and that when combined with +190G/C polymorphism, may act as an additive effect on the synthesis of HSP70 protein.
The present study has several strengths. First, our population was highly homogenous with respect to ethnicity and geographic regions, which minimizes the possible biases related to population stratification. In addition, the large number of cases and controls provided sufficient power to detect moderate effects of the genotypes and haplotypes. Finally, the possible patho-physiological significance of the +190G/C polymorphism was further confirmed by detailed functional assays in both endothelial and non-endothelial cell lines with consistent results. This strengthened the association between the HSPA1A gene polymorphisms and CHD revealed by the present study. However two limitations should also be acknowledged. First, because this was a retrospective study, a possible selection bias (inclusion of patients surviving CHD) and/or systemic error may exist. However, there was no evidence that these SNPs influence survival. Meanwhile, we selected controls that had normal electrocardiograms and no history and signs of CHD, but we can not exclude the possibility that some of them were affected by silent myocardial infarction because we did not perform coronary angiography on those control subjects. However, the prevalence of CHD in China is still low[32], especially among those without positive ECG test or clinical symptoms. All our controls were required to have normal ECG and no clinical symptoms before enrollment, thus the false negative cases in the controls are likely to be rare.
In conclusion, our results of the large-scale case-control study and reporter assays support the hypothesis that reduced expression of HSP70 protein induced by the HSPA1A +190C allele may dampens its cytoprotection role on endothelial cells and thus contributes to one's predisposition to CHD. Our study provides the first evidence that genetic variants in HSPA1A gene may potentially contribute to the susceptibility to CHD. However, our findings need to be replicated in additional studies, especially in large prospective cohort studies.
Materials and Methods
Human Subjects and General Characteristics
The study design for this investigation has been described elsewhere[33]. Briefly, the study population was composed of 1,003 cases and 1,003 age- and sex- frequency matched controls. All enrolled subjects were unrelated ethnic Han Chinese. Case patients, who were enrolled from three hospitals (Tongji Hospital, Union Hospital, and Wugang Hospital) in Wuhan City, Hubei province, were diagnosed as having CHD according to WHO criteria or by coronary angiography (significant coronary artery stenoses ≥50% in at least one major coronary artery). A total of 1,078 patients diagnosed as having CHD were recruited; 1,003 of them (93.0%) consented to participate in the study and provided questionnaire information and blood samples. The control subjects, residing in the same communities as the cases, were judged to be free of CHD and peripheral atherosclerotic arterial disease by medical history, clinical examinations, and electrocardiography (ECG). The response rate for the controls was 92.4% (1,003 of 1,085). Subjects with severe liver and/or kidney disease were excluded. Subjects were classified as nonsmokers, former, or current smokers. Pack-years were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person had smoked. BMI was calculated as weight in kilograms divided by the square of height in meters. Subjects were considered to be hypertensive if their systolic blood pressure was ≥140 mmHg and/or diastolic pressure ≥90 mmHg or they were already being treated with antihypertensive drugs. Medical history, socio-demographic information, family history of cardiovascular disease, medication use, home environment, and lifestyle factors were obtained through questionnaire interview. All subjects gave written consent after receiving a full explanation of the study. The Ethics Committee of Tongji Medical College approved the study.
Screening for SNPs in 5′ Flanking Region of HSPA1A Gene in Chinese
DNA samples extracted from whole blood of 60 randomly selected healthy subjects were used to identify SNPs in the 5′ flanking region of HSPA1A gene (GenBank accession no. NM_005345) in the Han Chinese population. Sequencing reactions were carried out on an ABI 3100 genetic analyzer. All primers and reaction conditions are listed in Table S1.
Genotyping of HSPA1A polymorphisms
Genotyping of HSPA1A was performed with 5′ nuclease TaqMan allelic discrimination assay on an ABI 7900HT real-time quantitative PCR system (Applied Biosystems), in 384-well format. For −110A/C polymorphism, the TaqMan primers were 5′- GCCTCTGATTGGTCCAAGGAA-3′ and 5′-GCTGCCAGGTCGGGAATAT-3′,while probes were FAM - AGGCGAAACCCCTGG-MGB for −110C and VIC- AGGCGAAAACCCTGG –MGB for −110A. The catalog number for +190G/C polymorphism was C-11917510-10. Finally, genotyping failed in 22 (2.20%) cases in +190G/C locus, 23 (2.30%) cases in −110A/C locus owing to DNA quantity or quality.
Biological variables determination
Fasting blood glucose (FBG), total cholesterol (TC), and triglyceride (TG) were assayed using standard laboratory procedures at the Department of Clinical Laboratory at the Wuhan Union Hospital.
Construction of Reporter Plasmids
We constructed six reporter plasmids based on pGL3-Basic vector (Promega, Madison, Wisconsin, USA), including four fragments compassing from −513 to +216 according to their haplotypes (−110A/+190G, −110A/+190C, −110C/+190G and −110C/+190C, respectively) and two core promoter regions from −513 to −1 containing either −110A or −110C allele. The position “+1” indicates the transcription start site (Figure 1a). They were all inserted into Kpn I/Hind III enzyme sites of pGL3-Basic. We also inserted the full-length 5′UTR containing +190G or +190C allele into the Hind III /Nco I enzyme sites of pGL3-Control vector. Primer pairs designed for plasmid constructs and site-specific mutagenesis are listed in Table S2. The direction and sequence authenticity of the above constructs were validated by restriction analysis and direct sequencing.
Transient Transfection and Luciferase Reporter Assays
Hela cells were cultured with RPMI 1640 containing 10% fetal bovine serum and seeded into 96-well plates at a density of 3×104 cells per well respectively, while 4×104 human umbilical vein endothelial cells (HUVEC) were seeded into 48-well plates. Twenty-four hours later when cells had grown to about 70% confluence, each well was co-transfected with 100 ng pGL3-Basic plasmids or its constructs (defined as −110A, −110A/+190G, −110A/+190C, −110C, −110C/+190G, −110C/+190C) and 1 ng pRL-SV40 (Promega) using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) according to the manufacturer's protocol. The Hela and HUVEC cells were harvested 24 hours after transfection, whereas in the heat-shocked group, cells were placed at 42°C for 1 hour in a water bath and then allowed to recover at 37°C for 4 hours before measurement. Then luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega) on a TD-20/20n luminometer (Turner Design, Promega). Each construct was tested in six assays and the transfection experiments were performed three times independently. As for the pGL3-Control and its constructs, the same transfection procedures were done. The result was denoted as relative luciferase activity (RLA) since the luciferase activity was normalized by Renilla activity and the empty pGL3-Basic or pGL3-Control vector.
Statistical Analysis
Normal distribution of data was checked by the Komogorov-Smirnov normality test. Data with a normal distribution were compared by Student's t-test, and those with unequal variance or without a normal distribution were analyzed by a nonparametric Mann-Whitney rank sum test. Different RLA of four haplotypes contained plasmids were determined by one-way ANOVA and Student-Newman-Keuls test. A Chi-square test was applied to compare categorical variables and the Hardy-Weinberg equilibrium of the polymorphisms. The linkage relationship between the two SNPs in HSPA1A genes was measured by linkage disequilibrium analyzer (LDA) program[34]. All genotype data for each sample were taken to infer the haplotypes by using the PHASE 2.0 program[35], a software for reconstruction of haplotypes from population genotype data by Bayesian statistical method. The associations between variants and CHD risk were estimated by odds ratios (ORs) and 95% confidence intervals (CIs) by using the unconditional logistic regression analyses with adjustment for multiple cardiovascular risk factors such as age, sex, pack-year of smoking, drinking activity, hypertension, diabetes mellitus (DM), and family history of CHD. A P value of less than 0.05 was considered significant. All data analyses were carried out with statistical analysis software package SPSS 12.0 (SPSS Inc., Chicago, Illinois, USA).
Supporting Information
(0.04 MB DOC)
(0.03 MB DOC)
Acknowledgments
The authors wish to thank all individuals who voluntarily participated in the present study and the medical assistants of Tongji Hospital, Union Hospital and Wugang Hospital in Wuhan city, Hubei Province, China. We also appreciate the technical supports and helpful comments of members of our lab and work team of Prof. Dongxin Lin in Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the National Natural Science Foundation of China (NSFC 30525031 and 30430590), NSFC-CIHR Collaborative Research Grant and NSFC (30711120579). Dr. Frank B. Hu's work was supported in part by the American Heart Association Established Investigator Award. TW and RMT also acknowledge support from a NNSFC-CIHR (Canadian Institutes of Health Research) collaborative research grant (CIHR CCI-85673). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World health orgnization. World Health Statistics Annual. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 2. http://www.moh.gov.cn.
- 3.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 4.Durand E, Scoazec A, Lafont A, Boddaert J, Al Hajzen A, et al. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation. 2004;109:2503–2506. doi: 10.1161/01.CIR.0000130172.62481.90. [DOI] [PubMed] [Google Scholar]
- 5.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 7.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 8.Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70:254–263. doi: 10.1016/j.cardiores.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, et al. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–51499. doi: 10.1074/jbc.M401314200. [DOI] [PubMed] [Google Scholar]
- 11.Clemons NJ, Buzzard K, Steel R, Anderson RL. Hsp72 inhibits Fas-mediated apoptosis upstream of the mitochondria in type II cells. J Biol Chem. 2005;280:9005–9012. doi: 10.1074/jbc.M414165200. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AD, Berberian PA, Tytell M, Bond MG. Differential distribution of 70-kD heat shock protein in atherosclerosis. Its potential role in arterial SMC survival. Arterioscler Thromb Vasc Biol. 1995;15:27–36. doi: 10.1161/01.atv.15.1.27. [DOI] [PubMed] [Google Scholar]
- 13.Kabakov AE, Budagova KR, Bryantsev AL, Latchman DS. Heat shock protein 70 or heat shock protein 27 overexpressed in human endothelial cells during posthypoxic reoxygenation can protect from delayed apoptosis. Cell Stress Chaperones. 2003;8:335–347. doi: 10.1379/1466-1268(2003)008<0335:hspohs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radford NB, Fina M, Benjamin IJ, Moreadith RW, Graves KH, et al. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994;89:355–360. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- 17.Currie RW, Tanguay RM, Kingma JG., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Sawa Y, Kagisaki K, Taketani S, Ichikawa H, et al. Reduction in myocardial apoptosis associated with overexpression of heat shock protein 70. Basic Res Cardiol. 2000;95:397–403. doi: 10.1007/s003950070039. [DOI] [PubMed] [Google Scholar]
- 19.Milner CM, Campbell RD. Polymorphic analysis of the three MHC-linked HSP70 genes. Immunogenetics. 1992;36:357–362. doi: 10.1007/BF00218042. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese A, Awdeh ZL, Galluzzo A, Yunis EJ, Alper CA, et al. No independent association between HSP70 gene polymorphism and IDDM. Diabetes. 1992;41:788–791. doi: 10.2337/diab.41.7.788. [DOI] [PubMed] [Google Scholar]
- 21.Jarjour W, Reed AM, Gauthier J, Hunt S, 3rd, Winfield JB. The 8.5-kb PstI allele of the stress protein gene, Hsp70-2: an independent risk factor for systemic lupus erythematosus in African Americans? Hum Immunol. 1996;45:59–63. doi: 10.1016/0198-8859(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 22.Favatier F, Bornman L, Hightower LE, Gunther E, Polla BS. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraile A, Nieto A, Mataran L, Martin J. HSP70 gene polymorphisms in ankylosing spondylitis. Tissue Antigens. 1998;51:382–385. doi: 10.1111/j.1399-0039.1998.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Alarcon G, Londono JD, Hernandez-Pacheco G, Gamboa R, Castillo E, et al. Heat shock protein 70 gene polymorphisms in Mexican patients with spondyloarthropathies. Ann Rheum Dis. 2002;61:48–51. doi: 10.1136/ard.61.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YR, Wang CK, Chen CM, Hsu Y, Lin SJ, et al. Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson's disease. Hum Genet. 2004;114:236–241. doi: 10.1007/s00439-003-1050-1. [DOI] [PubMed] [Google Scholar]
- 26.Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- 27.Brocchieri L, Conway de Macario E, Macario AJ. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolla MK, Miller GJ, Yellon DM, Evans A, Luc G, et al. Analysis of the association of a heat shock protein70-1 gene promoter polymorphism with myocardial infarction and coronary risk traits. Dis Markers. 1998;13:227–235. doi: 10.1155/1998/235151. [DOI] [PubMed] [Google Scholar]
- 29.Vivinus S, Baulande S, van Zanten M, Campbell F, Topley P, et al. An element within the 5′ untranslated region of human Hsp70 mRNA which acts as a general enhancer of mRNA translation. Eur J Biochem. 2001;268:1908–1917. doi: 10.1046/j.1432-1327.2001.02064.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubtsova MP, Sizova DV, Dmitriev SE, Ivanov DS, Prassolov VS, et al. Distinctive properties of the 5′-untranslated region of human hsp70 mRNA. J Biol Chem. 2003;278:22350–22356. doi: 10.1074/jbc.M303213200. [DOI] [PubMed] [Google Scholar]
- 31.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Liu X, Li X, Li Y, Zhao L, et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114:2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 33.He MA, Zhang X, Wang J, Cheng L, Zhou L, et al. Genetic variation in heat shock protein 60 gene and coronary heart disease in China: tagging-SNP haplotype analysis in a case-control study. Cell Stress Chaperones. 2008;13:231–238. doi: 10.1007/s12192-008-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding K, Zhou K, He F, Shen Y. LDA–a java-based linkage disequilibrium analyzer. Bioinformatics. 2003;19:2147–2148. doi: 10.1093/bioinformatics/btg276. [DOI] [PubMed] [Google Scholar]
- 35.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. http://www.ncbi.nlm.nih.gov/SNP.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.04 MB DOC)
(0.03 MB DOC)